Introduction
Extensive land use of woodland over thousands of years has created very specific semi-natural semi-open biotopes in northern Europe, the so-called wooded meadows (Kukk & Kull Reference Kukk and Kull1997; Poska & Saarse Reference Poska and Saarse1999; Pärtel et al. Reference Pärtel, Mändla and Zobel1999, Reference Pärtel, Helm, Reitalu, Liira and Zobel2007; Eriksson et al. Reference Eriksson, Cousins and Bruun2002). Continuous long-term management, mostly by cattle and sheep grazing with hay mowing and firewood gathering, have created habitats characterized by their high diversity within different taxonomical groups, such as vascular plants (Kull & Zobel Reference Kull and Zobel1991; Cousins & Eriksson Reference Cousins and Eriksson2001, Reference Cousins and Eriksson2002), bryophytes (Ingerpuu et al. Reference Ingerpuu, Kull and Vellak1998), agarics (Kalamees Reference Kalamees and Kukk2004), ectomycorrhizal fungi (Tedersoo et al. Reference Tedersoo, Suvi, Larsson and Kõljalg2006), lichens (Leppik & Jüriado Reference Leppik and Jüriado2008; Jönsson et al. Reference Jönsson, Thor and Johansson2011; Thor et al. Reference Thor, Johansson and Jönsson2010) or insects (Talvi Reference Talvi1995). Unfortunately, the area of these semi-natural habitats has decreased greatly over the last century because of the cessation of traditional management. This rate of loss has even accelerated since the 1940s and 1950s (Eriksson et al. Reference Eriksson, Cousins and Bruun2002; Poschlod & WallisDeVries Reference Poschlod and WallisDeVries2002; Pykälä et al. Reference Pykälä, Luoto, Heikkinen and Kontula2005). Therefore, in present-day Estonia, traditionally-managed wooded meadows with scattered trees, shrubs and meadow glades maintain only a miniscule proportion (0·2%) of their former area (850 000 ha in the 19th century) (Kukk & Sammul Reference Kukk, Sammul and Sammul2006), and the mosaic-structured wooded meadows have been replaced by cultivated fields or abandoned to brush-woods. In abandoned wooded meadows the diversity of vascular plants decreases rapidly (Hæggström Reference Hæggström1983; Hansson & Fogelfors Reference Hansson and Fogelfors2000; Pykälä et al. Reference Pykälä, Luoto, Heikkinen and Kontula2005; Aavik et al. Reference Aavik, Jõgar, Liira, Tulva and Zobel2008; Škornik et al. Reference Škornik, Šajna, Kramberger, Kaligarič and Kaligarič2008).
Although wooded meadows are listed as endangered habitats (Paal Reference Paal1998), studies of epiphytic lichens in them are scarce (Randlane Reference Randlane and Kukk2004). Wooded meadows are mostly distributed in the countries around the Baltic Sea and particularly on the islands (e.g. Estonia, Sweden and the southern part of Finland) (Hæggström Reference Hæggström1983; Kukk & Kull Reference Kukk and Kull1997). Regional, that is local, studies of epiphytic lichen communities in wooded meadows in each individual country are most relevant because of locally specific tradition of woodland management. For example, the Scandinavian tradition includes the pollarding of trees (Hæggström Reference Hæggström1983; Moe & Botnen Reference Moe and Botnen2000), while in Estonia this is not common. Regionally-restricted singularities in the species pool and its composition have also been described as about 58% of lichen species in wooded meadows are shared between the meadows in Gotland, Sweden and in Estonia (Thor et al. Reference Thor, Johansson and Jönsson2010). At present, only a few studies have been published on the effects of land use change on epiphytic lichen communities in wooded meadows. In Norway, Moe & Botnen (Reference Moe and Botnen1997, Reference Moe and Botnen2000) demonstrated that lichen species composition on pollarded trees changes with changing land use intensity. In Estonia, the cessation of the traditional management of wooded meadows and the successional development of these meadows into deciduous forests has resulted in the impoverishment of lichen communities in terms of species richness and species composition on the woodland scale (Leppik & Jüriado Reference Leppik and Jüriado2008). In Sweden Jönsson et al. (Reference Jönsson, Thor and Johansson2011) also showed that epiphytic lichen species richness is higher in managed wooded meadows than in unmanaged sites and, in addition, lichen species richness is dependent on the area of the wooded meadow and its historic canopy cover.
A multitude of factors should be considered in analyzing changes in epiphytic lichen communities. The evaluation of the drivers of the lichen community is complicated because the abiotic and biotic factors act on multiple spatial and temporal scales, and their spatial and temporal effects are also interrelated (Jesberger & Sheard Reference Jesberger and Sheard1973; McCune Reference McCune1993; Giordani Reference Giordani2006). For instance, the formation of an epiphytic lichen community depends on habitat history (Fritz et al. Reference Fritz, Gustafsson and Larsson2008; Ranius et al. Reference Ranius, Eliasson and Johansson2008a) and stand management (Aude & Poulsen Reference Aude and Poulsen2000; Jüriado et al. Reference Jüriado, Paal and Liira2003; Friedel et al. Reference Friedel, Oheimb, Dengler and Härdtle2006), host tree properties (Boudreault et al. Reference Boudreault, Coxon, Vincent, Bergeron and Marsh2008; Jüriado et al. Reference Jüriado, Liira, Paal and Suija2009a, b), lichen community successional stage and competition with other epiphytes (Stone Reference Stone1989; Ruchty et al. Reference Ruchty, Rosso and McCune2001).
Environmental factors, particularly those related to habitat openness or shadiness, have complex effects on lichen communities. That is because in addition to light availability, openness alters habitat conditions in terms of moisture, temperature and ventilation (McCune & Antos Reference McCune and Antos1982; Palmqvist & Sundberg Reference Palmqvist and Sundberg2000; Gauslaa et al. Reference Gauslaa, Lie, Solhaug and Ohlson2006; Stevenson & Coxson Reference Aavik, Jõgar, Liira, Tulva and Zobel2007). Site illumination (light availability) can influence epiphytic lichens directly upon photosynthesis and indirectly upon transpiration; temperature is affected by light and has a strong influence on evaporation. In the case of epiphytic lichens, water supply is determined not only by precipitation, but also by evaporation, inundation and atmospheric humidity. Higher wind speed in semi-open habitats increases evaporation and also has a stronger mechanical destroying effect (Barkman Reference Barkman1958; Nash Reference Nash and Nash1996). Therefore, structurally diverse wooded meadows, composed of patchily scattered trees and bushes in the landscape, offer epiphytes a habitat with a heterogeneous environment consisting of a mosaic of different light, moisture and wind conditions within one meadow.
In earlier studies of epiphytic lichen communities in wooded meadows, considerable attention has been devoted to the effect of environmental and historical factors on the habitat-scale composition and diversity of epiphytic lichens, while at the scale of the individual tree trunk, the relationships of lichen species diversity with tree species and trunk diameter has been studied (Leppik & Jüriado Reference Leppik and Jüriado2008; Jönsson et al. Reference Jönsson, Thor and Johansson2011; Thor et al. Reference Thor, Johansson and Jönsson2010). In this study, we shall evaluate the effect of environmental conditions on lichen communities at the scale of the individual tree trunk, with special emphasis on the tree species, substratum properties and light conditions near each sample tree. Specifically, we intended 1) to estimate how the cessation of traditional management and the transformation of wooded meadows into deciduous forests influences lichen species composition at tree-level and 2) to assess the effects of habitat and substratum properties (e.g. trunk diameter, bark structure and bark pH) on individual epiphytic lichens and their composition.
Materials and Methods
Study sites and data collection
Estonia is located in the hemi-boreal sub-zone of the boreal forest zone – this is the transitional area between the southern boreal forest subzone and the spruce-hardwood subzone (Laasimer & Masing Reference Laasimer, Masing and Raukas1995). Around 50% of the country is covered by forest (Adermann Reference Adermann2009); conifers Picea abies (L.) H. Karst. and Pinus sylvestris L., as well as birches Betula pendula Roth and B. pubescens Ehrh., are the dominant tree species (Adermann Reference Adermann2009). The proportion of deciduous woodland with temperate broad-leaved trees (e.g. Quercus robur, Acer platanoides, Fraxinus excelsior, Ulmus glabra) is almost insignificant in Estonia, mainly because the soil conditions in the habitats of these tree species are suitable for agricultural land use (Kaar Reference Kaar, Valk and Eilart1974; Paal Reference Paal1998). Characteristic tree species in wooded meadows of Estonia are temperate broad-leaved trees, such as English oak (Quercus robur L.) and European ash (Fraxinus excelsior L.), with common aspen (Populus tremula L.); birches and black alder [Alnus glutinosa (L.) Gaertn.] are also quite common (Kukk & Kull Reference Kukk and Kull1997).
The field work was performed in Estonia during the summer of 2006. The selection of wooded meadow fragments (study sites) was made according to the general distribution of wooded meadows in Estonia (Kukk & Kull Reference Kukk and Kull1997). We selected 12 study sites in three regions of Estonia (variable ‘Region’), five study sites on the island of Saaremaa (‘Region 1’), three sites in the western part of the Estonian mainland (‘Region 2’) and four sites in north-eastern Estonia (‘Region 3’) (Fig. 1; Table 1).
Table 1. Location and main characteristics of the 12 studied wooded meadows of Estonia

* 1 – island of Saaremaa, 2 – western part of Estonian mainland, 3 – north-eastern Estonia.
† number of trees studied and tree species (Ag – Alnus glutinosa; Ai – Alnus incana; B – Betula pendula or B. pubescens; Fe – Fraxinus excelsior; Pa – Picea abies; Ps – Pinus sylvestris; Pt – Populus tremula; Qr – Quercus robur; Tc – Tilia cordata)

Fig. 1. Location of the study stands in three sub-regions in Estonia: Reg. 1 – Region 1 (island of Saaremaa), Reg. 2 – Region 2 (western part of Estonian mainland), Reg. 3 – Region 3 (north-eastern Estonia).
Habitat fragments were selected according to their management history; six sites were regularly or irregularly mowed and had no stand undergrowth (bushes and young trees); six sites had not been mown or otherwise managed for, approximately, the last 50 years and had dense undergrowth (variable Management status ‘Overgrown’; Table 1). Our first goal was to determine the effect of the overgrowing of a wooded meadow by trees and brushwood on epiphytic lichens. In order to achieve this we: 1) estimated the percentage of canopy cover (variable ‘Canopy cover’) for each site using orthophotos (Web Map Server of Estonian Land Board 2008); 2) estimated, in the field, the percentage of undergrowth in the range of 10 m around each sample tree (variable ‘Undergrowth’); 3) measured light conditions using hemispherical digital photographs taken around each studied tree trunk in four cardinal directions with a Nikon Coolpix 950 camera and an FC-E8 fish-eye lens converter. The hemispherical photographs were analyzed using the WinSCANOPY2001A program (Régent Instruments Inc. 2001) to obtain an estimate of Total Site Factor (TSF), the proportional combination of direct and diffuse radiation. The maximum radiation coefficient for each tree (variable ‘Max TSF’) was used in the analyses.
We described lichen communities on 12 sample trees at every site. Trees were selected proportionally to the composition of the tree layer in an area of one hectare. Epiphytic lichen communities were investigated on 89 oaks (variable ‘Oak’), 24 ashes (‘Ash’), 23 birches (Betula pendula or B. pubescens, ‘Birch’), 5 aspens and 3 lime trees (Tilia cordata Mill.). Due to the low representation of aspen and lime trees in the studied sites, these tree species were excluded from further analyses. Vertically elongated sample plots (10 × 50 cm with five unit areas of 10 × 10 cm each) were placed on trees in four cardinal directions, with the upper margin of plots at 1·5 m (method by Asta et al. Reference Asta, Erhardt, Ferretti, Fornasier, Kirschbaum, Nimis, Purvis, Pirintsos, Scheidegger, van Haluwyn, Wirth, Nimis, Scheidegger and Wolseley2002 and Scheidegger et al. Reference Scheidegger, Groner, Keller, Stofer, Nimis, Scheidegger and Wolseley2002). The occurrence of lichen species in every unit area was recorded and summed for each sample tree (the maximum abundance score is 20 per sample tree). This abundance measure of lichen species per tree was used in all analyses.
To assess the effects of substratum properties on lichen species composition and on individual lichen species (goal 2) we recorded several tree scale parameters. The diameter of each sample tree (variable ‘DBH’) was measured at 1·3 m above ground, and a bark sample was taken at the same height to measure bark pH. Bark acidity (variable ‘Bark pH’) was measured with a flathead electrode (Consort C532), applying a technique suggested by Schmidt et al. (Reference Aavik, Jõgar, Liira, Tulva and Zobel2001) and Kricke (Reference Kricke, Nimis, Scheidegger and Wolseley2002), with slight modifications by Jüriado et al. (Reference Aavik, Jõgar, Liira, Tulva and Zobel2009b). The roughness of bark (variable ‘Bark structure’) was divided into three categories: ‘Smooth’ (< 1 cm deep bark crevices), ‘Intermediate’ (1–2 cm crevices) and ‘Deep’ (> 2 cm crevices). The occurrence of bryophytes (variable ‘Bryophyte abundance’) was recorded using a similar method as that used for lichens.
The lichen specimens that were difficult to identify in the field were collected for further analyses. A stereomicroscope, light microscope, UV light and standardized thin-layer chromatography (Culberson & Kristinsson Reference Culberson and Kristinsson1970; Culberson Reference Culberson1972) were used for the identification of lichen specimens in the laboratory. The reference material is deposited in the lichenological herbarium at the Natural History Museum of the University of Tartu (TU). The nomenclature of lichens follows Randlane et al. (Reference Randlane, Saag and Suija2008).
Statistical analyses
Spearman rank order correlations (implemented in the program Statistica 7.1; Statsoft Inc. 2005) were estimated among the environmental variables, and thereafter only one variable was selected amongst variables with overly strong correlations (correlation coefficient > 0·7) for further analyses. Geographical coordinates were excluded, because they were strongly correlated with the variable ‘Region’. The variable ‘Overgrown’ correlated strongly with ‘Canopy cover’ and was therefore excluded from the analyses. In all statistical analyses the explanatory variables ‘Max TSF’ and ‘DBH’ were log-transformed to obtain the linearity of relationships.
Prior to analyses of species composition, lichen species registered in fewer than three unit areas (10 × 10 cm) were removed from the data in order to reduce information noise. A non-parametric statistical method of the Multi-Response Permutation Procedure (MRPP) (Mielke Reference Mielke, Krishnaiah and Sen1984) with Euclidean distance, implemented in the program PC-ORD ver. 4.25 (McCune & Mefford Reference McCune and Mefford1999) was used to test differences in lichen species composition among the studied tree species and study regions.
The relationships between lichen community composition and the environmental parameters were more specifically analyzed using partial Canonical Correspondence Analysis (pCCA) (ter Braak Reference ter Braak1986) in the program CANOCO ver. 4.5 (ter Braak & Šmilauer Reference ter Braak and Šmilauer2002). In order to evaluate the explanatory power of different sets of variables, we performed the variation partitioning approach (Borcard et al. Reference Borcard, Legendre and Drapeau1992) in pCCA. Variables were grouped accordingly: G – geographical variables (‘Region 1’, ‘Region 2’ and ‘Region 3’); E – environmental variables related to site openness and light conditions (‘Canopy cover’, ‘Max TSF’ and ‘Undergrowth’); T – tree species-specific indicator variables (‘Ash’, ‘Birch’, ‘Oak’) and ‘Bark pH’; and O – other tree level variables (‘Bark structure’, ‘Bryophyte abundance’ and ‘DBH’). Explanatory power was estimated in two ways: as the main effect of a trait group without considering the explanatory effect of other trait groups, and the ‘unique’ contribution of each trait group after conditioning the effect of other factors. The variables had the value of inflation factors less than four, that is far below the suggested limit value of 20 (ter Braak & Šmilauer Reference ter Braak and Šmilauer2002).
In the graphic presentation of the results of the pCCA, only the most important variables were presented. The forward selection procedure with randomization tests (Monte-Carlo permutation test, 1000 unrestricted permutations) was employed to select the statistically significant environmental variables influencing species composition, retaining the variables with a conditional contribution below the level of P < 0·05 (‘Ash’, ‘Birch’, ‘Canopy cover’, ‘DBH’, ‘Oak’, ‘Bark pH’, ‘Region 1’, ‘Region 2’ and ‘Region 3’). Variables ‘Bark structure’, ‘Bryophyte abundance’, ‘Max TSF’ and ‘Undergrowth’ had P > 0·05 and were left out. In order to demonstrate specifically the influence of environmental factors and to reduce the variation in species data caused or explained by site geographical location, geographical variables (‘Region 1’, ‘Region 2’ and ‘Region 3’) were treated as co-variables. Due to the unbalanced sample design (more oaks in ‘Region 3’), it is difficult to distinguish the effect of tree species from the effect of the region on lichen species composition at individual trees. By removing the effect of geographical variables as covariates, however, the remaining variation, explained by tree species, can be seen as a unique effect of tree species regarding unbalanced sampling. Since the tree species-specific variables (‘Ash’, ‘Birch’, ‘Oak’ and ‘Bark pH’) also proved to be strong determinants of lichen communities, we moved them to the co-variable list with geographical variables in the second pCCA, in order to analyze the effect of habitat openness (variable ‘Canopy cover’) and tree level features on species composition. Variable ‘Bark pH’ was also moved to the co-variable list, because it has proven to be tree species specific.
To evaluate the effect of variables specifically on individual lichen species, we performed General Linear Model (GLM) with the forward stepwise selection procedure implemented in the program Statistica 7.1 (Statsoft Inc. 2005). The influence of variables ‘Region’ (levels: ‘Region 1’, ‘Region 2’ and ‘Region 3’), ‘Tree species’ (‘Ash’, ‘Birch’ and ‘Oak’), ‘Bark structure’ (‘Smooth’, ‘Intermediate’ and ‘Deep’), ‘Canopy cover,’ ‘DBH,’ ‘Bark pH’ and ‘Undergrowth’ on the abundance (square-root transformed) of the 35 most frequent lichen species was analyzed. GLM analyses were performed separately for each species. The Mallow Cp criterion was used to find the optimal model according to predictive power and to avoid over-parameterization (Shao Reference Shao1997). The Tukey HSD multiple comparison test was used to determine the significant differences of mean values between groups of categorical factors in the analysis.
Results
Lichen species composition is influenced most strongly by tree species specific variables
A total of 113 lichen species was recorded from 136 trees; oaks (n = 89) had 97 lichen species, ashes (n = 24) 57 and birches (n = 23) 40 lichen species (Appendix 1). According to the MRPP test results, the species composition of lichens is distinctive among ash, birch and oak trees (A = 0·0667, P < 0·0001; pairwise post-hoc comparisons P < 0·0001) and also among the regions (A = 0·0668, P < 0·0001; pairwise post-hoc comparisons P < 0·0001).
We performed a partial CCA in order to estimate the amount of variation explained by different sets of explanatory variables. The full model explained 30·5% of the total inertia (7·34 inertia units). Variance partitioning by pCCA indicated that the largest amount of variance in the composition of lichen communities is explained by the set of tree species-specific variables, that is tree species and bark pH (10·3% of the variation in the data set), followed by the set of geographical variables (8·8%), the set of other tree level variables (6·9%) and the set of environmental variables concerning site openness and light conditions (6·5%, Table 2). The variance uniquely described by the set of tree species-specific variables was 8·3% of the variation in the data set, when the other sets of variables were used as co-variables. The unique variation components of other sets of variables were lower and described a more or less equal amount of variation, for example 4·2% of the total variation was described uniquely by the set of other tree level variables (‘Bark structure’, ‘Bryophyte abundance’ and ‘DBH’; Table 2).
Table 2. Variation partitioning in epiphytic lichen community composition in wooded meadows among four sets of explanatory variables (split-plot design & pure main effect conditioning on other factors) using variation partitioning analysis in partial canonical correspondence analysis (pCCA)

* G – geographical variables (‘Region 1’, ‘Region 2’ and ‘Region 3’); E – environmental variables concerning site openness and light conditions (‘Canopy cover’, ‘Undergrowth’ and ‘Max TSF’); O – other tree level variables (‘Bark structure’, ‘Bryophyte abundance’ and ‘DBH’); T – tree species-specific variables (‘Ash’, ‘Birch’, ‘Oak’ and ‘Bark pH’); the symbol ‘∣’ means conditioning, i.e. variables which have been covaried out; ‘∪’ refers to the union of variable sets.
† The sum of all canonical eigenvalues is given in inertia units (IU); the system's total inertia is 7·343.
Habitat openness and diameter of the tree trunk determine differences in lichen composition
As our intention was to evaluate the effect of management intensity and tree level traits on the lichen community, we eliminated the effect of region by setting the geographical variable ‘Region’ as covariate in the first pCCA. The eigenvalues of the first three ordination axes are 0·42, 0·26 and 0·16, respectively. The first axis of pCCA (with geographical variables as co-variables) is described mainly by indicator variables of tree species (‘Ash’, ‘Birch’, and ‘Oak’). Dimerella pineti, Lecanora conizaeoides, Micarea prasina and Placynthiella icmalea are located at the right positive end of the first ordination axis and grow more frequently on birch trees (Fig. 2). The second axis is explained by environmental factors involving site openness, and the variable ‘Canopy cover’ describes the denser tree canopy at the positive end of the gradient (Fig. 2). The variable ‘DBH’ is also correlated with the second axis (Fig. 2). The third axis is explained by bark pH and tree species (variables ‘Ash’ and ‘Oak’), indicating that lichen species Acrocordia gemmata, Lecanora rugosella, Opegrapha rufescens and O. varia grow more frequently on ash trees with high pH (not shown).
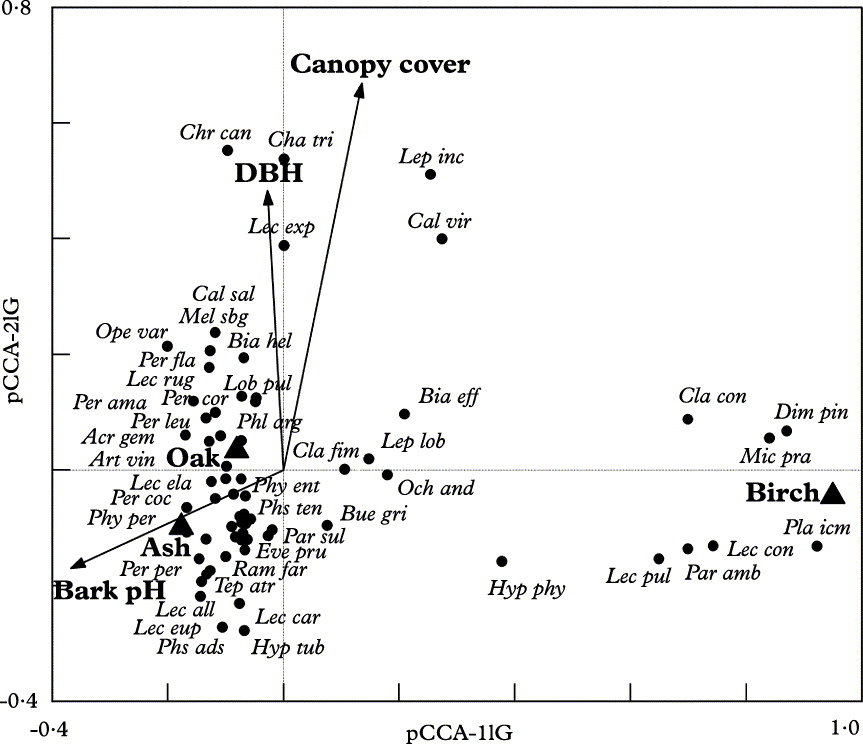
Fig. 2. Lichen species and environmental variables on the biplot of partial canonical correspondence analysis (pCCA) of the first and second axes. The symbol ‘l’ in the titles of the axes means conditioning, i.e. variable ‘Region’ (G) has been covaried out. DBH = diameter of tree trunk at breast height; abbreviations of species names are given in Appendix 1.
As tree species proved to be strong determinants of lichen communities, in the second pCCA we moved them into the co-variable list with geographical variables, in order to demonstrate the effect of ‘Canopy cover’ and also the effect of ‘DBH’. Variable ‘Bark pH’ was also moved to the co-variable list, because it was tree-species-specific. The eigenvalue of the first ordination axis is 0·25, and the eigenvalue of the second axis 0·17. Most of the lichen species that have a foliose and fruticose growth form (e.g. Evernia prunastri, Hypogymnia physodes, Parmelia sulcata, Parmeliopsis ambigua, Physcia tenella and Ramalina farinacea) grow in well-lit wooded meadows with more open canopies and are located on the lower left part of the second pCCA ordination plot (Fig. 3). In additionto macrolichens, a similar placement in ordination space can be observed in the case of many microlichen species, which also prefer improved light conditions (e.g. Amandinea punctata, Biatora globulosa, Candelariella xanthostigma and Pertusaria coccodes; Fig. 3). Only Biatora efflorescens, B. helvola, Graphis scripta, Lobaria pulmonaria and Micarea prasina grow more abundantly in shady conditions, that is have a positive dependence on canopy closure and are therefore located on the upper right part of the pCCA diagram (Fig. 3). The diameter of a tree trunk forms another gradient placing lichen species that grow on bigger trees (e.g. Calicium viride, Chrysothrix candelaris, Opegrapha varia and Pertusaria albescens) in the lower right part of the ordination axes (Fig. 3).

Fig. 3. Lichen species and environmental variables on the biplot of partial canonical correspondence analysis (pCCA) of the first and second axes. The symbol ‘l’ in the titles of the axes means conditioning, i.e. variable ‘Region’ (G) and tree species-specific variables (T – ‘Tree species’ and ‘Bark pH’) have been covaried out. DBH = diameter of tree trunk at breast height; abbreviations of species names are given in Appendix 1.
The variables ‘Bark structure’, ‘Bryophyte abundance’, ‘Max TSF’ and ‘Undergrowth’ proved to be insignificant for general composition of community, according to the forward selection procedure of factors in pCCA (P > 0·05).
Effects of habitat and substratum properties on individual epiphytic lichen species
The results of GLM analysis, where the effect of the studied variables was analyzed for the abundance of the 35 most frequent lichen species individually, confirmed that variables ‘Region’, ‘Canopy cover’, ‘DBH’ and ‘Tree species’ are the most frequently significant drivers of lichen abundance (Table 3). Variables such as ‘Bark structure’, ‘Bark pH’ and ‘Undergrowth’ appeared to be significant predictors for some lichen species' abundance. Seven lichen species are more abundant on the island of Saaremaa (‘Region 1’), six species are more abundant in the western part of the Estonian mainland (‘Region 2’), and another six lichen species prefer to grow in north-eastern Estonia (‘Region 3’). The model's results revealed 12 lichen species that prefer to grow on certain tree species: five species prefer to grow on ash, three on birch and one on oak (Table 3). Three lichen species prefer to grow on both ash and oak trees. Opegrapha rufescens and Tephromela atra are common on ash trees, Hypogymnia physodes and Lecanora pulicaris grow on birch and Pertusaria amara is restricted to oak. The abundances of seven lichen species are influenced by bark pH, for example Buellia griseovirens and Lepraria incana are species that grow on more acidic bark. More than half (21) of the 35 species analyzed are influenced by tree canopy cover in the wooded meadow or the percentage of undergrowth in the vicinity of the sampled tree (Table 3). Evernia prunastri, Hypogymnia physodes, Melanelia subaurifera, Parmelia sulcata and Ramalina farinacea are the macrolichens that prefer to grow in well-lit conditions as well as microlichens Buellia griseovirens, Lecanora pulicaris and Tephromela atra. Only a few microlichens (e.g. Lepraria incana and Chaenotheca trichialis) grow in shady habitats where canopy cover is denser. Local light conditions around the tree trunk reveal that the microlichen, Phlyctis argena, is more common on trees enclosed with high bush cover, whereas the macrolichens, Evernia prunastri, Parmelia sulcata and Ramalina farinacea, prefer habitats with limited undergrowth cover. The abundances of 14 lichen species are influenced by tree diameter. Lepraria incana and Parmelia sulcata are more common on large-diameter trees and Buellia griseovirens, Phlyctis argena and Tephromela atra are common on small-diameter trees. Eight lichen species are influenced by bark structure, for example Lecanora pulicaris often grows on trees with smooth bark.
Table 3. The results of the general linear model (GLM) for the influence of environmental variables on the abundance of the 35 most frequent lichen species in 12 studied wooded meadows of Estonia

The adjusted coefficient of determination (adjusted R 2) and significance value (P) are presented. The species with the model's adjR 2 ≥ 0·3 are presented in bold. Letter labels denote homogeneity groups according to the results of the Tukey HSD multiple comparison test.
* DBH – diameter of tree trunk at breast height (log-transformed), M – macrolichen (lichen with foliose or fruticose growth form).
† 1 – island of Saaremaa, 2 – western part of Estonian mainland, 3 – north-eastern Estonia.
Based on all of the performed GLM analyses, the variables ‘Bryophyte abundance’ and ‘Max TSF’ appeared to have an insignificant influence on lichen abundance on trees.
Discussion
Our study demonstrates that the variation in the epiphytic lichen species composition of wooded meadows is determined by a complex of factors, including regional, habitat and tree level variables. In wooded meadows, as in forest communities (Aude & Poulsen Reference Aude and Poulsen2000; Löbel et al. Reference Löbel, Snäll and Rydin2006; Belinchón et al. Reference Belinchón, Martínez, Escudero, Aragón and Valladares2007; Jüriado et al. Reference Aavik, Jõgar, Liira, Tulva and Zobel2009a), the tree level variables explained the largest fraction of variation in the composition of epiphytic lichen species. In accordance with an earlier study (Leppik & Jüriado Reference Leppik and Jüriado2008), we have demonstrated that habitat management and habitat openness are also significant drivers of epiphytic lichen species composition in wooded meadows. The cessation of traditional management consisting of hay mowing and selective cutting causes changes in stand structure and the openness of wooded meadows, and thereafter affects lichen species composition. We have demonstrated that the abandonment of wooded meadows and the overgrowing of their stands have led to the succession of epiphytic communities with light-demanding species toward communities with impoverished composition characteristic to secondary forests.
It has been suggested that before human interference and the introduction of agriculture in northern Europe, the natural vegetation predominantly consisted of closed forests (Laasimer Reference Laasimer1965; Svenning Reference Svenning2002). The pollen data of tree species in Europe, however, suggests that the original vegetation in the lowlands of Europe had more of an open than an extensively dense stand structure (Vera Reference Vera2000). Eriksson et al. (Reference Cousins and Eriksson2002) suggested that traditionally managed wooded meadows resemble pre-agricultural wooded ecosystems in northern Europe. Today, forests are intensively managed, mostly mono-cultured and evenly aged with dense canopies. These managed forests lack many natural elements of old-growth stands such as gaps and large-diameter old trees (Esseen et al. Reference Esseen, Ehnström, Ericson, Sjöberg and Hansson1992; Bengtsson et al. Reference Bengtsson, Nilsson, Franc and Menozzi2000; Liira & Sepp Reference Liira and Sepp2009). Therefore managed wooded meadows could be potential refugia for species originating from ancient semi-opened landscapes (Rose Reference Rose, Bates and Farmer1992; Svenning Reference Svenning2002).
Microclimatic conditions in the wooded meadow changed after the meadow's stand canopy became denser with brushwood. Changes in microclimate could be the main reason for the distinction of epiphytic lichen communities between open and dense canopy wooded meadows. Our results support the statement of Barkman (Reference Barkman1958) that most epiphytic macrolichens with a green photobiont and with foliose and fruticose growth form are highly dependent on good illumination conditions. We found that the majority of epiphytic macrolichen species were negatively affected by increasing canopy cover and only a few common forest microlichen species, including Chaenotheca trichialis, Dimerella pineti Micarea prasina and Lepraria incana, were abundant under the dense canopy in unmanaged wooded meadows (Fig. 3, Table 3). This trend was also demonstrated in large-scale studies at the habitat level in wooded meadows where decreasing richness of epiphytic lichen species with increasing canopy cover was detected (Leppik & Jüriado Reference Leppik and Jüriado2008; Jönsson et al. Reference Jönsson, Thor and Johansson2011).
However, our results contrast with the findings by Jönsson et al. (Reference Jönsson, Thor and Johansson2011), who found no differences in lichen species composition in relation to land use practices in wooded meadows. Jönsson et al. (Reference Jönsson, Thor and Johansson2011) suggested that the homogeneity of lichen species composition in managed and unmanaged wooded meadows could be caused by lichen species that are able to persist in unmanaged sites. Woodland management history and the time since the cessation of management of wooded meadows could differ in Estonia and Sweden (Kukk & Kull Reference Kukk and Kull1997; Eriksson et al. Reference Eriksson, Cousins and Bruun2002), and apparently the lichen species growing on trees in open wooded meadows have already disappeared in unmanaged wooded meadows in Estonia. During the field work we expected to find species confined to managed wooded meadows, for example Caloplaca chrysophthalma, C. lucifuga, Lecidella flavosorediata and Pertusaria flavida (Ekman et al. Reference Ekman, Fröberg, Kärnefelt, Sundin and Thor1991; Leppik & Jüriado Reference Leppik and Jüriado2008), but the frequency of these species in the wooded meadows studied proved to be so low that we were unable to analyze their ecology.
We have shown that light availability has an effect on species composition and on individual species on two scales, as two variables proved to be significant: canopy cover estimated at stand level and the percentage of undergrowth around each individual tree trunk. Surprisingly, the supposedly precise light availability estimates using hemispherical digital photographs taken under each studied tree had remarkably lower descriptive power. It has been shown in earlier studies that in open habitats, environmental factors have a strong interaction in their effect: the wetting and drying cycles are more rapid because of the higher amount of direct sunlight amplified by the improved air circulation, whereas in closed habitats micro-environmental fluctuations are smoother (Barkman Reference Barkman1958; McCune & Antos Reference McCune and Antos1982). Unfortunately we had no opportunity to measure the effect of altered moisture and temperature conditions at the site in general and near each tree trunk, and therefore we cannot emphasize the effect of light over the effect of moisture in wooded meadows.
In accordance with earlier studies, we have shown that lichen community composition is largely determined by the identity of tree species. In particular, the marked difference between the epiphytic lichen communities on birch (Betula pendula and B. pubescens) and those on other deciduous tree species in the temperate region is quite well known (Barkman Reference Barkman1958; Cieśliński et al. Reference Cieśliński, Czyżewska, Klama and Żarnowiec1996; Lõhmus Reference Lõhmus2003). The most probable causes of such differentiation between tree species are lichen responses to tree-species-specific bark properties (Culberson Reference Culberson1955; Barkman Reference Barkman1958; Rose Reference Rose, Morris and Perring1974; Bates & Brown Reference Bates and Brown1981). For example, Acrocordia gemmata and Opegrapha varia are common on sub-neutral barked ash (Jüriado et al. Reference Jüriado, Paal and Liira2003, 2009b). In our study we frequently also found them on oak, but in sites where the oak's bark pH was closer to neutral than usual. Similarly, Buellia griseovirens and Lepraria incana were frequent on trunks with acidic bark, independent of tree species. Bark roughness is another characteristic that affects lichens. For example, we found deep bark roughness to be important for Calicium salicinum, Chrysothrix candelaris and Pertusaria coronata, while smooth bark predicts the abundance of Lecanora pulicaris and Pertusaria leucostoma, regardless of tree species.
Tree diameter and also bark roughness are known to be age-dependent factors of trees (Johansson et al. Reference Johansson, Rydin and Thor2007; Ranius et al. Reference Ranius, Eliasson and Johansson2008b; Fritz et al. Reference Fritz, Niklasson and Churski2009). The observed variation in the composition of lichen communities along the gradient of trunk diameter (i.e. tree age) is in accordance with many other studies (Hedenås & Ericson Reference Hedenås and Ericson2000; Friedel et al. Reference Friedel, Oheimb, Dengler and Härdtle2006; Fritz et al. Reference Fritz, Niklasson and Churski2009; Jüriado et al. Reference Aavik, Jõgar, Liira, Tulva and Zobel2009a, b; Jönsson et al. Reference Jönsson, Thor and Johansson2011; Thor et al. Reference Thor, Johansson and Jönsson2010). It has been shown that the variation of physical and chemical properties of bark during tree ageing is correlated with the variation in lichen communities (Ellis & Coppins Reference Aavik, Jõgar, Liira, Tulva and Zobel2007b).
With regard to the tree species and tree age specificity of epiphytic lichen communities, the preservation of tree species diversity and different age (diameter) groups is vital in order to maintain the high diversity of epiphytic lichens in wooded meadows. We propose that the selective cutting of trees and undergrowth, retaining a mosaic of semi-open structure with the trees of various tree species and age classes and some clumps of bushes, will create heterogeneous microclimatic conditions for a diverse community of epiphytic lichens. The continuous traditional management or restorations should be performed in the first order in regions where wooded meadows have a long term historical background, as stand historical continuity and stand age have frequently been shown to be major determinants for epiphytic lichen diversity (Boudreault et al. Reference Boudreault, Gauthier and Bergeron2000; Jüriado et al. Reference Jüriado, Paal and Liira2003; Ellis & Coppins 2007a; Fritz et al. Reference Fritz, Gustafsson and Larsson2008).
In addition to checking the tree layer of the stands, the management of wooded meadows in terms of annual mowing or grazing has been prescribed as the vitally important actions preserving the semi-open structure, and particularly the high species diversity (Pykälä et al. Reference Pykälä, Luoto, Heikkinen and Kontula2005; Aavik et al. Reference Aavik, Jõgar, Liira, Tulva and Zobel2008; Jönsson et al. Reference Jönsson, Thor and Johansson2011). Grazing, however, might enrich the epiphytic lichen community with nitrophytic species due to fertilization of the tree trunks by cattle (Benfield Reference Benfield1994; van Herk Reference van Herk1999; Ruisi et al. Reference Ruisi, Zucconi, Fornasier, Paoli, Frati and Loppi2005), and therefore it is suggested that grazing is only beneficial for overall lichen diversity if it has been undertaken with limited intensity (Rose Reference Rose and Fletcher2001; Sanderson & Wolseley Reference Sanderson, Wolseley and Fletcher2001).
The authors would like to thank the following colleagues from the Institute of Ecology and Earth Sciences of the University of Tartu: Lauri Saag for help in planning the study and determining some sterile lichen specimens, Ave Suija and Piret Lõhmus for determining some difficult lichen specimens and Tiina Randlane for offering helpful comments on the manuscript. Ingmar Tulva and Eve Eensalu are thanked for help with the hemispherical digital photographs. Alexander Harding kindly revised the English. Financial support was received from the Estonian Science Foundation (grants no 7470, 7816 and 7878), the Estonian Ministry of Education and Research (targeted financing Nos SF0180153s08 and SF0180012s09), the EEA Financial Mechanism (grant EMP9) and the European Union, through the European Regional Development Fund (FIBIR Center of Excellence).
Appendix 1. List of the recorded lichen species and their abundance (percentage) on studied trees of ash, birch and oak in wooded meadows of Estonia. Abbreviations are provided for the species that have been used in ordination analyses

* macrolichen (lichen with foliose or fruticose growth form).
† Ash – Fraxinus excelsior, Birch – Betula pendula or B. pubescens and Oak – Quercus robur.









