Introduction
The genus Arctoparmelia Hale was separated from the genus Xanthoparmelia (Vain.) Hale and formally described by Hale (Reference Hale1986). Arctoparmelia taxa differ from Xanthoparmelia s. lat. species by containing alectoronic acid, having a velvety, ivory white to purplish lower surface, and being distributed across the boreal-arctic region (Hale Reference Hale1986). In contrast, Xanthoparmelia species do not contain alectoronic acid (except for Xanthoparmelia alectoronica Hale), have a plane or canaliculate, pale brown to black lower surface, and as a general rule are most abundant in semi-arid to arid regions with extensive exposures of granite and sandstone (Hale Reference Hale1990). Despite the morphological and chemical similarity with some Xanthoparmelia species, Arctoparmelia is more closely related to hypogymnioid species and belongs to the same clade as the genera Brodoa, Hypogymnia and Pseudevernia (Blanco et al. Reference Blanco, Crespo, Divakar, Esslinger, Hawksworth and Lumbsch2004; Crespo et al. Reference Crespo, Kauff, Divakar, del Prado, Pérez-Ortega, Amo de Paz, Ferencova, Blanco, Roca-Valiente and Núñez-Zapata2010; Thell et al. Reference Thell, Crespo, Divakar, Kärnefelt, Leavitt, Lumbsch and Seaward2012; Divakar et al. Reference Divakar, Crespo, Kraichak, Leavitt, Singh, Schmitt and Lumbsch2017).
Currently, Arctoparmelia includes four species: A. centrifuga (L.) Hale, A. incurva (Pers.) Hale, A. separata (Th. Fr.) Hale and A. subcentrifuga (Oxner) Hale. Additionally, Hale (Reference Hale1986) recognized a fifth species, A. aleuritica (Nyl.) Hale, which was considered by Hasselrot (Reference Hasselrot1953), Poelt & Vězda (Reference Poelt and Vězda1981) and Santesson (Reference Santesson1984) as an usnic acid-deficient mutant of A. centrifuga in Scandinavia. Later, the usnic acid-deficient specimens previously referred to as A. aleuritica were synonymized with A. centrifuga by Clayden (Reference Clayden1992), which was followed by Moberg & Thell (Reference Moberg, Thell, Thell and Moberg2011).
All four species of Arctoparmelia are known from Russia and three of them, A. centrifuga, A. incurva and A. separata, are described as common (see, for example, Hale Reference Hale1986; Tchabanenko Reference Tchabanenko2002; Urbanavichus & Urbanavichene Reference Urbanavichus, Urbanavichene and Korneeva2004; Makryi & Lishtva Reference Makryi, Lishtva, Bardunov, Chechetkina, Makryi, Malyshev, Petrov, Lishtva, Lopatovskaya and Maksimova2005; Poryadina Reference Poryadina and Danilova2005; Kuznetsova et al. Reference Kuznetsova, Ahti and Himelbrant2007; Kharpukhaeva Reference Kharpukhaeva2010; Kristinsson et al. Reference Kristinsson, Zhurbenko and Hansen2010; Sedelnikova Reference Sedelnikova2013; Himelbrant et al. Reference Himelbrant, Stepanchikova, Kuznetsova and Yu. Neshataeva2014), whereas A. subcentrifuga is less frequently recorded there (Hale Reference Hale1986; Vitikainen & Dudoreva Reference Vitikainen and Dudoreva2003; Urbanavichene & Urbanavichus Reference Urbanavichene, Urbanavichus, Vinogradova, Gagarina, Kovalenko, Kurbatova, Luknitskaya, Novozholov, Potemkin, Predtechenskaya, Titiov and Urbanavichene2008; Urbanavichus et al. Reference Urbanavichus, Urbanavichene and Melekhin2013; Urbanavichus & Fadeeva Reference Urbanavichus and Fadeeva2013; Urbanavichus & Urbanavichene Reference Urbanavichus and Urbanavichene2017). On the other hand, the usnic acid-deficient chemotype of Arctoparmelia was reported only once as Parmelia centrifuga f. dealbata Th. Fr. in the ‘Handbook of the Lichens of the USSR’ (Rassadina Reference Rassadina and Abramov1971), with no location recorded. None of the usnic acid-deficient chemotypes of Arctoparmelia have been reported from Russia since then. Also, no single herbarium specimen of the usnic acid-deficient chemotype of Arctoparmelia from Russia is known.
After finding usnic acid-deficient Arctoparmelia specimens in Siberia during fieldwork in 2014–2017, we decided to study these specimens using molecular and chemotaxonomic methods and compare them with other Arctoparmelia specimens. As a result, based on morphological, chemical and molecular data, we describe this material as Arctoparmelia collatolica S. Chesnokov & I. Prokopiev, a species new to science.
Materials and Methods
Field collection, revision and morphological analysis
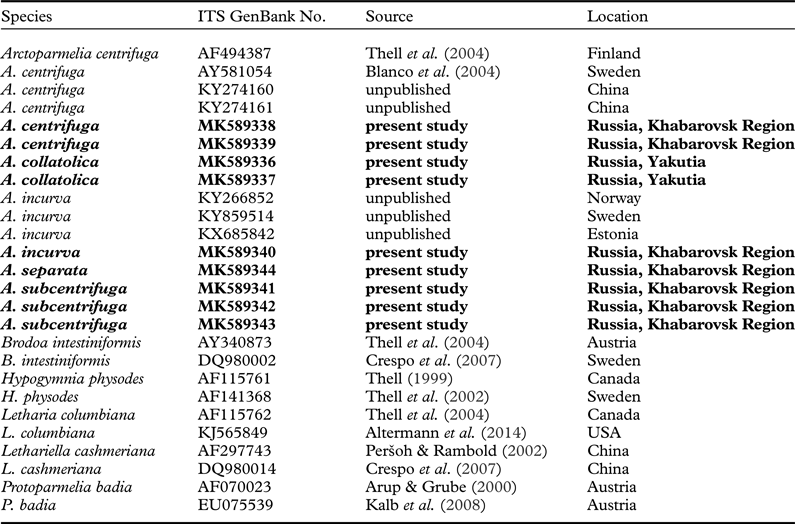
We revised specimens from the genus Arctoparmelia deposited in the following herbaria: Komarov Botanical Institute of the Russian Academy of Sciences (LE), University of Helsinki (H), Polar-Alpine Botanical Garden and Institution (KPABG), Institute of Experimental Botany at the National Academy of Sciences of Belarus (MSK), Institute for Biological Problems of the Cryolithozone, Siberian Branch, at the Russian Academy of Sciences (SASY) and Institute of Biological Problems of the North, Far East Branch, at the Russian Academy of Sciences (MAG). We identified the specimens using morphological features and standard colour reactions detected in 10% potassium hydroxide (KOH or K), sodium hypochlorite (C), K followed by C on the same fragment (KC) and para-phenylenediamine (PD) (Smith et al. Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). We performed metabolomic analyses on seven specimens of Arctoparmelia collatolica, 10 specimens of usnic acid-deficient and 49 specimens of usnic acid-containing chemotypes of A. centrifuga, 21 specimens of A. incurva, 23 specimens of A. separata and 17 specimens of A. subcentrifuga. This analysis also included some specimens of both chemotypes of A. centrifuga from Canada and Europe studied by Clayden (Reference Clayden1992), which are stored in the herbarium H. For molecular analysis, we used specimens collected by L. Konoreva and S. Chesnokov in the Sakha Republic (Yakutia) in 2017, and in the Khabarovsk Region in 2018 (Table 1). Type specimens of Arctoparmelia collatolica are stored in the herbaria LE and H.
Table 1. GenBank Accession numbers and additional information for the specimens used in the phylogenetic analysis in Fig. 1. Newly generated sequences are in bold.
Liquid chromatography-mass spectrometry (LC-MS) metabolite profiling
For the LC-MS analysis, 10 mg of air-dry lichen was ground up. The secondary substances from each sample were extracted with 1 ml of acetone. Extraction was carried out with constant stirring for 24 h at 20–25 °C. The high performance liquid chromatography (HPLC) analysis was carried out using an Agilent 1290 instrument. The molecular mass of ions was recorded on an Agilent 6538 UHD quadrupole-time-of-flight (qTOF) mass spectrometer with electrospray ionization (ESI). Elution was carried out in the isocratic mode. A mixture of acetonitrile and 0·1% formic acid aqueous solution in a ratio of 80:20 was used as the mobile phase. The analysis was carried out for 30 min at a flow rate of 100 μl min−1 and a column temperature of 25 °C. For separation, we used a ZORBAX SB-C18 reversed phase column, 80 Å, 150 × 0·5 mm × 5 μm in size. The injection volume was 1 μl and the UV detection wavelength was 254 nm. The voltage on the capillary at the ESI was 2·5 kV; capillary temperature 350 °С; atomizer gas pressure 45 psi; desiccant gas temperature (nitrogen) 225 °C; desiccant gas flow rate 5 μl min−1. Only negatively charged ions were registered, in the mass range of 100–1000 m/z. The resulting chromatograms were processed with the MassHunter WorkStation v. B.04.00 software package (Agilent, USA).
To identify lichen substances, we compared the measured molecular masses and retention times with the lichen substances standards from the V. L. Komarov Botanical Institute collection. The quantitative content of lichen substances was determined by the calibration curves of corresponding standard compounds.
Molecular data generation and analyses
Extraction of DNA and PCR amplification were performed following Cubero et al. (Reference Cubero, Crespo, Fatehi and Bridge1999). We used the primer pair ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) for the amplification of the ITS rDNA gene. Chromatograms were edited in FinchTV 1.4.0 (Geospiza, Inc., Seattle, WA, USA), then sequences were assembled in BioEdit 7.2.5 (Hall Reference Hall1999) and aligned online by MAFFT v.7 (Katoh & Standley Reference Katoh and Standley2013) with the L-INS-i method (Katoh et al. Reference Katoh, Kuma, Tohand and Miyata2005). The alignment was manually checked and adjusted in BioEdit 7.2.5. Newly generated sequences were submitted to the NCBI (GenBank); Accession numbers are provided in Table 1. The ITS rDNA sequences were aligned with all the Arctoparmelia ITS rDNA sequences available in GenBank (Table 1). We carried out maximum likelihood (ML) reconstruction using RAxML v8.2.10 (Stamatakis Reference Stamatakis2014). Optimum partitioning of the data set and the optimum substitution models per partition were calculated with the PartitionFinder2 program (Guindon et al. Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010; Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012, Reference Lanfear, Frandsen, Wright, Senfeld and Calcott2016). The analysis included two partitions: ITS1 + ITS2 and 5.8S, both with the GTR + G substitution model. Bootstrap support values were calculated on 500 bootstrap replicates. The analysis was performed on the CIPRES Web Portal (http://www.phylo.org/portal2/). Protoparmelia badia was chosen as an outgroup as the earliest diverging clade in the family Parmeliaceae (Divakar et al. Reference Divakar, Crespo, Kraichak, Leavitt, Singh, Schmitt and Lumbsch2017). The closest outgroup lineages (species of Brodoa, Hypogymnia, Letharia and Lethariella) were chosen according to the phylogeny of Parmeliaceae proposed by Divakar et al. (Reference Divakar, Crespo, Kraichak, Leavitt, Singh, Schmitt and Lumbsch2017).
Results and Discussion
We analyzed the chemical composition of Arctoparmelia collatolica and the A. centrifuga usnic acid-containing and usnic acid-deficient chemotypes using LC/MS assay. We also included in this analysis some specimens of both chemotypes of A. centrifuga revised by Clayden (Reference Clayden1992). For comparison, we examined samples of A. incurva, A. separata and A. subcentrifuga. The results are presented in Table 2.
Table 2. Content (% of dry mass) of secondary metabolites in Arctoparmelia lichens.

* substances previously unknown in the genus Arctoparmelia.
(+) = content <0·1%; (-) = compound not detected. Results are presented as mean ± standard deviation.
We detected usnic, α-alectoronic, β-alectoronic, physodic, 4-O-methylphysodic acids and atranorin in all revised specimens of Arctoparmelia, except for A. incurva, where atranorin was absent. We did not find usnic acid in any of the analyzed samples of A. collatolica or the usnic acid-deficient chemotypes of A. centrifuga. The main metabolites produced by A. collatolica (α-collatolic, β-collatolic and dehydrocollatolic acids) were not found in any other Arctoparmelia species. Interestingly, European and Canadian specimens of the usnic acid-containing and usnic acid-deficient chemotypes of A. centrifuga, analyzed here, contain an unidentified substance unk372, that was absent in A. collatolica.
Hale (Reference Hale1986) reported alectoronic and α-collatolic acids as major metabolites for the genus Arctoparmelia, distinguishing it from the genus Xanthoparmelia. However, we found α-collatolic acid only in the A. collatolica specimens from Siberia. Similar results to ours were obtained by Clayden (Reference Clayden1992).
Clayden (Reference Clayden1992) compared the chemistries of the usnic acid-deficient and usnic acid-containing chemotypes of A. centrifuga from Canada and Europe using thin-layer chromatography (TLC). He concluded that Canadian and European populations of this species (both usnic acid-deficient and usnic acid-containing specimens) belonged to different chemotypes because of the presence of different fatty acids: the Canadian chemotype (R f classes fatty acid: A = 3–4(?), B = 5, C = 5–6) differed from the European chemotype (R f classes fatty acid: A = 3(?), B = 4–5, C = 4). The author did not identify these substances. We studied several specimens analyzed by Clayden (Reference Clayden1992) but did not find the fatty acids that he reported. Clayden did not resolve the taxonomic position of these fatty acid chemotypes. Based on the distribution of the usniс acid-deficient chemotype of Arctoparmelia in Canada and Europe, he concluded that this chemotype had multiple origins and the presence or absence of usnic acid is not a taxonomically reliable feature in this case.
Based on morphology and secondary metabolites, we concur with the opinion of other researchers that ‘A. aleuritica’ is the usnic acid-deficient chemotype of A. centrifuga. However, further studies on this chemotype, supported by molecular analyses, are needed to confirm this observation.
Our phylogenetic reconstruction (Fig. 1) shows that two sequences of A. collatolica form a highly-supported clade well separated from other species of the genus. This result supports our designation of the new species based on morphology, chemistry and ecology. Unfortunately, we used only the usnic acid-containing chemotype of A. centrifuga in our analyses because the specimens of the usnic acid-deficient chemotype were too old to be used in the molecular analyses.

Fig. 1. A maximum likelihood (ML) phylogeny of the genus Arctoparmelia inferred from ITS sequences. Maximum likelihood bootstrap values ≥75% are shown above internal branches. Newly sequenced samples are indicated in bold.
Arctoparmelia centrifuga and A. incurva form their own supported clades. Arctoparmelia subcentrifuga also forms a supported clade closely related to A. separata. Relationships between these two species require further investigation.
Taxonomy
Arctoparmelia collatolica S. Chesnokov & I. Prokopiev sp. nov.
MycoBank No.: MB 830143
Similar to the usnic acid-deficient chemotype of Arctoparmelia centrifuga but differing in the grey-brown to brown upper surface in the central part and ivory white to pale brown rhizines. The species contains collatolic acid and its derivatives.
Type: Russia, Republic of Sakha (Yakutia), Ust-Maysky District, Tarbaganakh Mountain, 61°10′8·2″N, 138°21′46·2″E, alt. 1388 m, slope with boulders and thicket of Pinus pumila, on stone, 17 July 2017, S. V. Chesnokov 26 (LE-L15136—holotype; H—isotype). GenBank Accession numbers: ITS: MK589336, MK589337.
(Fig. 2A–F)

Fig. 2. Arctoparmelia collatolica, a species new to science from Siberia, Russia. A, thallus with preserved central part (holotype); B, colour of the upper surface at the end of lobes and in the central part of the thallus (holotype); C, lower surface with rhizines (holotype); D, concentric circles with preserved central part of the thallus; E, A. centrifuga (1) and A. collatolica (2) growing on the same stone; F, A. collatolica from Trans-Baikal Territory (LE-L15141). Scale: C = 1 mm.
Thallus rosette-forming or irregularly shaped, closely attached to the substratum. The central part of the thallus persists for a long time, sometimes forming huge thalli up to 30 cm diam. (Fig. 2A). If the central part of the thallus breaks down, the concentric circles follow closely one after another without exposing the substratum (Fig. 2D). Lobes flat to weakly convex, up to 2 mm wide. Upper surface whitish grey at the ends of lobes to grey-brown, brown in the central part (Fig. 2B). Lower surface ivory white to pale brown. Rhizines ivory white to pale brown, having the same colour as the lower surface (Fig. 2C).
Apothecia unknown.
Chemistry
Cortex C−, K+ yellow, KC−, PD− (atranorin). Medulla C+ pink, K−, KC+ pink, PD− containing α-alectoronic, β-alectoronic, α-collatolic, β-collatolic, dehydrocollatolic, physodic and methylphysodic acids.
Etymology
The species name comes from the presence of collatolic acid and its derivatives as major secondary metabolites.
Ecology and distribution
The new species is currently known from several locations in Russia, in the Trans-Baikal Territory (the Kodar Ridge) and in Yakutia (surroundings of the Tarbaganakh Mountain). The largest population was recorded near the Tarbaganakh Mountain, where several hundred individuals were observed. Just a small number of specimens were found on the Kodar Ridge. The species is a saxicolous lichen which grows on acidic rocks in the alpine and subalpine zones. Arctoparmelia collatolica apparently prefers shaded conditions. In Yakutia, we observed specimens of the new species growing at the same locality and on the same substratum as A. centrifuga and A. separata but restricted to the shaded slopes only (Fig. 2E), whereas the latter two species occurred in shaded and sunlit conditions. Despite our efforts, we did not find A. collatolica on sun-exposed slopes.
Notes
Only one of the studied specimens had black rhizines, similar to A. centrifuga, at the end of the lobes. However, they become ivory white to pale brown towards the centre of the thallus.
Many authors (Fries Reference Fries1871; Nylander Reference Nylander1875; Hillmann Reference Hillmann1926; Clayden Reference Clayden1992; Oxner Reference Oxner1993) have noted that the usnic acid-deficient chemotype of A. centrifuga (Fig. 3A & B) differs from the usnic acid-containing chemotype of that species by the grey thallus and upper cortex K ± yellow (atranorin). In contrast, the usnic acid-containing chemotype (Fig. 3D & E) has a yellow-green to yellow-grey thallus and is K+ yellow (atranorin and usnic acid). We also noticed in all the European and Canadian specimens, that the usnic acid-deficient specimens had a grey upper surface at the margins of the lobes, to lead grey in the central part of the thallus. However, the colour of the upper surface in A. collatolica specimens varies from white-grey at the margins of the lobes to grey-brown and brown in the central part of the thallus (Table 3). In addition, the specimens of A. collatolica differ from A. centrifuga in the colour of the rhizines. The rhizines of A. collatolica are mostly the same colour as the lower surface (Fig. 2C), while rhizines of usnic acid-deficient (Fig. 3C) and usnic acid-containing specimens of A. centrifuga (Fig. 3F) are brown to black all over contrasting with the lower surface.

Fig. 3. A–C, morphology of the usnic acid-deficient chemotype of Arctoparmelia centrifuga; A & B, grey upper surface; C, lower surface with rhizines. D–F, morphology of the usnic acid-containing chemotype of A. centrifuga; D, concentric circles with dying central part; E, yellow-green to yellow-grey upper surface; F, lower surface with rhizines. Source: B & C, Malme exsiccate (Malme, Lichenes suecici exsiccati 855, LE); F, Yakutia (LE-L15225); A, D & E, uncollected specimens photographed during field work in Murmansk region (Pechenga district). Scales: B = 1 cm; C & F = 1 mm.
Table 3. The most important morphological features of Arctoparmelia species.

Based on the field observations in Yakutia (Ust-Maya District), A. collatolica differs from A. centrifuga in the degree of destruction of the central part of the thallus. The central parts of A. centrifuga thalli deteriorate rather quickly. The thalli form concentric circles where the ‘inner circle’ does not reach the ‘outer circle’ and there is always some free space between them (Fig. 3D). In contrast, in A. collatolica the central part of the thallus is preserved for quite a long time, sometimes forming huge thalli that cover the substratum completely (Fig. 2A & D). If the central part deteriorates, the ‘inner circle’ reaches the ‘outer circle’ and begins to grow over it (Fig. 2D). Unfortunately, this difference can be observed only in the field.
Selected specimens examined
Russia: Republic of Sakha (Yakutia): Ust-Maysky District, Tarbaganakh Mountain, 61°10′48·4″N, 138°24′26·3″E, alt. 1959 m, on shaded stone, 17 July 2017, S. V. Chesnokov (LE-L15137, H). Trans-Baikal Territory: Kalarsky District, Kodar Ridge, Azarova glacier, 56°53′58·1″N, 117°34′59·2″E, alt. 2053 m, glacial deposits, on stone, 13 June 2014, L. A. Konoreva (LE-L15138, L15139); Medvezhy Creek, 56°54′51·7″N, 117°37′45·9″E, alt. 1709 m, thickets of Pinus pumila on the right bank, near Surprizniy Creek, on stone, 14 June 2014, L. A. Konoreva (LE-L15140); headwaters of Oleniy Rog, 56°48′31·1″N, 117°24′52·7″E, alt. 1971 m, mountain tundra with stone rubble, on stone, 16 June 2015, L. A. Konoreva (H), S. V. Chesnokov (LE-L15141) (Fig. 2F).
Key to Arctoparmelia species
1 Thallus with vegetative propagules (soralia or soredia-like structures)………………2
Thallus without vegetative propagules.………………3
2(1) Upper surface smooth, without cracks; lobes narrow, convex; soralia globular………………
………………A. incurva
Upper surface fissured, wrinkled to pustular; lobes wide due to pustules, unevenly convex; soredia-like structures formed in places of pustule destruction………………A. subcentrifuga
3(1) Upper surface yellowish green, pale green to greenish grey (usnic acid present)………………4
Upper surface pale grey, grey, dark grey or grey-brown (usnic acid absent)………………6
4(3) Lower surface pale yellow to pale brown, without violet colour………………
………………A. centrifuga (usnic acid-containing chemotype)
Lower surface grey to dark violet.………………5
5(4) Upper surface flat, smooth, never eroding; lobes flat ………………A. separata
Upper surface wrinkled to pustular, eroding; lobes wide due to pustules, unevenly
convex………………A. subcentrifuga
6(3) Rhizines ivory white to pale brown, concolorous with the lower surface, collatolic
acid present………………A. collatolica
Rhizines brown to black, looking like small black dots, collatolic acid absent………………
………………A. centrifuga (usnic acid-deficient chemotype)
The study was financially supported by the Russian Foundation for Basic Research (grants 17-04-01483 and 18-34-00332) and by the following institutional research projects: ‘Cryptogamic biota of Pacific Asia: taxonomy, biodiversity, species distribution’, Botanical Garden-Institute of the Far Eastern Branch of the Russian Academy of Sciences; ‘Flora of lichens and bryophytes of Russia and phytogeographically important regions’ (no. АААА-А19-119020690077-4) and ‘Assessment of changes in the correlation structure of metabolite networks in the process of growth and development of fungi and plants from the standpoint of system biology’ (no. АААА-А18-118032390136-5), Komarov Botanical Institute, Russian Academy of Sciences; ‘Flora of lichens, cyanoprokaryotes, bryophytes and vascular plants of the European Arctic and Subarctic’ (no. АААА-А18-118050490088-0), Avrorin Polar-Alpine Botanical Garden-Institute of the Russian Academy of Sciences; and ‘Development of biological products from tissues of plants and animals of Yakutia based on the study of the characteristics of their biochemical composition and mechanisms of adaptation to the conditions of the North’ (no. АААА-А17-117020110055-3), Institute for Biological Problems of the Cryolithozone, Siberian Branch, Russian Academy of Sciences. Ivan Frolov worked in the frame of the national project of the Botanical Garden (Russian Academy of Sciences, Urals Branch). Thanks to Mikhail Okun (Thermo Fisher Scientific, St. Petersburg) for his advice on the analyses of different ITS partitions.








