Introduction
The ‘textbook lichen’ is composed of regular, horizontal layers; an upper fungal cortex overlies an internal algal layer, which may in turn overlay a fungal medullary layer. The majority of lichens do indeed correspond to this tidy picture, but in others the arrangement and abundance of the photobiont cells may be different (e.g. in homoiomerous cyanolichens). Even closely related species growing in different habitats may show marked differences in this respect, in response to their different ecology (Poelt & Romauch Reference Poelt, Romauch, Frey, Hurka and Oberwinkler1977). Growth activity of the fungal partner can also influence patterns in the algal layer (Honegger Reference Honegger1987).
Since the environment can influence the pattern of algal cells, it would not be surprising if lichens from extreme habitats (e.g., those that are especially cold, hot, dry or insolated) were to show particularly unusual patterns. Our research on lichens from the deserts of Central Asia, a region in which all four of these extreme conditions are commonly experienced, shows that algal cells in many of these are organized in vertical stacks, rather than as an even horizontal layer. These algal stacks are separated by what we have termed fungal stacks: regions of vertically-oriented, translucent fungal plectenchyma that extend downwards from the upper cortex. Further comparisons have shown that lichens with both algal and fungal stacks occur in dry, strongly insolated areas all over the world; this anatomical structure must have evolved many times independently.
We suggest that the purpose of stacks may be twofold. 1) They might increase the active (wet) period during which photosynthesis can occur. Lichens in dry and sunny habitats have rather limited opportunity for photosynthesis, as they are not usually sufficiently hydrated (Pintado et al. Reference Pintado, Sancho, Blanquer, Green and Lazaro2010). The hypothesis that lichens with algal stacks and fungal stacks dry out slower than other lichens from the same habitat was only touched on by our experiments (see Discussion), and our results are not conclusive. However, we suggest that lichens with stacks extend the period of CO2 assimilation due to dynamic use of photobiont cells at different heights. 2) Stacks might represent an adaptation for high insolation. Lower algal cells in stacks must be better employed at high insolation, when light is effectively transferred to lower algal cells by the fungal stacks. We tested the hypothesis that under conditions of high irradiance, lichens with algal stacks and fungal stacks have a higher area-based light saturated photosynthetic rate (Amax) compared with lichens from the same habitats that lack those features.
Materials and Methods
Cross-sections were made of various saxicolous lichen taxa from various territories of Eurasia to find examples of lichens with algal and fungal stacks. Examples from other continents are adopted from the literature sources cited. For physiological experiments, we selected three taxa with stacks, which had been collected in large amounts from sun-exposed limestone outcrops in western Kazakhstan semi-deserts: Aspicilia desertorum, Acarospora ‘cervina’ and Caloplaca ‘variabilis’. The material was collected in summer 2009 and deposited in a dry and dark state in paper envelopes. Voucher material is deposited in the public herbarium CBFS. Measurements were performed the following summer. Viability of samples measured was evaluated by maximum quantum yield of chlorophyll fluorescence (F v/F m; Maxwell & Johnson Reference Maxwell and Johnson2000) after hydration and dim-light recovery pretreatment. Vital, photosynthetically-active samples reached F v/F m>0·5 within several tens of minutes after hydration, whereas dead samples did not. Only photosynthetically-active material containing Acarospora, Aspicilia and Caloplaca with stacks was used for gas exchange measurements.
Physiological investigations
CO2 assimilation was measured using an LI-6400 portable photosynthesis system (Licor, Nebraska, USA) with a sample accommodation chamber of our own construction. This chamber was developed specifically for measurements on lichens and bryophytes, and is better suited to this work than chambers that are commercially available as it allows better environmental control and more sensitive gas exchange measurements. The chamber is rounded, with a surface area of 64 cm2, and cooled by a peltier-module. Illumination is by dimmable red/blue LEDs (90% of red photons set). The relative humidity inside is close to 100%, and desiccation during the measurement period (of around 1 h) is negligible. Measurements of CO2 assimilation were made on six rock samples bearing saxicolous lichens. Lichens with stacks and lichens without stacks were present in all samples. The species with algal stacks occupied 30% (in the sample with Acarospora), 15, 25, 30% (in three samples with Aspicilia), and 25 and 60% (in two samples of Caloplaca) of the lichen-covered area. The species without algal stacks were well-developed crusts from the genera Caloplaca, Diplotomma and family Verrucariaceae. The area covered by each group (lichens with stacks vs other lichens) was calculated in Adobe Photoshop CS4 from size-calibrated images of these samples. Because lichens with algal stacks and other lichen crusts growing on the same rock sample cannot be detached from the substratum without injury, we first measured each sample with the whole lichen community and then again after careful removal of the lichens without algal stacks. Results for species without algal stacks were calculated (see the next paragraph for details).
All measurements were made at a temperature of 17±0·5°C and at ambient CO2 concentration (380 ppm). The lichens were kept on the substratum and hydrated for at least 48 h (including two 16 h photoperiods under a photosynthetic photon flux density of 100 µmol m–2 s–1) before measurement, until respiration and photosynthesis were stable; the lichens should then have been in a condition close to optimal for photosynthesis. Before measurement, samples were thoroughly watered again and external water blotted out via absorbent cellulose. This pretreatment ensures ± optimal water content in lichen samples, according to our previous measurements and evidence from the literature (Snelgar et al. Reference Snelgar, Green and Wilkins1981). We then measured CO2 uptake under seven different irradiance values (0, 40, 90, 175, 350, 750, 1500 µmol m–2 s–1). Two such measurements were made on each rock sample. We then removed all the lichens without stacks from the rock surface, so that only the species with stacks remained, and then repeated the measurements twice. The CO2 uptake by species without algal stacks was calculated from the difference between the two sets of measurements. Assimilation was expressed per unit area of each type of lichen, as mentioned above. The raw data for photosynthetic responses were processed in Photosynth Assistant 1.1 (Dundee Scientific, United Kingdom). The basic light response parameters (Fig. 5) were obtained by fitting a non-rectangular hyperbola to the measurements (Ögren Reference Ögren1993). The statistical significance of these parameters was tested by the non-parametric Mann-Whitney U test using STATISTICA 8 (Statsoft, Tulsa, USA).
Results
Morpho-anatomical description
The samples studied included lichens with a conventionally stratified thallus and others with algal stacks and fungal stacks. The former have a variously thick cortex formed of ± isodiametric fungal cells and a photobiont layer that is usually less than 200 µm thick (Fig. 1A). There is considerable anatomical variability among the species in the latter group, but all have a columnar arrangement of cortex and algal layer. The cortex itself is formed of long anticlinal hyphae. Algal stacks and fungal stacks are typically 200–500 µm tall, but occasionally more (Fig. 1B). Fungal stacks do, of course, reduce the proportion of the thallus surface area that is underlain by photobiont cells. In Caloplaca ‘variabilis’, the thallus surface underlain by algal cells is reduced to c. 50% of the whole lichen area (Fig. 2E & F).
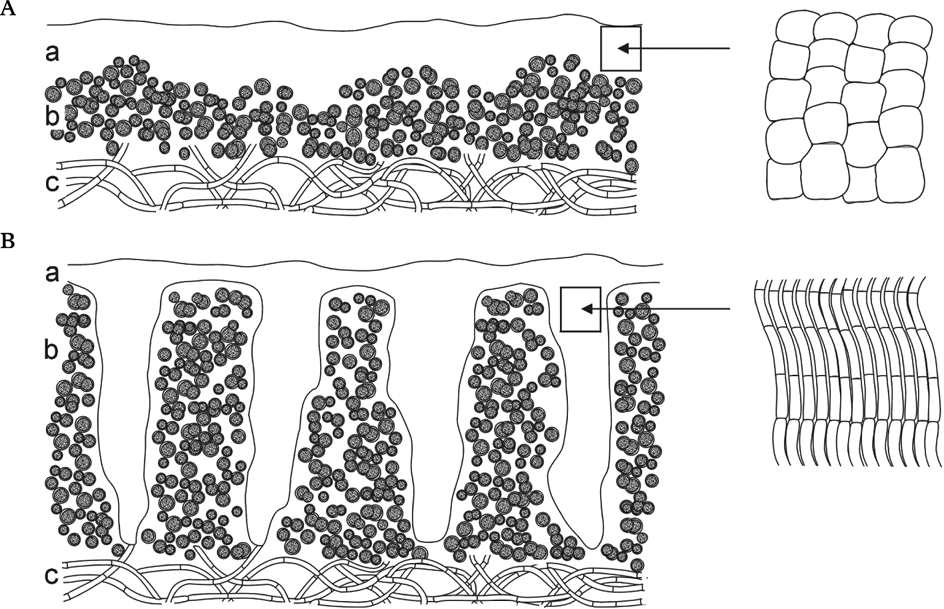
Fig. 1. Schematic vertical section through epilithic crustose lichens (a, cortex; b, algal layer; c, medulla). A, ‘textbook lichen’; B, lichen with algal stacks and fungal stacks; details of fungal cells on the right.
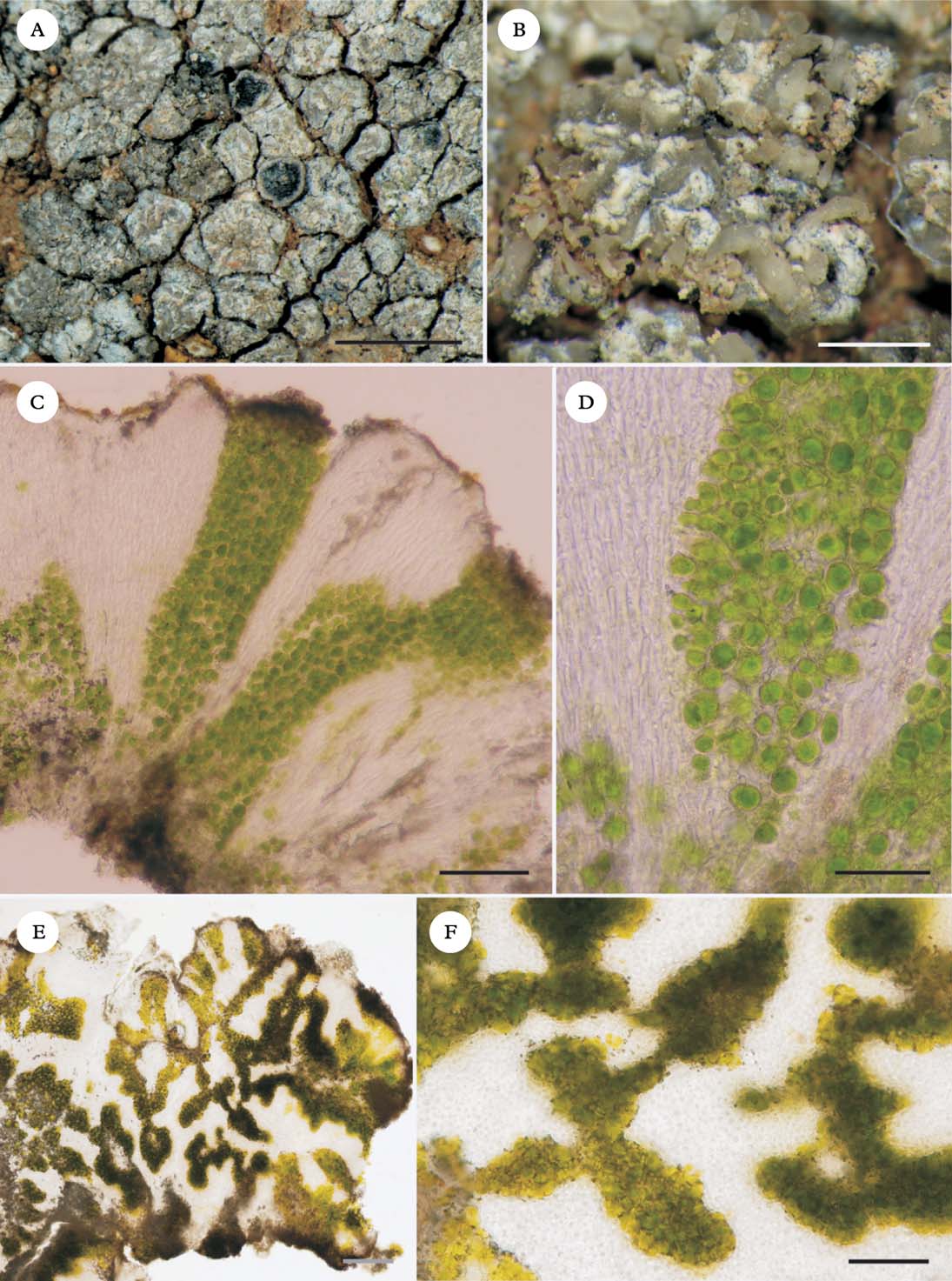
Fig. 2. Caloplaca variabilis coll. algal stacks and fungal stacks. A, outer morphology; B, thallus surface resembling clusters of rock crystals; C, vertical section of thallus; D, detail of fungal stacks; E & F, horizontal sections of thallus. Scales: A=2 mm; B=0·5 mm; C=100 µm; D=50 µm, E & F=20 µm.
Central Asian specimens of Caloplaca ‘variabilis’ (Fig. 2) have their algal stacks and fungal stacks better developed than other lichens used in the physiological experiments. In Caloplaca ‘variabilis’, a distinct fungal cortex is absent, and the walls of the hyphal tips contain the dark grey lichen pigment Sedifolia-grey (Meyer & Printzen Reference Meyer and Printzen2000), which presumably serves as a photoprotective role in the absence of a cortex. The algal stacks themselves are c. 500 µm tall. Fungal stacks surround the algal columns and are distinct on the thallus surface, resembling quartz crystals (Fig. 2B).
In some species of Acarospora (Fig. 3A), Aspicilia (Fig. 3B) and Caloplaca (e.g. Caloplaca gomerana), algal stacks and fungal stacks are developed to some degree. Well-developed examples are less frequent than transitional forms, where the upper cortex is of isodiametric cells but bundles of long hyphae extending down into the algal layer are more or less developed. The South African species Caloplaca elegantissima (Fig. 3C & D) has rather shallow but distinct algal stacks, c. 200 µm tall. The thick (200–300 µm) overlying cortex, which has orange anthraquinone pigments in its upper part, is formed of intricate long hyphae which extend downwards in bundles.
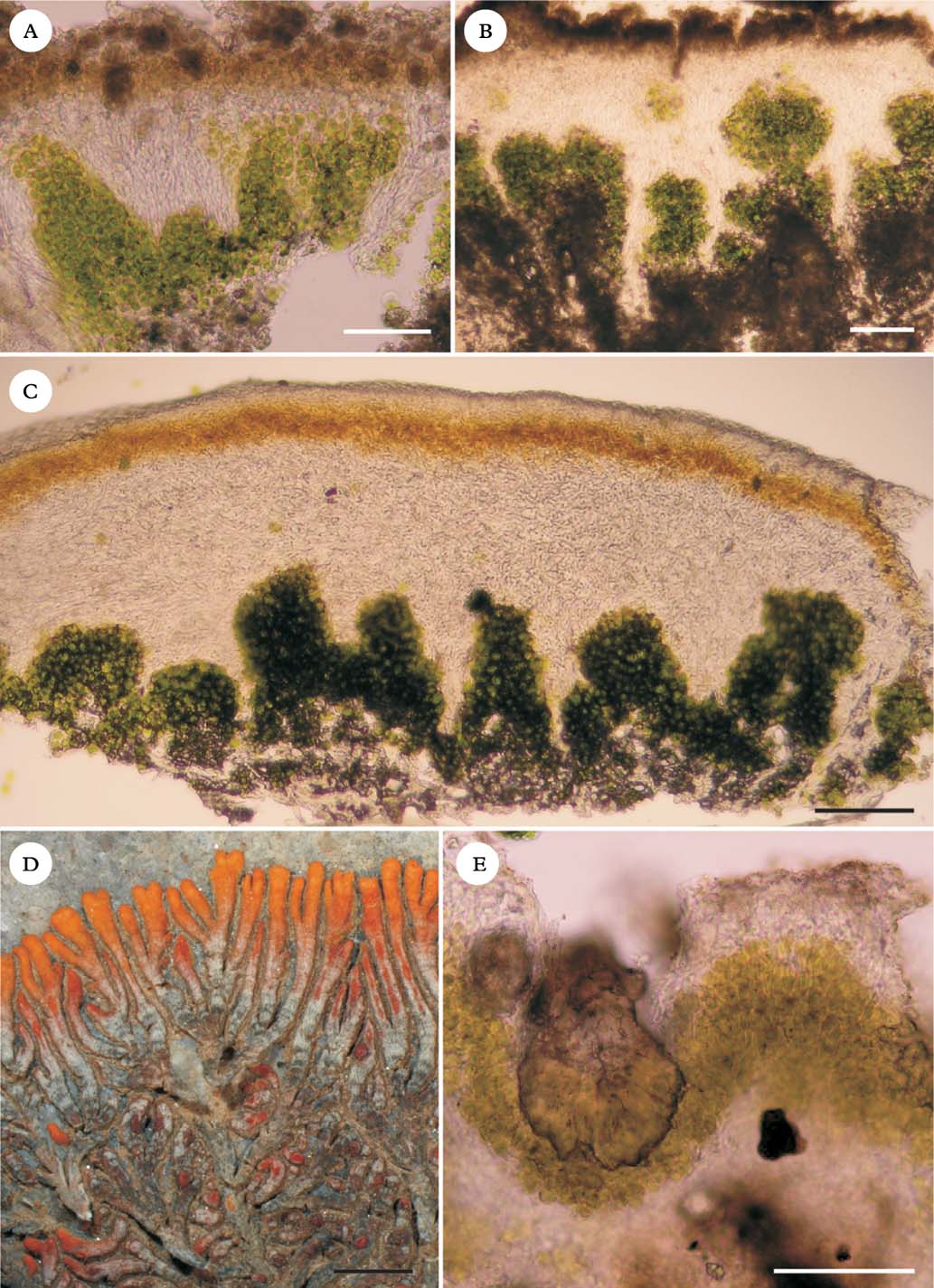
Fig. 3. Lichens with stacks. A, Acarospora aff. cervina, vertical section; B, Aspicilia desertorum, vertical section; C, Caloplaca elegantissima vertical section; D, C. elegantissima, outer morphology; E, Lecidella crystallina (Vězda: Lich. Sel. Exsiccate, 1332). Scales: A–C & E=100 µm; D=2 mm.
Photosynthetic light responses
Species with algal stacks and species without them clearly respond to light in different ways (Fig. 4). Species with algal stacks have both a higher photosynthetic capacity (Amax; CO2 assimilation when light-saturated) and higher dark respiration (Rdark) than species without algal stacks. The single exception was a sample of Acarospora in which the algal stacks were poorly developed (Fig. 3A); this sample did not display a particularly high photosynthetic capacity. Figure 5 shows distributions of the basic light response parameters. Lichens with algal stacks had significantly higher Amax, Rdark, and an especially higher compensation irradiance, that is the level of irradiance at which net CO2 assimilation is zero (Icomp), than species without algal stacks. However, maximal quantum yield of CO2 assimilation (Qmax, the initial slope of the light response curve) did not differ significantly between the two groups of species. The irradiance corresponding to 50% of photosynthetic capacity (50% of Amax) appears to be higher in lichens with algal stacks, but the difference is not statistically significant in our dataset.
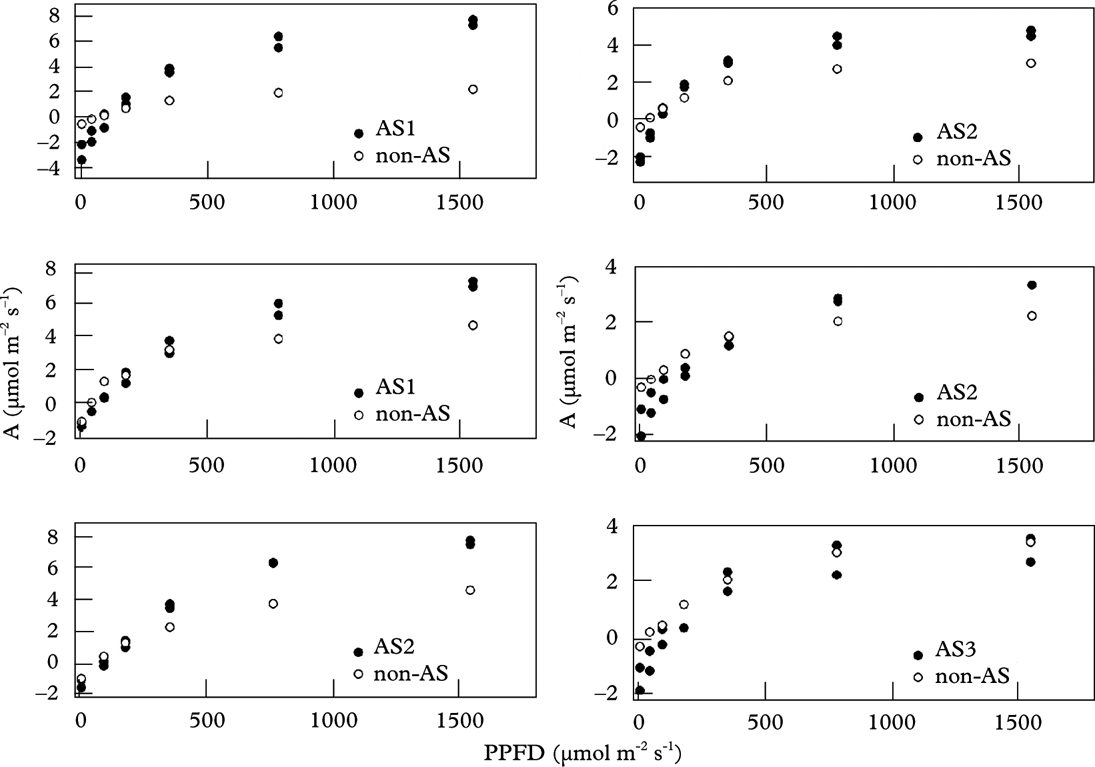
Fig. 4. Light response of CO2 assimilation in six rock samples bearing species with and species without algal stacks (AS); both are plotted separately (AS1 – Caloplaca variabilis coll.; AS2 – Aspicilia desertorum; AS3 – Acarospora aff. cervina; non-AS – other species). Two measurements for species with algal stacks refer to first and repeated measurements.
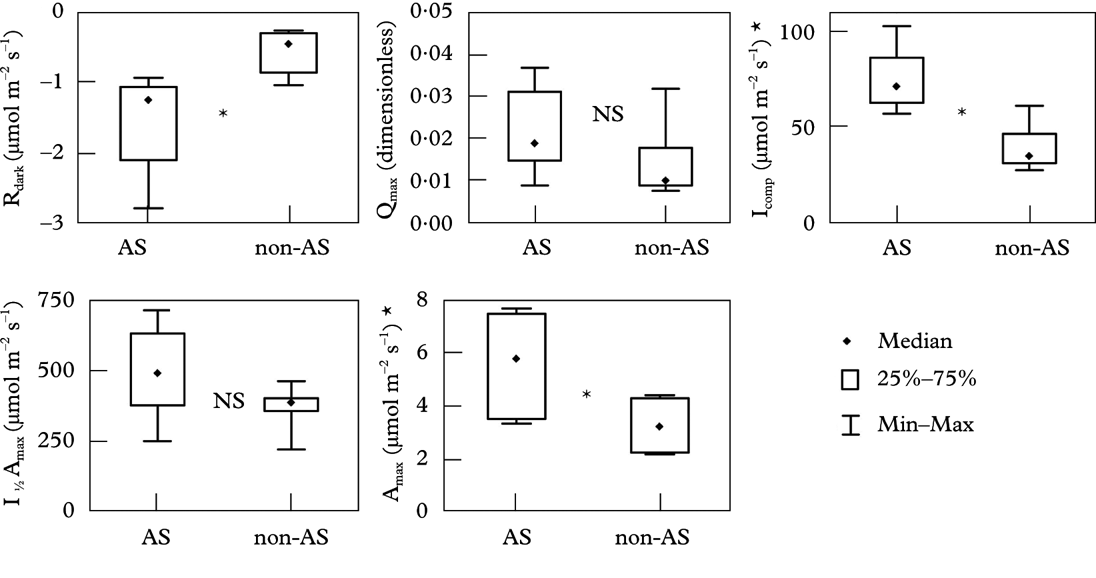
Fig. 5. Parameters of light response curves in lichens with algal stacks and fungal stacks (AS) and accompanying species (non-AS). Rdark, dark respiration after 10 min in dark and 17°C; Qmax, maximum quantum yield of CO2 assimilation; Icomp, compensation irradiance; I ½ Amax, irradiance producing 50% of light saturated assimilation; Amax, light saturated CO2 assimilation in ambient air. *: statistically significant difference (P<0·05); NS: not significant; n=6 in each group.
Phylogenetic considerations
An anatomy that corresponds, more or less, to what we have termed algal and fungal stacking occurs in at least seven families of lichenized ascomycetes, and presumably has evolved independently many times. It is common in various xerophilous Acarospora (Acarosporaceae) in North America (Knudsen Reference Knudsen, Nash, Gries and Bungartz2007) and Eurasia (J. Vondrák and J. Kubásek, personal observations), and in thick-crusted Aspicilia (Megasporaceae) from arid regions (our observations), including various vagrant species (Owe-Larsson et al. Reference Owe-Larsson, Nordin, Tibell, Nash, Gries and Bungartz2007; Sohrabi et al. Reference Sohrabi, Ahti and Litterski2011a , Reference Sohrabi, Stenroos, Högnabba, Nordin and Owe-larsson b ). In Caloplaca (Teloschistaceae), we have seen instances in C. variabilis coll., C. elegantissima, C. gomerana, C. scrobiculata and C. trachyphylla; these species are not closely related. We have also found well-developed algal stacks in alpine Miriquidica complanata and Psorinia (both Lecanoraceae). The phenomenon is also known in Diplotomma, Physciaceae (Follmann Reference Follmann1965), Psora crystallifera, Psoraceae (Vogel Reference Vogel1955), and Labyrintha, tentatively in Lecideaceae (Malcolm Reference Malcolm1995).
Geographical and ecological pattern
Lichens with stacks commonly occur on rocks in desert to steppe regions with high solar radiation, all over the world (Fig. 6), where they may be the predominant species in saxicolous communities. We found examples in southernmost Europe (southern Spain), North Africa and the Canary Islands, the Near East, Central Asia, Greenland, south-west North America, South America (Atacama Desert of Chile), southern Africa (Namib Desert), and Australia. Lichens with stacks also occur in high mountains; we observed them in alpine habitats in various European Mountains.
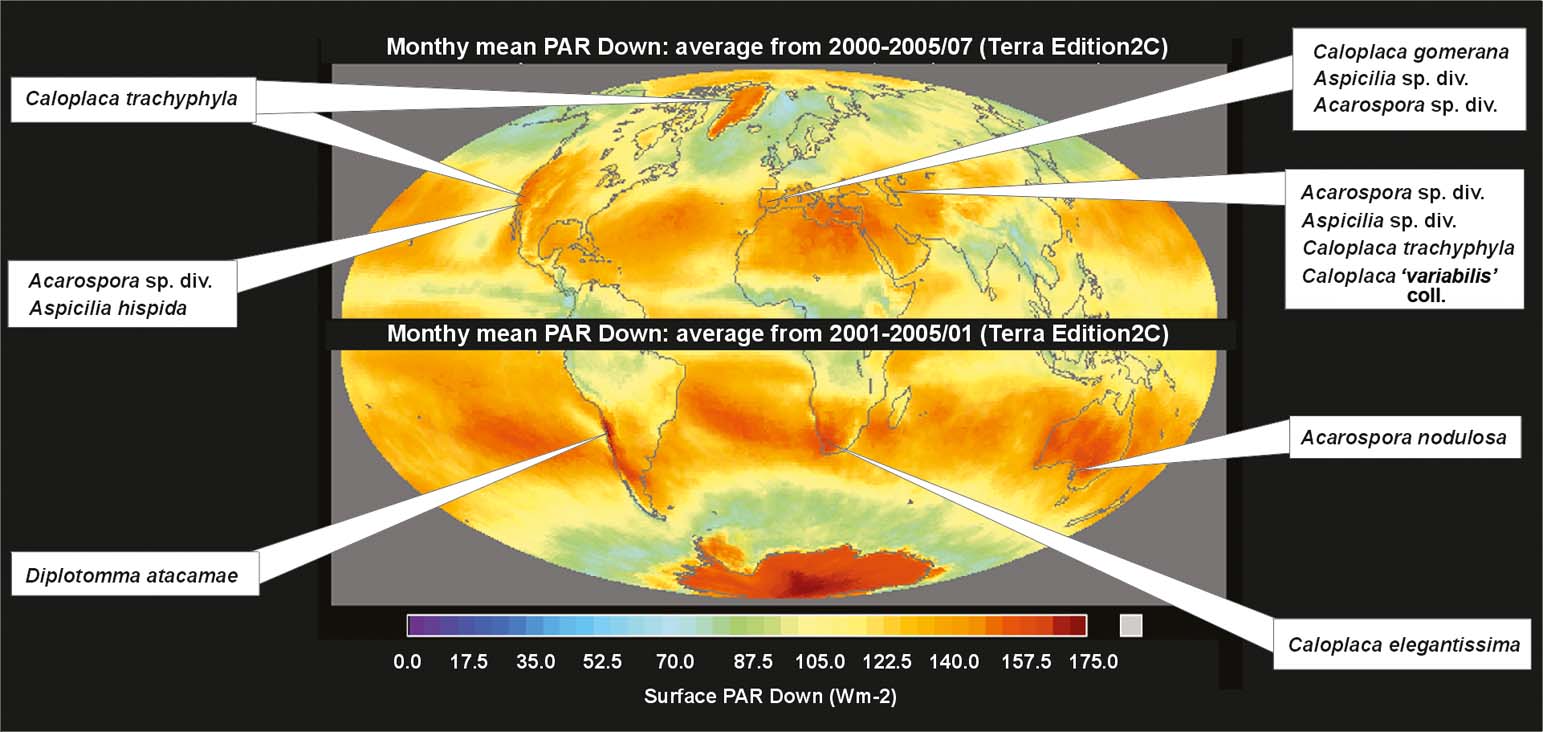
Fig. 6. World distribution of the semi-desert lichens with stacks on the map of summer solar radiation.
Discussion
Malcolm (Reference Malcolm1995) found a remarkable example of stacks in the monospecific genus Labyrintha, which is endemic to New Zealand. Although this species does occur in a rather dry, though certainly not desert-like, part of the South Island, it is also present in humid, oceanic areas such as Campbell Island (Galloway Reference Galloway2007). This species does not fit the pattern shown by the other lichens with tissue stacks discussed in this paper, i.e. occurrence in very dry or alpine habitats. The biology and evolutionary history of this enigmatic lichen merits further study.
Malcolm (Reference Malcolm1995) also measured the light transmission through translucent hyphal bundles (fungal stacks) and fully hydrated algal stacks of Labyrintha, and found that absorption rates were 8·6 times lower in the former (0·21% per µm of hyphal tissue). Malcolm did not make any physiological measurements, but suggested that hyphal bundles might increase illumination of the deeper algal cells.
Our first hypothesis, that algal stacks and fungal stacks extend the period of CO2 assimilation, was not supported by our experiments. On the contrary, Aspicilia desertorum with stacks desiccated faster than its accompanying species without stacks (data available from the authors). The fact that both kinds of lichen co-exist, apparently successfully, at the same site, and indeed on the same rock, suggests that neither anatomy has an overwhelming advantage. Presumably each type exploits the environment in a different way, perhaps corresponding to the rule “higher photosynthetic activity=shorter activity” and vice versa , reported for soil crust species (Pintado et al. Reference Pintado, Sancho, Green, Blanquer and Lazaro2005). However, our laboratory experiments did not disprove the hypothesis either, and more advanced field experiments are justified, as desiccation is a complex process that is affected by many variables, including relative humidity of the air, irradiance, wind speed, thermal properties of the substratum. Experiments on undisturbed lichens in actual field conditions would be particularly valuable.
Our experiments do support the second hypothesis tested, that lichens with stacks have a higher area-based light saturated photosynthetic rate than co-occurring lichens without stacks. High photosynthetic capacity would seem to be clearly advantageous in areas with high insolation but, equally clearly, high respiration must offset this advantage, especially in areas with generally lower insolation. Due to the higher dark respiration, compensation irradiance (irradiation when respiration=CO2 assimilation) is also higher for stacked lichens; it may be the crucial parameter limiting distribution of lichens with stacks. The result that the maximal quantum yield of CO2 assimilation (Qmax, the initial slope of the light response curve) did not differ significantly between lichens with and without stacks is rather surprising. It indicates a good efficiency in light-transferring fungal stacks even at low irradiances. Algal and fungal stacks may be a mechanism to overcome the CO2 expenditure by high respiration of thick lichen crusts. The second hypothesis is also supported, indirectly, by the observed geographical distribution of lichens with tissue stacks. They occur in areas of high insolation and the fact that they occur in these areas in unrelated groups suggests that this is not merely a coincidence: convergent evolution in response to the same selection pressure is a far more plausible explanation. The fact that lichens with algal stacks often dominate the communities in which they occur implies that this adaptation is a very successful one.
We examined a sample of South African Lecidella crystallina in which crystals (Fig. 3E) have a similar role to that of the fungal stacks discussed in the present paper. Vogel (Reference Vogel1955) also mentioned an unusual anatomy in South African Psora crystallifera, where the thick cortex is cracked into translucent crystal-like structures above the algal layer, for which he introduced the term “Fensterflechten” [window lichens]. Although P. crystallifera also has well-developed algal stacks below the cracked cortex, the term “Fensterflechten” does not explicitly refer to the anatomy with stacks. Fensterflechten have been reported also from the Atacama Desert (Follmann Reference Follmann1965). A real stacking anatomy but only slightly developed was described by Poelt (Reference Poelt1958), who introduced the term “Kegelrinden” [cortex cones] for conical extensions of cortex tissue into the algal layer (fungal stacks) in lobate species of Candelariella and Lecanora. Algal stacks in these species are, however, rather indistinct. Kegelrinden were later observed in other lichens (e.g. Timdal Reference Timdal1990).
Last but not least, lichens with stacks have a similar anatomy to the South African living stones (e.g. Lithops sp.), which are the most extreme examples of window-leaved plants; they grow more or less underground, obtaining light by stacks of translucent central mesophyll cells, and chlorophyll-containing cells are arranged below and at the margins of underground leaves (Krulik Reference Krulik1980). This anatomy is considered to be adaptive for conserving water and avoiding heat stress from sunshine (Rauh Reference Rauh1971), but Nobel (Reference Nobel1989) did not obtain substantial differences between plant and soil surface temperatures. Surprisingly, the real function of the window-leaves is still not clarified (Egbert & Martin Reference Egbert and Martin2000).
We are grateful to Linda in Arcadia for revising the English and for helpful discussions. Martin Grube and Toby Spribille provided some useful comments on the topic. Our research received support from the Visegrad Fund (grant 51100753). This study was also supported as a long-term research development project no. RVO 67985939. The work forms a part of research supported by the European Commission (project CzechGlobe contract no. CZ.1.05/1.1.00/02.0073) and by the National Infrastructure CzeCos/ICOS (LM2010007).








