Introduction
Tyrosinases are a widespread family of copper-containing oxidase proteins found in many higher plants, fungi and prokaryotes (Seo et al. Reference Seo, Sharma and Sharma2003; Claus & Decker Reference Claus and Decker2006). Copper-containing enzymes play important roles in the biological activation of oxygen, necessary for the oxidation of many different substrata. Specifically, tyrosinases catalyze the o-hydroxylation of monophenols (cresolase activity or ‘monophenolase’) and the subsequent oxidation of the resulting o-diphenols into reactive o-quinones (catecholase activity or ‘diphenolase’), both reactions using molecular oxygen (Halaouli et al. Reference Halaouli, Asther, Sigoillot, Hamdi and Lomascolo2006; Marusek et al. Reference Marusek, Trobaugh, Flurkey and Inlow2006). Subsequently, the o-quinones undergo non-enzymatic reactions with various nucleophiles, producing intermediates that associate spontaneously into dark brown melanin pigments (Selinheimo et al. Reference Selinheimo, NiEidhin, Steffensen, Nielsen, Lomascolo, Halaouli, Record, O’Beirne, Buchert and Kruus2007).
Tyrosinase activity in lichens has been identified in earlier work based on substratum specificity, sensitivity to inhibitors and molecular mass (Laufer et al. Reference Laufer, Beckett and Minibayeva2006 a; Zavarzina & Zavarzin Reference Zavarzina and Zavarzin2006). Activities were much higher in Peltigeralean lichens compared with lichens from other orders. The precise role of tyrosinases in lichens and free-living fungi remains unclear. In lichens, activity is increased following hydration (Beckett et al. Reference Beckett, Minibayeva and Liers2012), and wounding (Laufer et al. Reference Laufer, Beckett and Minibayeva2006 a). The most important role is often considered to be L-DOPA melanin biosynthesis (Mayer Reference Mayer2006), although many fungi produce melanins from 1,8-dihydroxynaphthalene (DHN) using polyketide synthases (Langfelder et. al. Reference Langfelder, Streibel, Jahn, Haase and Brakhagea2003; Eisenman & Casadevall Reference Eisenman and Casadevall2012; Solano Reference Solano2014). Furthermore, tyrosinase is not necessarily the only enzyme involved in L-DOPA metabolism. In the laboratory, it is easy to show that lichen peroxidases and laccases can also readily oxidize L-DOPA into dark pigments and therefore participate in melanization (Liers et al. Reference Liers, Ullrich, Hofrichter, Minibayeva and Beckett2011). Melanin formation increases the resistance of organisms to abiotic stresses such as UV radiation, free radicals, gamma rays, dehydration and extreme temperatures (Gostinčar et al. Reference Gostinčar, Grube, de Hoog, Zalar and Gunde-Cimerman2010; Solano Reference Solano2014). Melanins have also been suggested to improve the resistance of the cell walls to biotic stresses, for example by reducing digestion by hydrolytic enzymes secreted by pathogens (Bell & Wheeler Reference Bell and Wheeler1986). In lichens, cortical melanins are believed to protect the photobiont against excessive light (Nybakken & Julkunen-Tiitto Reference Nybakken and Julkunen-Tiitto2006; McEvoy et al. Reference McEvoy, Gauslaa and Solhaug2007; Nybakken et al. Reference Nybakken, Asplund, Solhaug and Gauslaa2007; Larsson et al. Reference Larsson, Večeřová, Cempírková, Solhaug and Gauslaa2009).
The types of melanins that lichens produce remain unknown, but seem likely to vary between species. In our earlier work we showed that, in general, Peltigeralean lichens display high tyrosinase and laccases activities, while activities are often much lower in non-Peltigeralean species (Laufer et al. Reference Laufer, Beckett and Minibayeva2006 a). Furthermore, Peltigeralean lichens often contain nitrogen-fixing photobionts. It seems likely, therefore, that Peltigeralean species are more likely to produce L-DOPA melanins. In non-Peltigeralean species, which usually cannot fix N2, and in which tyrosinase activity is very low, melanins are more likely to be of the DHN-type.
Surprisingly, there have been very few studies on the interaction between UV light, tyrosinase activity and melanization. However, Shang et al. (Reference Shang, Duan, Huang, Gao and Wang2012) showed that transforming the pathogenic fungus Beauveria bassiana with an exogenous tyrosinase gene increases both pigment formation and UV resistance. In the work presented here, we used a combination of laboratory and field experiments to better characterize Peltigeralean lichen tyrosinases, and to elucidate their role in lichen biology, specifically in melanin synthesis and photoprotection. We chose as our test species Lobaria pulmonaria (L.) Hoffm., a species with a wide distribution in Europe, Asia, North America and Africa. This species contains readily detectable tyrosinase activity, although activities tend to be towards the lower range compared with other Peltigeralean species (Beckett et al. Reference Beckett, Zavarzina and Liers2013 b). Lobaria pulmonaria prefers damp habitats with high rainfall, especially coastal areas (Geiser & McCune Reference Geiser and McCune1997), but when exposed to high levels of PAR and UV can readily synthesize melanin (Solhaug et al. Reference Solhaug, Gauslaa, Nybakken and Bilger2003; Nybakken et al. Reference Nybakken, Asplund, Solhaug and Gauslaa2007). To test the role of tyrosinases in melanization, we induced melanin synthesis by exposing thalli to various combinations of PAR and UV light, and then measured the activities of tyrosinase and other redox enzymes. To check whether the induced melanins were of the DOPA type, the N content of the melanins was measured; DOPA melanins have an N content of c. 10%, compared with 1% for other types (Loganathan & Kalyanasundaram Reference Loganathan and Kalyanasundaram1999).
Materials and Methods
Lichen material
Lobaria pulmonaria was randomly collected from moss-covered rocks under the canopy of an evergreen forest in the outskirts of Cape Town, Western Cape, South Africa, and from trees growing in the margins of a deciduous forest in Porsgrunn, Southern Norway. Material was air-dried, and stored in plastic bags at −18 °C for a maximum of six weeks before use.
Electrophoretic studies
The presence of specific oxidoreductases in South African material was tested electrophoretically in crude extracts of the thalli of Lobaria pulmonaria as described by Laufer et al. (Reference Laufer, Beckett and Minibayeva2006 a). A modified method of Laemmli (Reference Laemmli1970) was followed using SDS and native PAGE (5% and 12%). Tyrosinase activity was visualized by incubating gels in 10 mM L-DOPA (Sigma) in 0·1 M phosphate buffer pH 6. To test for the metabolism of L-DOPA by peroxidases, H2O2 (2 mM) was added and the gels left to stain for 12 h. Peroxidase and laccase activity was visualized by incubating gels in sodium acetate buffer (0·25 M, pH 5) containing glycerol (10%), and o-dianisidine (1 mM) with or without H2O2 (1 mM).
Enzymatic reactions
Enzymes from South African material were extracted by grinding thalli in 50 mM chilled phosphate buffer, pH 7. Extracts were centrifuged at 4000 g at 4 °C for 20 min, and then the pellet was discarded. Unless otherwise indicated, enzyme activities are expressed as units g−1 dry mass. Tyrosinase activity in the supernatant was estimated by the oxidation of 2 mM L-DOPA to 2-carboxy-2, 3-dihydroindole-5, 6-quinone in 50 mM phosphate buffer pH 6 (Horowitz et al. Reference Horowitz, Feldman and Pall1970) (ε475=3·6 mM−1 cm−1). Spontaneous rates of L-DOPA oxidation were negligible for the duration of the assays (2 min). While laccase may have contributed to some L-DOPA oxidation in our assays, laccase activity is low at pH 6 (see results below). L-DOPA peroxidase activity was measured as the stimulation in activity following the addition of 1 mM H2O2. Apparent kinetic constants for L-DOPA metabolism were measured by varying the concentration of L-DOPA from 30 to 200 µM and analyzing the rate of metabolism using the program ‘Hyper’. Laccase activity was determined by following the oxidation of 2,2-azino-bis(3-ethylthiazoline-6-sulfonate) (0·3 mM, ABTS, Eggert et al. Reference Eggert, Temp and Eriksson1996) (ε420=36 mM−1 cm−1) in Na-acetate buffer (100 mM, pH 4·5). Peroxidase activity was measured as the stimulation in activity following the addition of 0·1 mM H2O2. The effect of pH on enzyme activity was tested using 2 mM L-DOPA for tyrosinases, as described above. For laccase and peroxidase 1 mM 2,6-dimethoxylphenol (DMP) was used as a substratum, and the production of coerulignone measured (ε469=27·5 mM−1 cm−1) (Liers et al. Reference Liers, Ullrich, Hofrichter, Minibayeva and Beckett2011). For all enzymes, a 50 mM citrate-phosphate buffer in the pH range 3·0–8·0 was used.
Monophenolase activity was determined for phenol, tyrosine and tyramine using a method based on the coupling reaction between 3-methyl-2-benzothiazolinone hydrazone (MBTH) and the quinone products of the oxidation of monophenols in the presence of polyphenol oxidase (Espín et al. Reference Espín, Morales, García-Ruiz, Tudela and García-Cánovas1997). Samples were incubated in 10 mM MBTH and 50 mM phosphate buffer, pH 6, and 2 mM of substratum (monophenol). Extinction coefficients of the products were: from L-tyrosine, ε476=20·7 mM−1 cm−1; from tyramine, ε476=33·0 mM−1 cm−1; and from phenol, ε476=30·5 mM−1 cm−1. The thermostability of tyrosinase was tested by incubating crude extract at 40 °C for 200 min.
Effect of hydration on redox enzyme activity
Lichens were collected and dried in the dark between three layers of filter paper for a period of two days, and then 1 cm discs were cut from random places in the thalli. Discs were randomized, and then placed on wet non-cellulosic cloth in growth chambers at 10 °C in the dark. For each time point, three replicates comprising five 1 cm discs corresponding to c. 0·1 g dry mass were sampled, and the activities of laccase, peroxidase and tyrosinase were measured. Activity was measured in dry material and at intervals until 13 days after the start of the experiment.
Effect of UV light on melanization and enzyme activity
Whole thalli (125 from South Africa and 125 from Norway) were randomly selected, and three or four (depending on thallus size) were sewn with cotton thread to wooden frames (15·9×15·9 cm2) covered with nylon mesh netting. Three frames were placed 5 cm under different types of 70×70 cm filters. Each of the four light treatments comprised three replicate sets of three frames randomly arranged on an unshaded lawn in the grounds of the Norwegian University of Life Sciences, Ås, Norway, for three weeks during June 2015. The weather was mainly clear and sunny with only a couple of rainy days. The maximum photosynthetically active radiation (PAR) was between 1500 and 2000 µmol photons m−2 s−1 on the sunny days. The daily PAR dose was c. 30 mol photons m−2 day−1. PAR levels were measured with a quantum sensor (model S-LIA-M003; Onset Computer Corporation, Bourne, MA, USA) connected to a Hobo Microstation Datalogger (model H21-002; Onset Computer Corporation). The treatments were: first, no screen (normal ambient light); second, a wavelength-neutral acrylic screen that transmitted all light with a 15% reduction in intensity (Acrylic sunbed, 3 mm, Finn Løken AS, Ås, Norway); third, a polyester screen that removed UV-B (PET, 0·175 mm, Nordbergs Tekniska AB, Vallentuna, Sweden); and fourth a polycarbonate screen that removed both UV-A and UV-B (clear polycarbonate, 3 mm, Finn Løken AS, Ås, Norway). The second treatment was included to test the effect of the physical presence of a screen. The thalli were sprayed with deionized water each morning (c. 09:00) and evening after sunset (c. 23:00) to ensure that they were moist and photosynthetically active during the first part of the day and at night.
Lichens from the first frame in each replicate were used to determine the activities of the enzymes tyrosinase (with and without SDS), laccase and peroxidase as described above. Each replicate comprised at least four thalli with a combined dry mass of 0·5 g. Lichens from the second frame were used to measure the relative growth rate (derived from the increase in air-dry mass) throughout the experiment (Evans Reference Evans1972). Five additional thalli air-dried together with all experimental thalli were weighed before they were oven dried at 70 °C and reweighed. The reduction factor in dry mass in the sacrificed thalli was used to calculate the true dry mass for all thalli.
Lichens from the third frame were used to measure browning reflective index (BRI), a measure of melanization, F v/F m and chlorophyll content. Reflectance spectra (450–900 nm) were recorded non-destructively using an integrating sphere (ISP-50-REFL OceanOptics, Eerbeek, the Netherlands) placed directly on the upper surface of each thallus. A halogen lamp (DH2000, OceanOptics) illuminated the thallus via a 600 μm optical fibre, connected to the sphere. Reflectance was measured at one random position for each of the four or five thalli that comprised a replicate with a spectrometer (model SD2000, OceanOptics) connected to the output port of the sphere with a 400 μm-thick fibre. The percentage reflection was calculated on the basis of a dark spectrum and a reference spectrum from a white reference tile (WS-2, OceanOptics). BRI, calculated as (1/R550 – 1/R700)/R750 (Chivkunova et al. Reference Chivkunova, Solovchenko, Sokolova, Merzlyak, Reshetnikova and Gitelson2001), was used as a quantitative estimate of melanic compounds. Photosynthetic competence was assessed as maximum photosystem II efficiency (F v/F m) using the PAM-2000 fluorimeter (Walz, Effeltrich, Germany). Each of the four or five thalli comprising a replicate was first placed under 10 µmol m−2 s−1 for one day to recover from short-term photoinhibition, and then dark adapted for at least 15 min before measurement. After measuring BRI and F v/F m, chlorophyll was extracted as described by Palmqvist & Sundberg (Reference Palmqvist and Sundberg2002). Whole lichen thalli were ground to a fine powder in a ball mill, and extracted with MgCO3-saturated dimethyl sulfoxide (DMSO) in Eppendorf tubes at 60 °C for 40 min. Extracts were centrifuged, and absorbance measured at 649 and 665 nm. The amounts of chlorophyll a and b were calculated following Wellburn (Reference Wellburn1994). All the above parameters measured in the lichens attached to the three replicate frames were also measured in replicate thalli at the start of the experiment.
Chemical nature of the melanic pigments from L. pulmonaria
Melanins from replicate thalli from the above experiment corresponding to the ‘open’ treatment were extracted at the end of the experiment using a modification of the method of Ellis & Griffith (Reference Ellis and Griffith1974). Briefly, thalli were incubated overnight in 2 M NaOH, centrifuged at 4000 g for 20 min and the pellet discarded. The pH of the supernatant was adjusted to 1 using 5 M HCl, and melanins left to precipitate overnight. Samples were centrifuged at 4000 g for 20 min, and the supernatant discarded. The resulting pellet was washed sequentially in 5 ml of distilled water, chloroform, ethyl acetate and acetone, and then dried overnight at 80 °C. The C:N ratio of the melanins was then measured in 5 mg samples using the Vario Micro Cube (Elementar, Frankfurt).
Statistical analysis
Statistical analyses, where necessary, were performed using the software SPSS (SPSS Inc., Chicago, IL, USA). Where appropriate, each parameter was analyzed by a general linear model (GLM) univariate analysis of variance (ANOVA). Pairwise comparisons of treatment means were performed with a Duncan’s multiple range post hoc procedure with P<0·05 used as the statistical indication of significance.
Results
Screening the oxidoreductases of Lobaria pulmonaria by native PAGE
Native PAGE with L-DOPA as a substratum revealed that L. pulmonaria has a single tyrosinase isoform with a molecular mass of 45 kDa (Fig. 1). Staining gels with o-dianisidine and H2O2 revealed a single peroxidase isoform with a molecular mass of 35 kDa, while using a 5% gel and adding o-dianisidine alone revealed a single laccase isoform with a molecular mass of 170 kDa. Adding H2O2 and incubating gels in L-DOPA followed by staining overnight indicated that both peroxidases and laccases can also metabolize L-DOPA.

Fig. 1 Native PAGE of crude extracts from Lobaria pulmonaria. Tyrosinase (TYR) activity was visualized by incubation in 2 mM L-DOPA for 30 min, L-DOPA peroxidase (DOPA oxidase) activity by 2 mM L-DOPA and 10 mM H2O2 for 12 h, peroxidase (POX) activity by 1 mM o-dianisidine and 10 mM H2O2 for 30 min, and laccase (LAC) activity by o-dianisidine in the absence of H2O2. Molecular masses of standard proteins are indicated in kDa.
Enzyme kinetics
Tyrosinase activity increased by c. 20% following hydration for three to four days, and then declined slightly (Fig. 2) to initial values after 13 days. Latent form displayed the same trend, although the ratio of latent to active forms declined following hydration. Laccase and peroxidase activity rapidly increased during the first day following rehydration, and then progressively declined over the following 12 days. Tyrosinase activity was optimal at a pH of 6·5, while the optimum for L-DOPA oxidase and peroxidase was 4·5 (Fig. 3). Laccase activity displayed a rather broad peak between pH 3·5 and 5·5. The thermostability of tyrosinase at 40 °C was moderately high, with almost 50% of original activity remaining after 3 h (Fig. 4). Tyrosinases metabolized the monophenols tyrosine, tyramine and phenol at rates of 2·8, 2·2 and 1·9 µmoles g−1 dry mass min−1. Michaelis-Menten analysis of L-DOPA metabolism by tyrosinase indicated that at pH 6 the apparent K m was 125 µM (Fig. 5). The affinity of L-DOPA oxidase for L-DOPA at a pH of 4·5 was much higher, with an apparent K m of 20 µM.
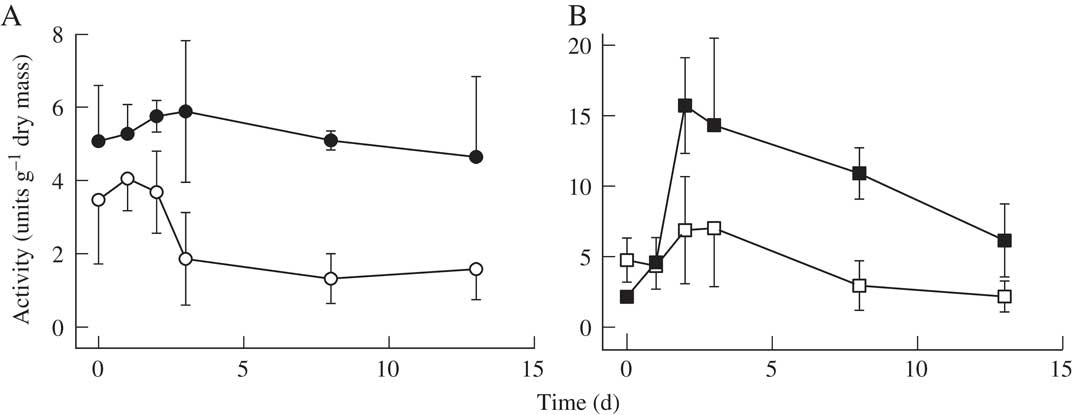
Fig. 2 The effect of rehydration on enzyme activity in Lobaria pulmonaria. A, tyrosinase activity measured with 2 mM DOPA (solid symbols) and stimulation of tyrosinase activity by SDS (latent forms, open symbols); B, laccase activity (solid symbols) and peroxidase activity (open symbols) measured with 0·3 mM ABTS in Lobaria pulmonaria. Activity was measured on day 0, and then material transferred to moist filter paper. Activity was then measured at intervals for the next 13 days. Plotted values are means ± 1S.E., n=3.

Fig. 3 The effect of pH on enzyme activity in Lobaria pulmonaria. A, tyrosinase (solid symbols) and L-DOPA peroxidase (open symbols) activities, measured with 2 mM DOPA; B, laccase (solid symbols) and peroxidase (open symbols) activities, measured with 1 mM DMP as the substrate. Mean values (n=3) are plotted; symbols exceed the size of the standard error bars.

Fig. 4 Thermostability at 40 °C of tyrosinase from Lobaria pulmonaria measured with 2 mM DOPA. Extracts were incubated at 40 °C for varying times, and then activity measured for 2 min at 20 °C. Values represent means ± S.E., n=3.

Fig. 5 Kinetic analysis of L-DOPA metabolism by tyrosinase (closed circles) at pH 6·0 and L-DOPA peroxidase (open circles) at pH 4·5 in a crude extract from Lobaria pulmonaria. The fitted lines were derived by back transforming linear regressions on a Lineweaver-Burke transformation of the raw data. Mean values (n=3) are plotted; symbols exceed the size of standard error bars.
The effects of UV on lichens
South African lichens placed in the field set-up in Norway rapidly bleached, and were therefore discarded. It is not entirely clear why this happened, although visual inspection at the time of collection suggested that the South African site was more shaded than the Norwegian one, and furthermore the South African site was shaded by evergreen trees, while the Norwegian site comprised deciduous species. South African material had a significantly lower chlorophyll a to b ratio (2·7 compared with 2·9 for Norwegian material); lower ratios generally indicate adaptation to shade (Boardman Reference Boardman1977). The Norwegian material gradually became melanized when placed on frames without a filter, and on frames beneath the wavelength-neutral filter that transmitted both photosynthetically active radiation and UV (Fig. 6). Analysis of this melanin indicated that the C:N ratio was 10·4 ± 0·3 (n=3), typical for L-DOPA melanin (‘eumelanin’). Melanization was significantly reduced by filtering UV-B, and was prevented by removing both UV-A and UV-B. The various treatments had no significant effect on the health of photobionts, assessed by chlorophyll content and photochemical efficiency (Table 1). However, lichens in the open treatment displayed a negative growth rate, while the growth rate of lichens with both UV-A and UV-B removed was significantly higher than in other treatments (Table 1). Enzyme activities tended to be rather variable (Fig. 7). In lichens without any filter, the activity of laccase significantly increased (c. × 8), while there was a small non-significant increase in peroxidase activity. Lichens placed under a wavelength-neutral filter displayed no changes in enzyme activity. Enzyme activity changed little from initial values when UV was filtered, although removing both UV-A and UV-B significantly increased active tyrosinase (Fig. 7A), but not latent forms (Fig. 7B).

Fig. 6 Effect on melanization of transferring shade-adapted Lobaria pulmonaria to an exposed site under no filter, a neutral filter, a filter that removes UV-B and a filter that removes both UV-A and UV-B. Melanization was measured as the browning reflectance index (BRI, see text for details). Mean values (n=3) are plotted (±1 SE), and columns with the same letters do not differ significantly (P<0·05).

Fig. 7 Effect on the activity of redox enzymes of transferring shade-adapted Lobaria pulmonaria to an exposed site under no filter, a neutral filter, a filter that removes UV-B and a filter that removes both UV-A and UV-B. Mean values (n=3) are plotted ±1 SE, and columns with the same letters do not differ significantly (P<0·05). A, tyrosinase activity measured with 2 mM L-DOPA (active forms); B, stimulation of tyrosinsase activity by SDS (latent forms); C, laccase activity; D, peroxidase activity. Laccase and peroxidase activities were measured with 0·3 mM ABTS.
Table 1 The effect of exposing shade-adapted Lobaria pulmonaria to various light treatments for 3 weeks on chlorophyll content, F v/F m and RGR when exposed. Figures in each column followed by the same letter are not significantly different (Duncan’s multiple range test, P<0·05, n=15)

Discussion
In the work presented here, both laboratory and field experiments were carried out to investigate the characteristics and roles of tyrosinases in the lichen L. pulmonaria. Lichen tyrosinases in many respects resemble those of free-living fungi. It is easy to show in the laboratory that tyrosinases, and indeed other redox enzymes such as laccases and peroxidases, can readily polymerize L-DOPA into melanic pigments (Liers et al. Reference Liers, Ullrich, Hofrichter, Minibayeva and Beckett2011; Beckett et al. Reference Beckett, Minibayeva and Liers2013 a). However, in the field experiment melanization was not accompanied by any increase in tyrosinase activity, although under one set of conditions laccase activity increased. This study therefore highlights the difficulty of extrapolating results from laboratory experiments to the field. It remains unclear whether tyrosinases are involved in melanin synthesis in the field, and the function of tyrosinases in lichens remains enigmatic.
Redox enzymes in L. pulmonaria
Redox enzymes in lichens typically comprise tyrosinases, peroxidases and laccases (Beckett et al. Reference Beckett, Zavarzina and Liers2013 b). Before carrying out more detailed kinetic analyses, we examined these enzymes in L. pulmonaria using gel electrophoresis (Fig. 1). Tyrosinase from L. pulmonaria has a molecular mass of c. 45 kDa, typical for free-living fungi (van Gelder et al. Reference van Gelder, Flurkey and Wichers1997). By contrast, our earlier results from a range of other species indicated that lichen tyrosinases have masses of c. 60 kDa (Laufer et al. Reference Laufer, Beckett and Minibayeva2006 a). The most likely explanation for the discrepancy is that tyrosinases comprise two units (Mauracher et al. Reference Mauracher, Molitor, Al-Oweini, Kortz and Rompel2014). The main, active, unit has a mass of 45 kDa, and the second is a 15 kDa subunit that renders the enzyme ‘latent’ when it binds to the main unit. Presumably, unlike L. pulmonaria, in the species tested by Laufer et al. (Reference Laufer, Beckett and Minibayeva2006 a) the complete enzyme reforms during electrophoresis, and for some unknown reason can display activity. Lobaria pulmonaria also contains a 36 kDa peroxidase and a 190 kDa laccase, similar masses to those reported for these enzymes in other lichens (Laufer et al. Reference Laufer, Beckett, Minibayeva, Lüthje and Böttger2009; Liers et al. Reference Liers, Ullrich, Hofrichter, Minibayeva and Beckett2011). The gels also indicated that even at pH 6, higher than their normal optimal pH values peroxidases and laccases were capable of polymerizing L-DOPA, suggesting that they might participate in melanization reactions.
Kinetics of tyrosinase and other redox enzymes
In general, results from kinetic studies indicate that the tyrosinases and other redox enzymes from L. pulmonaria resemble those from other lichens and free-living fungi. Initial experiments showed that the highest enzyme activities occur in thalli rehydrated for three to four days (Fig. 2), and thalli treated in this way were used to prepare extracts for use in all of the kinetic experiments described here. The pH optima for tyrosinase and laccase (Fig. 3) are similar to those reported for other lichens (Laufer et al. Reference Laufer, Beckett, Minibayeva, Lüthje and Böttger2006 b; Beckett et al. Reference Beckett, Minibayeva and Liers2012). However, while the peroxidase from Leptogium saturninum displays increasing activity with decreasing pH (Liers et al. Reference Liers, Ullrich, Hofrichter, Minibayeva and Beckett2011), the peroxidase from L. pulmonaria has a distinct optimum of c. 4·5. The pH optima of ‘L-DOPA peroxidase’, tested using a mixture of L-DOPA and H2O2, was identical to that of peroxidase measured with DMP and H2O2, suggesting that the same enzyme metabolizes both substrates. The thermostability of the tyrosinase from L. pulmonaria resembles that of the enzyme from free-living fungi (e.g. Duarte et al. Reference Duarte, Tiba, Santiago, Garcia and Bara2012) (Fig. 4). The affinity of the tyrosinase for L-DOPA, measured as apparent K m, was 125 µM (Fig. 5), and was more than five times lower than that of L-DOPA peroxidase (20 µM). The high affinity (lower K m) of the peroxidase for L-DOPA suggests that it may have a significant role in melanin synthesis. The affinities of laccases from free-living fungi for L-DOPA are similar to those of tyrosinase (c. 0·5 mM; e.g. Tĭsma et al. 2008). Finally, L. pulmonaria tyrosinase can metabolize monophenols, a characteristic shared with most tyrosinases from free-living fungi (Selinheimo et al. Reference Selinheimo, NiEidhin, Steffensen, Nielsen, Lomascolo, Halaouli, Record, O’Beirne, Buchert and Kruus2007). Taken together, results indicate that in L. pulmonaria tyrosinase is similar to the corresponding enzyme in other lichens, and also that in free-living fungi. The results also show that tyrosinase is not the only redox enzyme capable of metabolizing L-DOPA.
Role of tyrosinase in melanin synthesis
Melanization in humans is known to depend on the presence of tyrosinase, and UV upregulates tyrosinase activity (Khlgatian et al. Reference Khlgatian, Hadshiew, Asawanonda, Yaar, Eller, Fujita, Norris and Gilchrest2002); limited evidence suggests that the same may be true for free-living fungi (Shang et al. Reference Shang, Duan, Huang, Gao and Wang2012). In shade-adapted L. pulmonaria, melanin synthesis can be induced within three weeks by transplanting the material to an open area without a filter, or by placing it under a wavelength-neutral filter that transmits both PAR and UV (Fig. 6). The low C:N ratio of the melanins formed (10:1) indicates that, as in humans, they were almost certainly synthesized from L-DOPA rather than hydroxylated naphthalene-derived molecules that produce a melanin with a C:N ratio close to 100:1 (Loganathan & Kalyanasundaram Reference Loganathan and Kalyanasundaram1999; Solano Reference Solano2014). Possibly, this is a consequence of L. pulmonaria containing N2-fixing cephalodia that supply thalli with N. Filtering both UV-A and UV-B prevents melanization, while only limited melanization occurs in lichens receiving UV-A but not UV-B. This clearly indicates that UV is the signal that triggers melanization. It is well known that fungi contain a variety of UV receptors that are involved in signalling, for example the UV-B sensitive fungal cryptochromes (Heintzen Reference Heintzen2012).
In all four treatments the photobionts remained healthy, assessed by maximum photochemical efficiency of dark-adapted thalli (F v/F m), and their chlorophyll contents (Table 1). Melanization in L. pulmonaria is cortical (McEvoy et al. Reference McEvoy, Gauslaa and Solhaug2007), and probably protects the photobiont from UV. However, growth rates indicate that UV might have harmed the mycobiont; for example, UV is known to damage lichen DNA (Unal & Uyanikgil Reference Unal and Uyanikgil2011). Lichens in the open treatment displayed a negative growth rate, while the lichens placed under the wavelength-neutral filter grew significantly faster (Table 1). We observed that the physical presence of a screen reduces dew formation, and therefore the time that lichen thalli are metabolically active. While this will reduce the time available for photosynthesis, it will also effectively reduce the time that hydrated, active thalli are exposed to UV stress, possibly explaining the higher growth rate in these lichens. The highest growth rate occurred in thalli from which both UV-A and UV-B were filtered. While protecting the photobionts, it is possible that melanization reduced the PAR received by the photobionts, thus reducing photosynthesis. However, this appears unlikely, since similar growth rates occurred in both the strongly melanized lichens under the neutral filter and the slightly melanized lichens under the filter that removed UV-B. Results suggest that normal, ambient UV-A might directly harm the mycobiont, even if a thallus becomes melanized.
Our transplant experiment demonstrated that no simple correlations exist between melanization in the field and tyrosinase activity. Melanized lichens did not display increased tyrosinase activity, suggesting that these enzymes may not be responsible for melanization (Fig. 7). However, lichens in the unfiltered treatment had increased laccase activity. Laccases in free-living fungi have been suggested to play roles in melanization (Nagai et al. Reference Nagai, Kawata, Watanabe, Ogawa, Saito, Takesawa, Kanda and Sato2003). While it may be tempting to speculate that laccases are responsible for melanin synthesis, lichens placed under a wavelength-neutral filter also became melanized (Fig. 6), but displayed no increase in laccase activity (Fig. 7). As discussed above, lichens in the unfiltered treatment had the lowest growth rates, and the increase in laccase activity in these lichens may have been a stress response. It has been suggested that laccases are involved in a variety of general stress responses in fungi, particularly oxidative and nitrosative stresses (Missall et al. Reference Missall, Moran, Corbett and Lodge2005). There is no obvious explanation for the increase in tyrosinase activity of lichens placed under the filters that removed both UV-A and UV-B. However, taken together, the results suggest that increased tyrosinase activity is not essential for melanin synthesis in L. pulmonaria.
Conclusion
Work is currently underway to purify and sequence lichen tyrosinases so that they can be compared with their counterparts from free-living fungi at the molecular level. Intuitively, based on their substrate specificity determined in the laboratory, the main role for lichen tyrosinase is melanin synthesis. However, the absence of a correlation between tyrosinase activity and melanization reported here suggests that future studies are needed to determine whether melanization is controlled by the levels of melanin precursors such as tyrosine, and also the role of other redox enzymes in the process. Additional roles for tyrosinases need to be investigated, such as defence against pathogens. For example, infection of the basidiomycete Agaricus. bisporus by a pathogenic bacterium causes discoloration of the cap, and is accompanied by an induction of fungal tyrosinases (Soler-Rivas et al. Reference Soler-Rivas, Arpin, Olivier and Wichers2000). A further role for tyrosinases may be to synthesize the quinones needed for radical generating extracellular redox cycling that has been reported to occur in the lichen genus Usnea (Beckett et al. Reference Beckett, Ntombela, Scott, Gurjanov, Minibayeva and Liers2015).
SANCOOP, the NRF (South Africa) and the Russian Foundation for Basic Research (grant no. 14-04-93962) are thanked for financial support.










