Introduction
Lichenization is one of the most important life strategies among fungi in the Ascomycota, but only a comparatively small number of ascomycete lichen fungi (c. 1700 species; Rikkinen Reference Rikkinen2002) utilize cyanobacteria as photobionts. These lichens may still have a substantial impact on the ecosystems they inhabit by contributing fixed atmospheric nitrogen (Cornelissen et al. Reference Cornelissen, Lang, Soudzilovskaia and During2007; Nash Reference Nash2008; Campbell et al. Reference Campbell, Fredeen and Prescott2010), and many cyanobacterial lichens are sensitive to habitat disturbance such as changes in forest age, structure and composition (e.g. Price & Hochachka Reference Price and Hochachka2001; Scheidegger et al. Reference Scheidegger, Groner, Keller and Stofer2002; Hedenås & Ericson Reference Hedenås and Ericson2008; Fedrowitz et al. Reference Fedrowitz, Kuusinen and Snäll2012) and pollution (e.g. Goward & Arsenault Reference Goward and Arsenault2000; Jovan Reference Jovan2008). The largest group of lichenized Ascomycota featuring cyanobacteria as the main or sole photobiont is Peltigerales in the Lecanoromycetes (Wiklund & Wedin Reference Wiklund and Wedin2003; Lumbsch et al. Reference Lumbsch, Schmitt, Palice, Wiklund, Ekman and Wedin2004; Wedin et al. Reference Wedin, Wiklund, Crewe, Döring, Ekman, Nyberg, Schmitt and Lumbsch2005; Schoch et al. Reference Schoch, Sung, López-Giráldez, Townsend, Miądlikowska, Hofstetter, Robbertse, Matheny, Kauff and Wang2009; Miądlikowska et al. Reference Miądlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014; Rikkinen Reference Rikkinen2015). Peltigerales are a comparatively recent group of fungi, the ancestor of which diverged from its Lecidealean sister-group in the early Jurassic, and the group diversified towards the end of the Jurassic-early Cretaceous (Prieto & Wedin Reference Prieto and Wedin2013). Peltigerales currently includes ten families (Wedin et al. Reference Wedin, Jørgensen and Wiklund2007, Reference Wedin, Jørgensen and Ekman2011; Spribille & Muggia Reference Spribille and Muggia2013), one of which is Placynthiaceae.
Placynthiaceae comprises comparatively small, flat, rosette-forming, crustose to squamulose-lobate species where the thalli often produce a prothallus (Czeika & Czeika Reference Czeika and Czeika2007; Jørgensen Reference Jørgensen2007). After the recent reclassification of Vestergrenopsis into the newly described family Koerberiaceae (Spribille & Muggia Reference Spribille and Muggia2013), Placynthiaceae currently includes two genera: Placynthium with c. 30 species worldwide, and the monotypic Collolechia. Placynthium and Collolechia differ in thallus structure (squamulose vs. leprose-crustose thallus), ascus apex structure (apical caps/sheets vs. apical ring/tube) and spore shape and septation (ellipsoid, 1–3 septate in Placynthium vs. elongate, fusiform-acicular, pluriseptate in Collolechia; Jørgensen Reference Jørgensen2005, Reference Jørgensen2007). These observations were made on Placynthium nigrum (the type for Placynthium), and Collolechia caesia, respectively (Jørgensen Reference Jørgensen2005). Several authors, however, have observed tube structures in the asci of Placynthium, including in P. nigrum (Keuck Reference Keuck1977; Rambold & Triebel Reference Rambold and Triebel1992; Gilbert & James Reference Gilbert and James2009; Øvstedal et al. Reference Øvstedal, Tønsberg and Elvebakk2009; Wirth et al. Reference Wirth, Hauck and Schultz2013). The presence of a tube in Placynthiaceae was pointed out as supporting the sister-group relationship with Collemataceae by Wiklund & Wedin (Reference Wiklund and Wedin2003). Several authors have also noted large variation within the group, including cap- or sheet-like structures, and tube- or ring-structures (Czeika & Czeika Reference Czeika and Czeika2007; Spribille & Muggia Reference Spribille and Muggia2013).
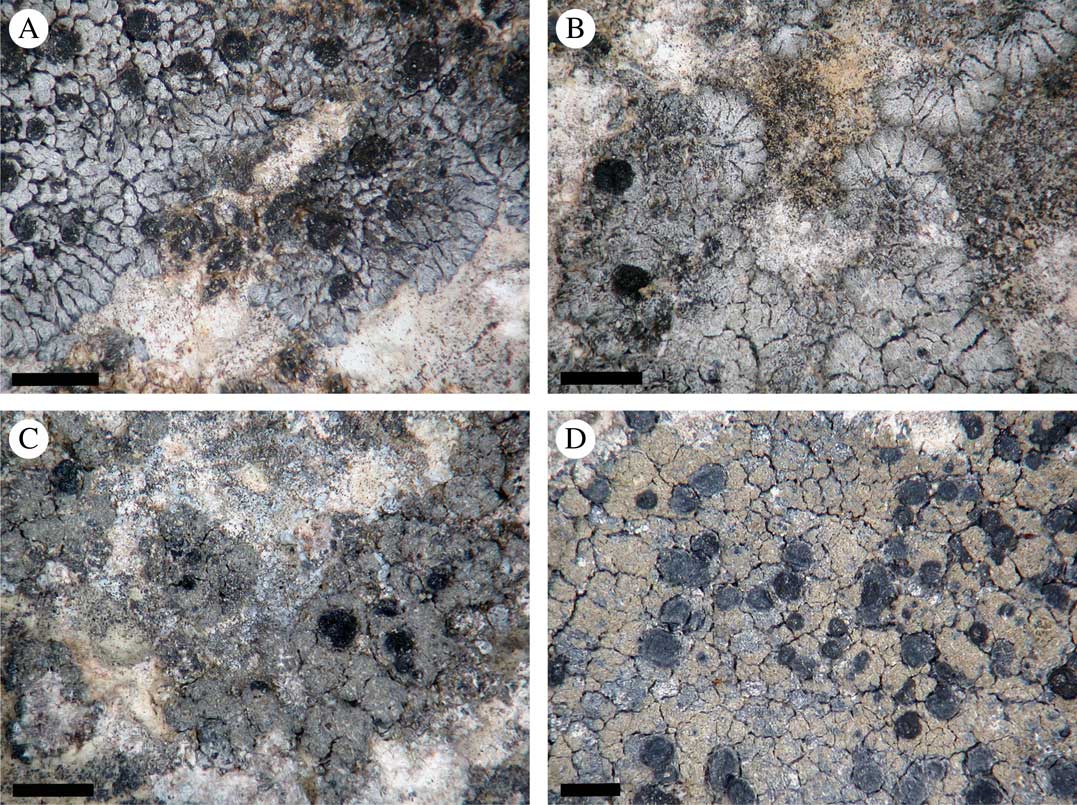
In Scandinavia, Collolechia caesia was for many years confused with Placynthium garovaglioi (Fig. 1). The distinction between the species was clarified by Jørgensen (Reference Jørgensen2005) who showed that in Scandinavia C. caesia was known only from a couple of localities on the Baltic island of Gotland, where it had not been collected since 1942. These are northern outposts for a species with an otherwise mainly southern warm-temperate distribution. True Placynthium garovaglioi is not known from Sweden. In 2014, we visited some of the known Swedish localities to assess the status of C. caesia, and collected fresh material for DNA-based studies. In this study, we investigated the phylogenetic relationships of Collolechia to test the current generic delimitations in Placynthiaceae. We reinvestigated herbarium material of Collolechia and P. garovaglioi, and studied the spore and ascus apex characteristics in these species and other selected Placynthiaceae in order to assess variation and potential natural groupings.
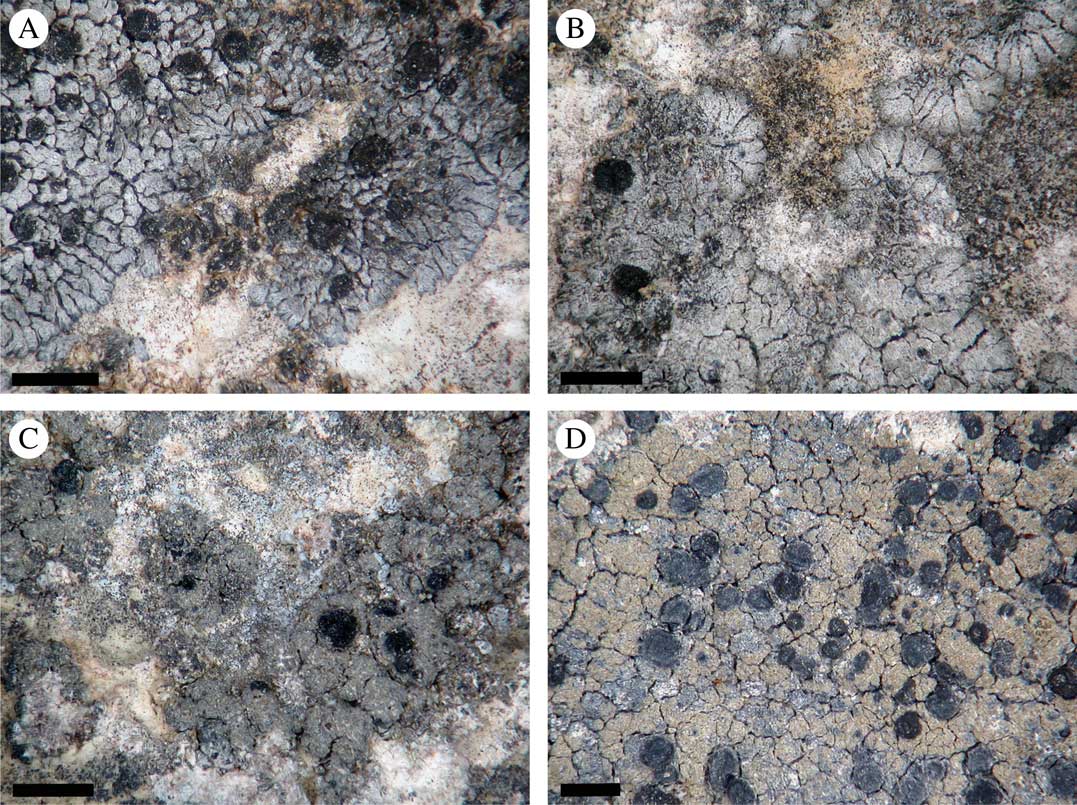
Fig. 1 Morphology of Placynthium garovaglioi and “Collolechia” caesia. A & B, thallus of Placynthium garovaglioi with distinct marginal lobes; C & D, “Collolechia” caesia with a leprose thallus lacking marginal lobes. Specimen origin. A, Palice 16564 (SAV); B, Palice 16954 (S); C, Košuthová GOT2 (S); D, Cleve s. n. (S). Scales=1 mm. In colour online.
Material and Methods
Taxon sampling and morphological analysis
Material utilized for the phylogenetic study was mainly freshly collected specimens but was also supplemented with herbarium material from S, SAV and UPS and the personal herbaria of Z. Palice (hb. Palice) and J. Malíček (hb. Malíček). Origin of the material is summarized in Table 1. Asci were studied under oil-immersion, in hand-cut sections of apothecia which were pretreated with and squashed in KOH and subsequently stained with Lugol’s solution.
Table 1 Specimen information and European Nucleotide Archive or GenBank accession numbers for the specimens included in the phylogenetic inference depicted in Figure 2. Sequences represented in bold font were generated in this study

DNA extraction, amplification and sequencing
Total DNA was extracted from fresh material and herbarium specimens, and isolated using the DNeasy Plant Mini Kit (Qiagen, Germany) following the manufacturer’s instructions. We amplified ≈0·6 kb of the small subunit of the mitochondrial rDNA (mtSSU) and ≈1·2 kb of nuclear mini-chromosome maintenance complex component 7 (Mcm7). Primer combinations used in this study were: mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999) for the mtSSU; and MCM7-709for and MCM7-1348rev for the Mcm7 (Schmitt et al. Reference Schmitt, Crespo, Divakar, Fankhauser, Herman-Sackett, Kalb, Nelsen, Nelson, Rivas-Plata and Shimp2009). Symmetric PCR amplifications were performed using IllustraTM Hot Start PCR beads, according to the manufacturer’s instructions. PCR reactions for mtSSU were performed using one of two cycling conditions, depending on what worked with particular samples. The first was 95°C for 5 min followed by 35–40 cycles (95°C for 1 min, 54°C for 50 s, and 72°C for 1 min), with a final extension of 72°C for 8 min. The second was as follows: 95°C for 5 min followed by 4 cycles (95°C for 1 min, 58°C for 1 min, and 72°C for 1 min), 4 cycles (95°C for 1 min, 56°C for 1 min, and 72°C for 1 min) and 34 cycles (95°C for 1 min, 54°C for 1 min, and 72°C for 1 min) with a final extension of 72°C for 8 min. For the amplification of the Mcm7, the following cycling conditions were used: 95°C for 5 min followed by 4 cycles (95°C for 1 min, 60°C for 1 min, and 72°C for 1 min), followed by 36 cycles (95°C for 1 min, 58°C for 1 min, and 72°C for 1 min), with a final extension of 72°C for 8 min. After examination by gel electrophoresis, amplification products were purified using ExoSAP-IT (USB Corp., USA). Sequencing of both strands was performed with the Big Dye Terminator technology kit v3.1 (ABI PRISM, USA) using the PCR primers, and the additional internal PCR primers mrSSU2 and mrSSU2R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999).
Sequence alignments and analyses
Sequence fragments were assembled and edited using Sequencer 4.9 (Gene Codes Corp., Ann Arbor, MI) and Geneious version R8 (http://www.geneious.com, Kearse et al. Reference Kearse, Moir, Wilson, Stones-Havas, Cheung, Sturrock, Buxton, Cooper, Markowitz and Duran2012), and were subjected to BLAST searches (Zhang et al. Reference Zhang, Schwartz, Wagner and Miller2000) for a first identity verification. Sequences were aligned manually in Aliview 1.09 (Larsson Reference Larsson2014). Introns and ambiguously aligned regions (sensu Lutzoni et al. Reference Lutzoni, Wagner, Reeb and Zoller2000) were delimited manually and excluded from the analyses. We analyzed the mtSSU and Mcm7 datasets separately using maximum likelihood (ML) as the optimization criterion, with GARLI v.2.0 (Zwickl Reference Zwickl2006). Models of molecular evolution were estimated for each locus using the Akaike information criterion correction for finite sample sizes (AICc; Akaike Reference Akaike1973) implemented in jModeltest v.0.1.1 (Guindon & Gascuel Reference Guindon and Gascuel2003; Posada Reference Posada2008). The models selected were TVM+I+G (Posada Reference Posada2003) for mtSSU, TrNef+I (Tamura & Nei Reference Tamura and Nei1993) for Mcm7 first codon position, F81+I (Felsenstein Reference Felsenstein1981) for Mcm7 second codon position, and K80+G (Kimura Reference Kimura1980) for Mcm7 third codon position. We performed ML searches setting the program to stop after 10 000 generations if no improvement of the Ln likelihood ≤0·01 was detected, with a maximum of 500 000. Topological incongruence between the two datasets was examined using the consensus trees from 1000 replicates of ML bootstrapping under the same models, on each locus separately (Mason-Gamer & Kellogg Reference Mason-Gamer and Kellogg1996). Because no incongruence was detected using a 70% reciprocal threshold, the two alignments were concatenated and one specimen (Placynthium garovaglioi AL148) for which we have only the mtSSU sequence included. The concatenated alignment was deposited in TreeBASE (accession number S18034).
Phylogenetic relationships and confidence were inferred on the combined dataset using ML and Bayesian inference (B). For the ML analysis, the same settings were used as in the individual gene analyses using GARLI v.2.0, with the same models specified for each partition, for both ML search and ML bootstrap analyses. The Bayesian inference of the phylogeny was carried out by a Metropolis coupled Markov chain Monte Carlo (MCMCMC), as implemented in MrBayes 3.2.3 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). The substitution models estimated using the AICc implemented in jModeltest v.0.1.1 were GTR+I+G (Tavaré Reference Tavaré1986) for mtSSU, SYM+I (Zharkikh Reference Zharkikh1994) for Mcm7 first codon position (MCM7_c1), F81+I for Mcm7 second codon position (MCM7_c2), and K80+G for Mcm7 third codon position (MCM7_c3). The prior distributions settings were: all topologies equally probable and branch lengths followed an unconstrained gamma distribution (1, 0·1, 1, 1); the state frequencies followed a (1, 1, 1, 1) Dirichlet distribution for mtSSU and MCM7_c2 and were equally probable for MCM7_c1 and MCM7_c3; the rate matrix for mtSSU and MCM7_c1 followed a (1, 1, 1, 1, 1, 1) Dirichlet distribution, for the transition-transversion rates for MCM7_c3 a beta (1, 1) distribution and was equally probable for MCM7_c2; when applicable, proportion of invariable sites followed a uniform distribution (0, 1). Two parallel runs with four independent chains each were conducted for 20 million generations, with trees sampled at intervals of 500 generations. A burn-in sample of the first 10 000 trees was discarded for each run and the remaining trees were used to estimate branch lengths and posterior probabilities (PP). Convergence was monitored with the diagnostic tool provided by MrBayes 3.2.3., including the average standard deviation of splits between runs. All analyses were run in the CIPRES Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010).
Hypothesis testing
We specified two hypotheses to be tested. One equals the classification where Placynthium and Collolechia are two separate accepted genera (H0: Placynthium monophyletic excluding Collolechia). The alternative hypothesis (H1) corresponds to the case where Collolechia is nested within a paraphyletic Placynthium. In order to contrast the hypotheses, we calculated Bayes factors by comparing the ratio of the marginal likelihoods of each hypothesis. One common approach is to estimate the marginal likelihoods from constrained and unconstrained Bayesian analyses (e.g., Nelsen & Gargas Reference Nelsen and Gargas2009; Otálora et al. Reference Otálora, Jørgensen and Wedin2014; Westberg et al. Reference Westberg, Millanes, Knudsen and Wedin2015). Here, however, we followed the novel approach proposed in Bergsten et al. (Reference Bergsten, Nilsson and Ronquist2013), in which the marginal likelihoods are calculated from two alternative topologies after the specification of equally informed priors (constraints). Interpretation of Bayes factor values followed Kass & Raftery (Reference Kass and Raftery1995). We calculated the marginal likelihoods using the stepping-stone sampling algorithm implemented in MrBayes 3.2.3., which has proved to be a more accurate estimator of the model likelihoods than the harmonic mean estimator calculated in the MCMC output (Ronquist et al. Reference Ronquist, Huelsenbeck and Teslenko2011; Bergsten et al. Reference Bergsten, Nilsson and Ronquist2013). We ran the stepping-stone sampling taking 50 steps for a total of 10 200 000 generations, sampling every 100th generation, and discarding the first 200 000 generations as burn-in. The contribution to the marginal likelihood in each step was estimated from a sample size of 2000.
Results
New sequences from two loci were produced for the 19 specimens, except for Placynthium garovaglioi AL148 for which only mtSSU was obtained. These were aligned with 10 sequences from five taxa representing several families of the order Peltigerales (i.e., Collemataceae, Lobariaceae and Pannariaceae), retrieved from GenBank. Lobaria pulmonaria was selected as outgroup to root the tree. Voucher information for newly produced sequences and accession numbers are listed in Table 1. The matrix of aligned sequences included 1588 sites (635 for Mcm7 and 953 for mtSSU), which was reduced to 1311 sites (of which 297 were parsimony-informative) after the exclusion of the flanking primer regions, introns, and ambiguously aligned regions.
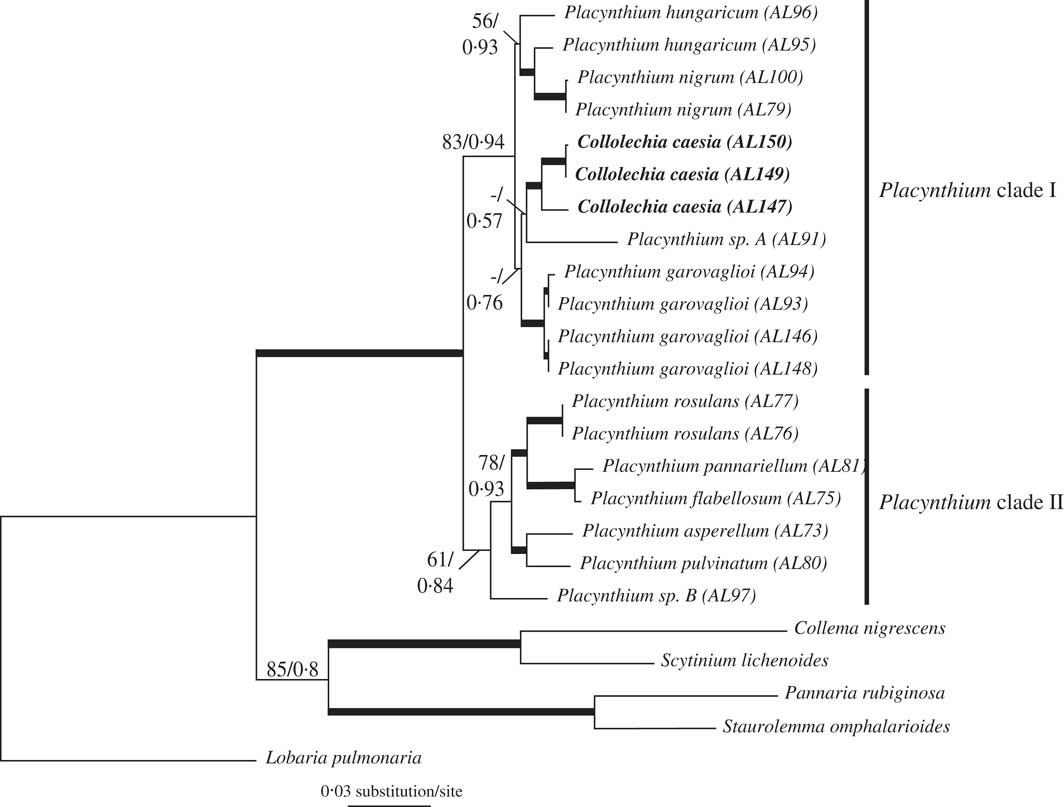
The most likely tree from ML (Fig. 2) with ln likelihood=−6126·8160 recovered a topology with 22 resolved internodes, of which 17 were significantly supported (i.e., ML-BS≥70%). In the Bayesian analysis, the value of the standard deviation of splits between runs was 0·000632, below the threshold of 0·01 established for convergence (Ronquist et al. Reference Ronquist, Huelsenbeck and Teslenko2011). This was further confirmed as the PSRF of all parameters and bipartitions was close to 1·0. The 50% majority-rule consensus trees of the 60 000 trees showed 21 resolved internodes, of which 14 were significantly supported (PP≥0·95). As the topologies had no significant conflicts, only the ML tree is shown in Fig. 2, with the support indicated for both analyses. In both phylogenetic analyses, Collolechia was recovered as monophyletic with strong support (BS=95%, PP=1·00), and nested within Placynthium (BS=99%, PP=1·00).
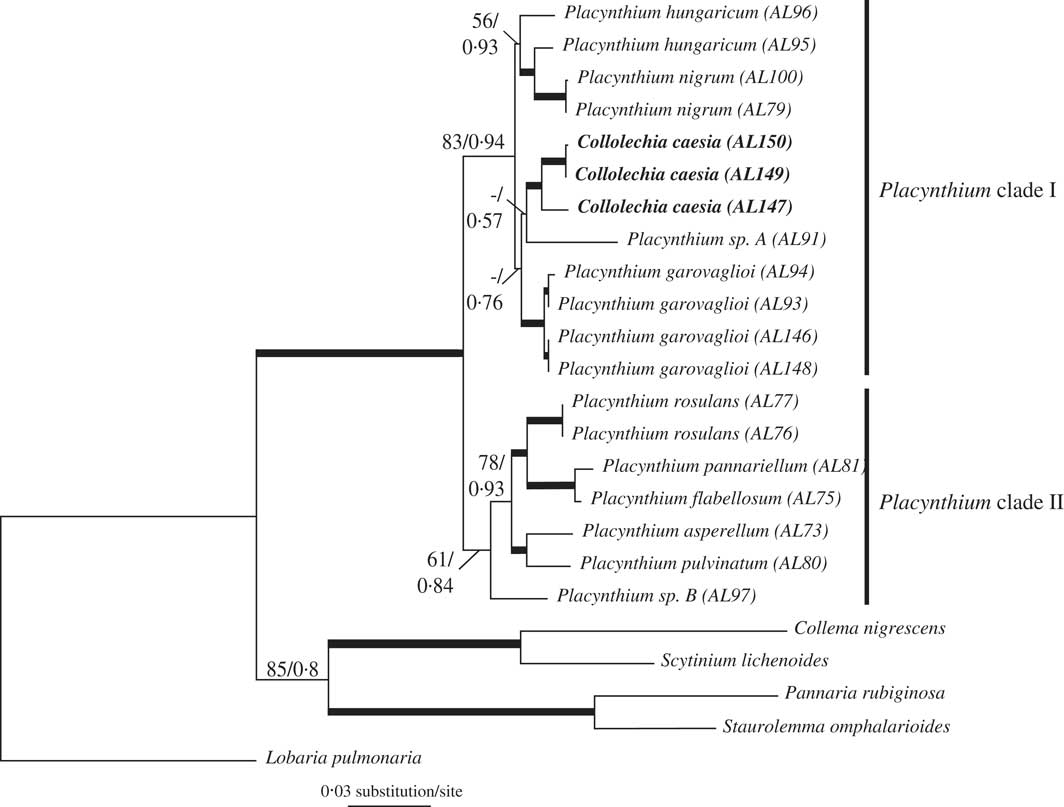
Fig. 2 Most likely tree (ln likelihood=−6126·8160) based on a combined matrix of mtSSU and Mcm7 showing Collolechia nested within Placynthium. Internodes with bootstrap values ≥70% and posterior probabilities ≥0·95 are represented by thick lines. Numbers above other internodes indicate ML bootstrap support (only when values ≥50%) followed by posterior probabilities (only when values ≥0·5%) for the Bayesian analysis.
The two independent runs for each stepping-stone MCMC sampling conducted to calculate the marginal likelihood of each alternative topology reached convergence, as shown by the values of split frequencies <0·01. The estimation of the marginal log likelihood was −6123·60 for the hypothesis in which Placynthium was constrained to be monophyletic and −6114·30 for the alternative hypothesis in which Collolechia was nested within Placynthium. The Bayes factor value was 18·6. We compared this value to the reference table provided by Kass & Raftery (Reference Kass and Raftery1995, p.777) that states 2×loge (BF10) values >10 as strong evidence against H0.
Discussion
Here, we show that Collolechia is clearly nested within Placynthium (Fig. 2), which is composed of two monophyletic subgroups, one of which contains both Collolechia and the type species of Placynthium, P. nigrum, as well as P. garovaglioi, P. hungaricum, and the potentially undescribed Placynthium sp. A. As a consequence, Collolechia caesia should be classified in Placynthium. The nomenclature of “Collolechia” caesia is complicated, but fortunately Jørgensen (Reference Jørgensen2005) has clarified the situation and the reader is referred to this work for details regarding author citation, typification, and synonymy. When treated in Placynthium, the correct name for this species is Placynthium caesium (Fr.) Jatta.
Placynthium caesium (Fr.) Jatta
Syll. Lich. Ital.: 38 (1900).—Lecidea contigua var. caesia Fr., Lich. Eur.: 302 (1831).—Collolechia caesia (Fr.) A. Massal. Geneac. Lich.: 7 (1854); type: (France) Gallia merid., Dufour (UPS!— lectotypus, designated by Jørgensen Reference Jørgensen2005).
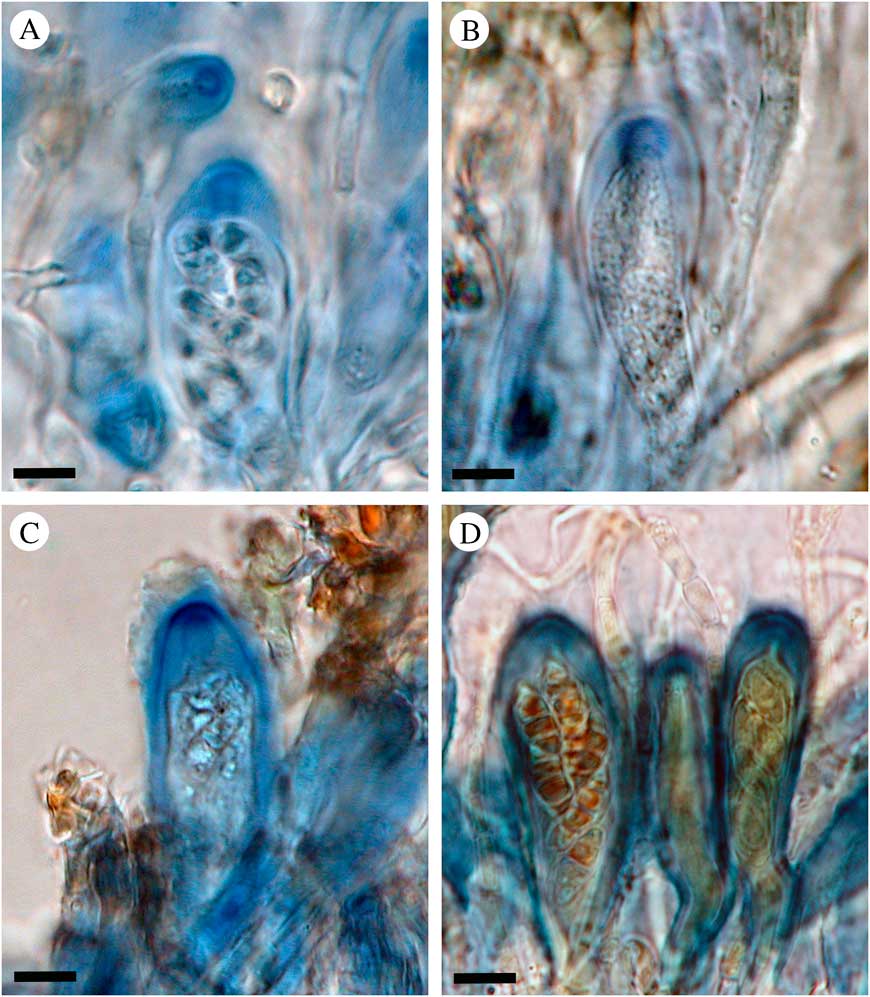
This re-synonymization contradicts the suggestion by Jørgensen (Reference Jørgensen2005), who justified treating the two genera as distinct based on their different ascus, spore and thallus characteristics. The ascus characters are difficult to study in many Placynthium species; the asci are small and the structures indistinct, and the variation between ascus developmental stages within one hymenium is frequently quite confusing. We can still confirm that several Placynthium species do have a tube structure in their asci, a trait considered characteristic of “Collolechia” (Jørgensen Reference Jørgensen2005). Placynthium caesium, P. garovaglioi, P. hungaricum, Placynthium sp. A, and P. nigrum (the type of Placynthium) have a distinct amyloid tube structure in the ascus apex (Fig. 3). Spribille & Muggia (Reference Spribille and Muggia2013) provided a very useful overview of the ascus structures in Peltigerales, and included the amyloid tube structure in the Micarea-type. A tube structure is reported in a number of Placynthium species by Keuck (Reference Keuck1977) and Czeika & Czeika (Reference Czeika and Czeika2007), and in the Collemataceae (Rambold & Triebel Reference Rambold and Triebel1992), and hence could be seen as a synapomorphy for the Placynthiaceae and Collemataceae (Wiklund & Wedin Reference Wiklund and Wedin2003). The tube in Placynthium is frequently flaring and the apical opening is often visible only from above (Fig. 3A). Our observations confirm that other Placynthium species sampled here (i.e. P. asperellum, P. flabellosum, and P. rosulans) have an amyloid cap-like structure (Fig. 3D), corresponding to the Vahliella-type of Spribille & Muggia (Reference Spribille and Muggia2013), as previously reported by Keuck (Reference Keuck1977) and Spribille & Muggia (Reference Spribille and Muggia2013). The asci in the potentially undescribed Placynthium sp. B were very difficult to interpret and we await more material to study this further. We have not found any apothecia in the samples of P. pannariellum and P. pulvinatum, both of which are very rarely fertile. Although we have only investigated a fraction of the species in Placynthium, each ascus character state is correlated with one of the two monophyletic groups identified within the genus. This, however, needs further study to confirm.

Fig. 3 Ascus characteristics in Placynthium. A–C, tube structures of the “Micarea-type”; D, cap-like structure of the “Vahliella-type”. A, Placynthium nigrum, type species of Placynthium (Nordin 5860, UPS); B, Placynthium (“Collolechia”) caesium (Košuthová GOT2, S); C, Placynthium garovaglioi (Palice 16954, S); D, Placynthium flabellosum (Nordin 5666, UPS). Scales=10 µm. In colour online.
All investigated samples of Placynthium caesium and P. garovaglioi have long spores (c. 26–38×3–5 µm in P. caesium and c. 24–35×3·5–6·5 µm in P. garovaglioi) with 3(–5) septa. The difference in spore length between the two species appears less conspicuous than proposed by Jørgensen (Reference Jørgensen2005). Long, pluriseptate spores are also produced in several other Placynthium species, and in our material, P. flabellosum, P. rosulans, and Placynthium sp. B have more than one septum. Placynthium pulvinatum which is shown here (Fig. 2) to be only distantly related to P. caesium is, according to the original description (Øvstedal et al. Reference Øvstedal, Tønsberg and Elvebakk2009), another species with long, pluriseptate spores, again suggesting that this character state is widespread in the genus. Placynthium nigrum has shorter (c. 10–12×5 µm), mainly 1(–3)-septate spores.
In conclusion, when the phylogeny suggests that the two groups are not distinct and the claimed differences in ascus and spore do not hold up to scrutiny, it appears that “Collolechia” caesia should be better treated as a Placynthium species with a crustose-leprose thallus structure. It is not unusual to find examples of closely related lichens, including cyanolichens, which differ in thallus structure. Caloplaca chrysodeta and Micarea leprosula (Tønsberg Reference Tønsberg1992) are both examples of leprose representatives in green-algal crustose genera, and the cyanolichen “Moelleropsis” nebulosa was recently shown to be a leprose Fuscopannaria (Ekman et al. Reference Ekman, Wedin, Lindblom and Jørgensen2014). Also, in the spore and ascus characteristics, Placynthium caesium is not unique compared to other Placynthium species and the results of the molecular phylogeny are consistent with the morphology.
Material investigated (Placynthium caesium): France: Gallia merid., Dufour (UPS L104293, lectotype).—Germany: Bayern: ad saxa jurassica prope in valle Wiesentthal Bavariae, Arnold s. n., (S F155260, F155943); Obersdorf (Tiefenbach) in Algäu, Rehm s. n. (S F155268); Streitberg Oberfranken, 1865, (S F15524); Eichstätt, 1956, (S F155248); Muggendorf, Arnold s. n. (S F155266); 1954, (S F155259).—Italy: Massalongo s. n. (S F155283).—Slovakia: Žilinský kraj, Kraľovany, 1882, Lojka s. n. (S F155283); Muránska planina Mts, Pohronská Polhora – Bánovo, 2014, Guttová & Fačkovcová s. n. (SAV).—Sweden: Gotland: Ardre par., Tviburg (v. Torsburgen), 1943, Degelius s. n. (S F155213); 1963, Degelius s. n. (UPS L159251); Hangvar par., Ire, Floderus (UPS L130317); Irevik, 2014, Košuthová GOT2 (S); Kräklingbo par., Torsburgen, 1857, Stenhammar & Floderus s. n. (S F155224); 1857, Lönnroth s. n. (S F155216, F155217, F155221); 1864, Cleve s. n. (S F155209, UPS L137454); 1871, Molér s. n. (S F155218, S F155215, UPS L137459); 1874, Elmqvist s. n. (S F155225, UPS L137458); 1880, Blomberg s. n. (S F155223; UPS L137452); 1889, Hellborn s. n. (S F155222); 1918, Malme, Malme, Lich. Suec. Exs no 743 (S F155214, UPS L109332); 2014, Košuthová GOT1 (S); Lojsta par., Lojsta, Lönnroth s. n. (S F155210); Lojstabergen, Lönnroth s. n. (S F155934; UPS L137455); Stenkyrka par., Lickershamn, 1869, Laurer s. n. (UPS L137460); Vesterheide par., Hallbros alvar, 1917, Malme (S F155220); 1918, Malme (S F155211); Kneippbyn, 1918, Magnusson 2330 (UPS L160437); Kneippbyn, 2014, Košuthová GOT3 (S).—Switzerland: Schwyz, Pilatus, Hegetschweiler & Hegetschweiler s.n. (S F155227).—Turkey: Bucak, 2013, Yazici 1037 (S).
Material investigated for comparison (Placynthium garovaglioi): Slovakia: W. Carpathians, Dolina Siedmych, 2013, Palice 16564 (SAV); W. Carpathians, Tisovec, 2013, Palice 16954 (S).—Spain: Navarra: Estella, 1983, Santesson 30775 (UPS L160445).—Poland: Malopolskie: Tatry, Giewont 1954, Tobolewski 76 (UPS L160450).—Turkey: Burdur: 2012, Yazici 1123 (S), 1125 (S).
This study was supported by funding from the Swedish Research Council grant 621-2012-3990, to M. Wedin. M. Westberg was funded by the Swedish Taxonomy Initiative, and A. Košuthová was funded by Štefan Schwarz Fund SAS, and EEA and Norway grants no. NF-CZ07-ICP-3-104-2015. We are grateful to the staff at the Molecular Systematics Laboratory at the Swedish Museum of Natural History, in particular Bodil Cronholm, for laboratory assistance. We thank Dr Johannes Bergsten for his helpful discussions and guidance regarding the hypothesis testing analyses. The staff at S, SAV, and UPS, and Z. Palice and J. Malíček, are gratefully thanked for the loan of material, and A. Aptroot kindly gave us samples from Turkey.