Introduction
The concept of the evolution of life is linked to Darwin’s visit to the Galapagos Islands (Sulloway Reference Sulloway1982; Weiner Reference Weiner1994; Grant & Estes Reference Grant and Estes2009). However, the true laboratory of evolution is found in the Hawaiian archipelago, arguably the most remote place on Earth, being over 4000 km to the closest continental land mass (Wagner & Funk Reference Wagner and Funk1995; Fleischer et al. Reference Fleischer, McIntosh and Tarr1998). Notably, Darwin himself wrote in a letter to Joseph Dalton Hooker in 1850: “Of all places in the world I would like to see a good flora of the Sandwich Islands. I would subscribe 50 pounds to any collector to go there and work at these islands.” (Burkhardt & Smith Reference Burkhardt and Smith1984; Kay Reference Kay1997). Hawaii is home to some of the most striking evolutionary radiations in vascular plants, such as the Hawaiian lobeliads in the family Campanulaceae, with 126 species in six genera having evolved from a single colonization event c. 13 mya (Givnish et al. Reference Givnish, Millam, Theim, Mast, Patterson, Hipp, Henss, Smith, Wood and Sytsma2009), and the silversword alliance in the family Asteraceae (Baldwin et al. Reference Baldwin, Kyhos, Dvorak and Carr1991; Baldwin & Sanderson Reference Baldwin and Sanderson1998; Barrier et al. Reference Barrier, Robichaux and Purugganan2001; Carlquist et al. Reference Carlquist, Baldwin and Carr2003). Island biota also often evolved unique forms. Thus, endemic species of Geranium L., a genus elsewhere known only as herbaceous, form woody stems in Hawaii (Fosberg Reference Fosberg1936; Pax et al. Reference Pax, Price and Michaels1997; Kidd Reference Kidd2005). The two native raspberry species (Rubus hawaiensis A. Gray and R. macraei A. Gray) lack defensive thorns (Morden et al. Reference Morden, Weniger and Gardner2003), and endemic species of the mint family lost the characteristic scent based on oily substances (Morden & Loeffler Reference Morden and Loeffler1999), possibly due to the absence of native herbivores.
Unfortunately, this unique environment has been almost overwhelmed by human influence, including land use change and a large number of invasive species (Kirch Reference Kirch1982; Olson & James Reference Olson and James1982; Stone et al. Reference Stone, Smith and Tunison1992; Asquith Reference Asquith1995; Pratt et al. Reference Pratt, Atkinson, Banko, Jacobi and Woodworth2009). The 1386 native plant species (Imada Reference Imada2012) are opposed by close to 10 000 introduced taxa, with some, such as the Kahili Ginger (Hedychium gardnerianum Griff.) and Strawberry Guava (Psidium cattleianum Sabine), threatening to take over entire forests (Department of Land and Natural Resources 2007). Such human-induced alteration of island floras is a global phenomenon (Castro et al. Reference Castro, Daehler, Silva, Torres-Santana, Reyes-Betancort, Atkinson, Jaramillo, Guezou and Jaksic2010). In Hawaii, major efforts are directed at trying to contain the impact of alien influences while stabilizing and restoring native elements, particularly those close to extirpation (Stone & Scott Reference Stone and Scott1985). Nearly 40% of the original native Hawaiian flora is extinct or threatened with extinction (Sakai et al. Reference Sakai, Wagner and Mehrhoff2002; Wood Reference Wood2007, Reference Wood2012; US Fish and Wildlife Service 2010).
In contrast to vascular plants, Hawaiian bryophytes, lichens and fungi are believed to include mostly widespread species. While estimates of endemism reach 80% for vascular plants (Wagner et al. Reference Wagner, Herbst and Sohmer1999; Wagner & Herbst Reference Wagner and Herbst2002; Evenhuis & Eldredge 2002 Reference Evenhuis and Eldredgea , Reference Evenhuis and Eldredgeb ), they are substantially lower for cryptogams. Among bryophytes, two genera (1%) and 178 species are listed as endemic (Staples et al. Reference Staples, Imada, Hoe and Smith2004; Staples & Imada Reference Staples and Imada2006). Of the 880 lichens (Smith Reference Smith2013), up to 30% are considered endemic (Eldredge & Miller Reference Eldredge and Miller1995). Compared to vascular plants, Hawaiian lichens have generally received less attention (Smith Reference Smith1993). Only a small number of new taxa have been described in the past few decades (Esslinger Reference Esslinger1978; Kalb & Vězda Reference Kalb and Vězda1980; McCarthy Reference McCarthy1993; Moon et al. Reference Moon, Kurokawa and Kashiwadani2001; Kashiwadani et al. Reference Kashiwadani, Smith and Moon2002; Aptroot et al. Reference Aptroot, Schumm and Cáceres2012). Modern treatments have covered foliicolous taxa (Smith Reference Smith1977; Smith et al. Reference Smith, Gardner and Hoe1997; Sérusiaux & Lücking Reference Sérusiaux and Lücking2007), alectorioid and umbilicarioid lichens (Smith Reference Smith1984, Reference Smith2001), and parmelioid species (Smith Reference Smith1993). The last study in particular supported the view that Hawaiian lichens are generally widespread, a view expressed by other workers (Wirth Reference Wirth1997; Marbach Reference Marbach2000; Smith Reference Smith2001; Inoue Reference Inoue2002; Sérusiaux & Lücking Reference Sérusiaux and Lücking2007). In contrast, a biogeographical analysis of Cladoniaceae suggested 40% of species in that family are endemic (Stenroos & Smith Reference Stenroos and Smith1993). Smith (Reference Smith1995) provided an analysis of ascospore features of Hawaiian lichens and found a higher proportion of species with pigmented and/or large ascospores.
The Hawaiian biota is also notable for its biogeographical relationships and inferred dispersal routes. Most native vascular plants, such as forest trees of the genera Acacia Mill. (Fabaceae), Cheirodendron Nutt. ex Seem. (Araliaceae) and Metrosideros Banks ex Gaertn. (Myrtaceae), have Indopacific-Australasian relationships (Mueller-Dombois Reference Mueller-Dombois1987; Wright et al. Reference Wright, Yong, Wichman, Dawson and Gardner2001; Percy et al. Reference Percy, Garver, Wagner, James, Cunningham, Miller and Fleischer2008; Brown et al. Reference Brown, Murphy, Kidman and Ladiges2012; Mitchell et al. Reference Mitchell, Li, Brown, Schönberger and Wen2012), explained by the northern subtropical jet stream being the predominant dispersal agent (Geiger et al. Reference Geiger, Ranker, Neale and Klimas2007). Biogeographical relationships with North, Central and South America are less common; for example, the closest relative of the Hawaiian silverswords (genera Argyroxiphium DC., Dubautia Gaudich. and Wilkesia A. Gray) are the North American tarweeds (Baldwin et al. Reference Baldwin, Kyhos, Dvorak and Carr1991; Carlquist et al. Reference Carlquist, Baldwin and Carr2003). As so far studied, lichenized fungi, for example in the family Cladoniaceae (Stenroos & Smith Reference Stenroos and Smith1993) and the genus Pannaria, mostly have Australasian affinities, whereas few are linked to the neotropical realm. This was first supported with molecular data for the genus Pseudocyphellaria Vain. s. lat. (Lobariaceae), in which all Hawaiian species are related to palaeotropical taxa, with the exception of P. hawaiiensis H. Magn. (Moncada et al. Reference Moncada, Reidy and Lücking2014).
The genus Lobariella Yoshim. is entirely restricted to the Neotropics, with the notable exception of L. crenulata (Hook.) Yoshim. reported from Hawaii (Yoshimura Reference Yoshimura1984, Reference Yoshimura1998; Yoshimura & Arvidsson Reference Yoshimura and Arvidsson1994). The genus was recently revised using molecular data (Moncada et al. Reference Moncada, Lücking and Betancourt2013), showing that it is much more speciose than previously assumed and that secondary chemistry, previously thought to vary within species (Yoshimura Reference Yoshimura1984; Yoshimura & Arvidsson Reference Yoshimura and Arvidsson1994), is an important systematic character. For that study, we had revised material of L. crenulata at the US National Herbarium, collected in Hawaii by the late Mason Hale, and concluded that it represented at least two different species, based on morphology and chemistry: L. crenulata s. str. and L. subcrenulata Moncada & Lücking. Since these represented two unrelated lineages within the genus (Moncada et al. Reference Moncada, Lücking and Betancourt2013), this suggested that Hawaii was colonized at least two times independently from the Neotropics. In order to test this hypothesis, we obtained fresh material of Lobariella from Hawaii during a field trip in June 2013 and additional fieldwork in September 2016 and sequenced three loci of the nuclear and mitochondrial ribosomal DNA. We also revised numerous herbarium specimens. Much to our surprise, the material studied turned out to represent three species new to science, all closely related but morphologically disparate and including a phenotype never before observed in the genus, forming a well-supported clade different from the species believed to occur in Hawaii.
Material and Methods
Herbarium material of Lobariella originating from Hawaii was revised at the US National Herbarium, the herbarium of the University of Hawaii at Manoa (HAW) and the herbarium of the National Tropical Botanical Garden on Kauai (PTBG). We performed thin-layer chromatography in solvent C to determine the chemical constituents of each sample (Orange et al. Reference Orange, James and White2010).
New fresh material of Lobariella was collected in Hawaii by all authors during fieldwork in June 2013 and by the third author in September 2016. We obtained 42 new sequences of the nuclear ITS barcoding locus, including 16 specimens from Hawaii and the remainder from Colombia and Costa Rica, 12 new sequences of the nuclear large subunit (nuLSU), including two from Hawaii, and 15 new sequences of the mitochondrial small subunit (mtSSU) ribosomal DNA, including two from Hawaii. DNA was extracted using the QIAGEN DNeasy Plant Mini Kit. Dilutions of 10:1 were used for PCR amplifications, with the primer pairs ITS1F and ITS4 for the ITS (White et al. Reference White, Bruns, Lee and Taylor1990; Gardes & Bruns Reference Gardes and Bruns1993), mrSSU1 and MSU7 for the mtSSU (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999; Zhou & Stanosz Reference Zhou and Stanosz2001), and AL2R and LR6 for the nuLSU (Vilgalys & Hester Reference Vilgalys and Hester1990; Mangold et al. Reference Mangold, Martín, Lücking and Lumbsch2008). The 25 µl PCR reactions contained 2·5 µl buffer, 2·5 µl dNTP mix, 1 µl of each primer (10 µM), 5 µl BSA, 2 µl Taq, 2 µl genomic DNA extract and 9 µl distilled water. The thermal cycling parameters were set as follows: initial denaturation for 3 min at 95°C followed by 30 cycles of 1 min at 95°C, 1 min at 52°C, 1 min at 73°C, and a final elongation for 7 min at 73°C. Amplification products were mounted on 1% agarose gels stained with ethidium bromide and, after cutting of the target bands, purified using the QIAGEN QIAquick PCR Purification Kit or NucleoSpin DNA purification kit (Macherey-Nagel). Fragments were sequenced using the BigDye Terminator reaction kit (ABI PRISM, Applied Biosystems). Sequencing and PCR amplifications were performed using the same sets of primers. Cycle sequencing was executed with the following setting: 25 cycles of 95°C for 30 s, 48°C for 15 s and 60°C for 4 min. Sequenced products were precipitated with 10 µl of sterile dH2O, 2 µl of 3 M NaPA (sodium phenylacetate) and 50 µl of 95% EtOH, and subsequently loaded on an ABI 3100 (Applied Biosystems) automatic sequencer. Sequence fragments obtained were assembled with DNASTAR SeqMan 4.03, manually inspected and adjusted and, after quality control within the context of multiple alignments for each locus, submitted to GenBank (Table 1).
Table 1 Newly generated sequences (in bold) and sequences downloaded from GenBank used in this study. Specimens marked with an asterisk* are those used for the 3-locus and for the nuLSU molecular clock analysis

The sequences obtained were aligned with a previous data set analyzed by Moncada et al. (Reference Moncada, Lücking and Betancourt2013), using selected sequences of Lobariaceae from GenBank (Table 1). Alignments for each locus were assembled separately in BioEdit 7.0.9 (Hall Reference Hall1999) and automatically pre-aligned using ClustalW2 (Thompson et al. Reference Thompson, Higgins and Gibson1994) to detect potentially problematic sequences. Final alignments were made with MAFFT 6.850b (Katoh et al. Reference Katoh, Misawa, Kuma and Miyata2002, Reference Katoh, Asimenos and Toh2009) using the “--auto” option and subsequent manual inspection. The individual alignments were subjected to analysis of ambiguously aligned regions using the GUIDANCE webserver (Penn et al. 2010 Reference Penn, Privman, Ashkenazy, Landan, Graur and Pupkoa , Reference Penn, Privman, Landan, Graur and Pupkob ) and introns and regions aligned with low confidence (below 0·90) were removed. This resulted in an alignment length of 554 sites for the ITS, 999 for the nuLSU, and 831 for the mtSSU partition. The separate gene trees were tested for topological conflict using tree-branch comparison at a bootstrap support threshold of 70% (Mason-Gamer & Kellogg Reference Mason-Gamer and Kellogg1996; Miadlikowska & Lutzoni Reference Miadlikowska and Lutzoni2000; Kauff & Lutzoni Reference Kauff and Lutzoni2002), and three single- or multilocus data sets were generated: 1) a 3-locus data set of 18 species (one sample per species) of Lobariella, with Lobaria pulmonaria as outgroup, to determine the closest relative of the Hawaiian taxa; 2) a single-locus ITS data set of 65 samples of Lobariella, with three species of Yoshimuriella as outgroup, to assess species delimitations; and 3) a single-locus nuLSU data set of representative species of Lobariaceae, including 10 of the 12 currently recognized genera (Moncada et al. Reference Moncada, Lücking and Betancourt2013; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017), with Nephroma parile as outgroup, to perform a molecular clock analysis. Phylogenetic analyses were performed on each locus and the combined data set using maximum likelihood in RAxML 7.2.6 (Stamatakis Reference Stamatakis2006), with parametric bootstrapping using 500 replicates under the GTRGAMMA model. Trees were visualized in FigTree v.1.4.0 (Drummond & Rambaut Reference Drummond and Rambaut2007).
A relaxed, uncorrelated lognormal molecular clock model was employed to date the evolutionary origin of the Hawaiian Lobariella clade, using data set (3). The program BEAST v.1.7.5 (Drummond & Rambaut Reference Drummond and Rambaut2007) was used for this purpose, with the following specifications: 1) GTR substitution model with base frequencies estimated and Gamma and invariant sites with six Gamma categories; 2) speciation through a Yule process with the ‘yule.birthRate’ prior set to an exponential distribution with 0·7 as mean; 3) the ‘ucld.mean’ prior (mean substitution rate) set to an exponential distribution with 0·001 as mean; and 4) the ‘ucld.stdev’ prior (mean substitution rate) set to an exponential distribution with 0·6 as mean. All other priors except ‘tmrca’ and ‘treemodel.rootheight’ (see below) were held to default values. Estimation of priors was approximated by first running a strict clock and using the ‘meanRate’ posterior estimate as prior for a subsequent run applying a relaxed, lognormal, uncorrelated clock, with all other priors set to default values, and then further runs in an iterative manner, using the obtained posterior estimates as priors, until no substantial change was observed in the posteriors. The obtained final priors were: ac: shape=0·05, scale=10·0, offset=0·0; ag: shape=0·05, scale=20·0, offset=0·0; at: shape=0·05, scale=10·0, offset=0·0; cg: shape=0·05, scale=10·0, offset=0·0; gt: shape=0·05, scale=10·0, offset=0·0; frequencies: uniform, lower=0·0, upper=1·0; alpha: exponential, mean=0·5, offset=0·0; pInv: uniform, lower=0·0, upper=1·0; ucld.stdev: exponential, mean=0·7, offset=0·0; ucld.mean: exponential, mean=0·001, offset=0·0; treeModel.rootHeight: normal, mean=85·0, stdev=10·0; tmrca(Ingroup): normal, mean=62·0, stdev=10·0; yule.birthRate: 0·7, offset=0·0. All runs were performed with 50 million generations each and analyses were performed on the CIPRES Science Gateway server (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). The time to the most recent ancestor (‘tmrca’) for the ingroup node of Lobariaceae was calibrated at 62 mya, using a normal prior distribution with the standard deviation set to 10 my; in addition, the ‘treemodel.rootheight’ (Nephroma divergence) was calibrated at 85 mya, using a normal prior distribution with the standard deviation set to 10 my. Both values followed Rivas Plata (Reference Rivas Plata2011: 21), who performed an analysis across all Ascomycota using ten fungal fossils (Rivas Plata Reference Rivas Plata2011: 15). Notably, Rivas Plata (Reference Rivas Plata2011) estimated the stem node age of Peltigerales at 186 mya, very similar to Amo de Paz et al. (Reference Amo de Paz, Cubas, Divakar, Lumbsch and Crespo2011) and Prieto & Wedin (Reference Prieto and Wedin2013) with 185 mya and 184 mya, respectively. The latter two did not contain the Nephroma divergence for comparison; however, the very similar stem node estimates for Peltigerales across these studies support our selection for the two external calibration points. To test the resulting chronogram, we fitted a posteriori the age estimate of a fossil species of Lobaria similar in morphology to L. pulmonaria, estimated at 12–24 mya (Peterson Reference Peterson2000). The molecular clock trees were estimated using a constrained topology based on a previous multigene study (Moncada et al. Reference Moncada, Lücking and Betancourt2013). For that purpose, data set (3) was analyzed in RAxML with a constrained multifurcate tree and the resulting fully-resolved best tree was used as constraint for the molecular clock analysis. This was done by removing the tree swapping operators ‘subtreeSlide’, ‘narrowExchange’, ‘wideExchange’ and ‘wilsonBalding’ (Drummond & Rambaut Reference Drummond and Rambaut2007).
Results
In the 3-loci analysis, the genus Lobariella emerged as a long branch relative to the outgroup, Lobaria pulmonaria, but with comparatively short branches leading to the individual species and a lack of backbone support (Fig. 1). The following sister group relationships were supported: Lobariella crenulata vs. L. pallidocrenulata, L. pallida vs. L. reticulata, L. ecorticata vs. L. stenroosiae, L. botryoides vs. L. parmelioides and L. pseudocrenulata vs. L. rugulosa. In addition, we found strong support for a clade of five species, including two Hawaiian taxa, with L. nashii sister to a clade of four species in which L. flavomedullosa plus L. subcrenulata are supported as sister clade to the Hawaiian L. flynniana and L. sandwicensis, two of the three new species described below.
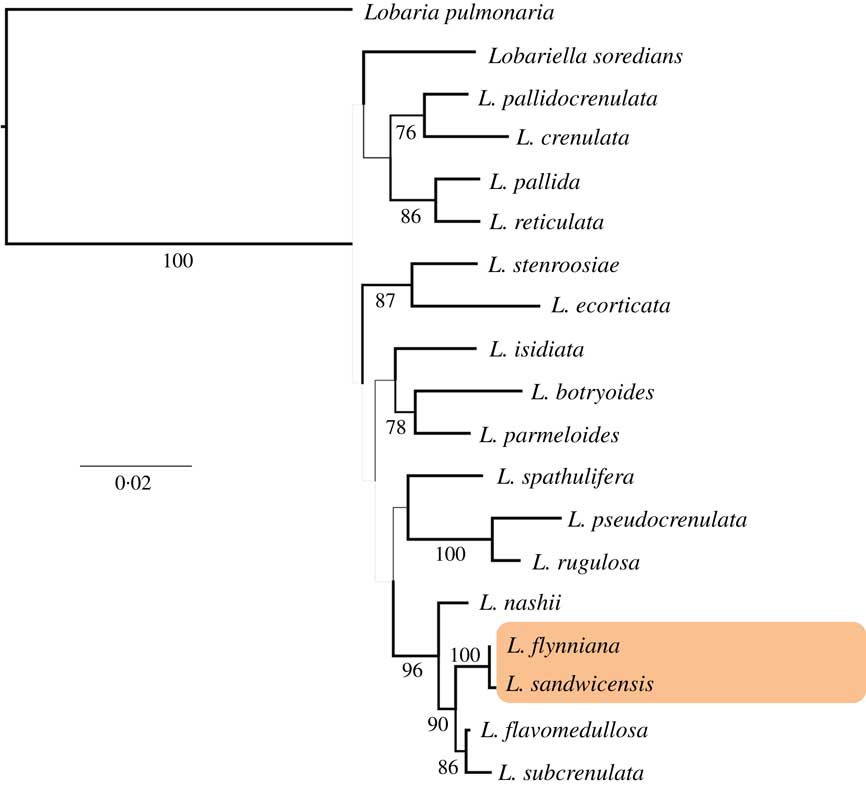
Fig. 1 Best-scoring maximum likelihood tree based on a 3-locus combined data set (mtSSU, nuLSU, ITS) of Lobariella highlighting the position of the Hawaiian species. Branch thickness is proportional to bootstrap support and bootstrap values of ≥70% are indicated below branches. Lobaria pulmonaria as the outgroup. For voucher information see Table 1.
The expanded taxon set of the single-locus ITS analysis (Fig. 2) recovered the L. pseudocrenulata-rugulosa clade, further including L. angustata and L. auriculata. The six species L. botryoides, L. crenulata, L. pallida, L. pallidocrenulata, L. parmelioides and L. reticulata, forming three supported sister clades in the 3-loci analysis, clustered in an unsupported clade in the ITS analysis, with the addition of L. sipmanii as strongly supported sister to L. reticulata. The clade of five species with L. nashii in a basal position and comprising the two Hawaiian species was recovered in the ITS analysis but did not have support; this clade further included L. spathulifera and L. stenroosiae. In both the 3-locus tree and the ITS tree, the sequenced Hawaiian taxa formed a well-supported, monophyletic clade.
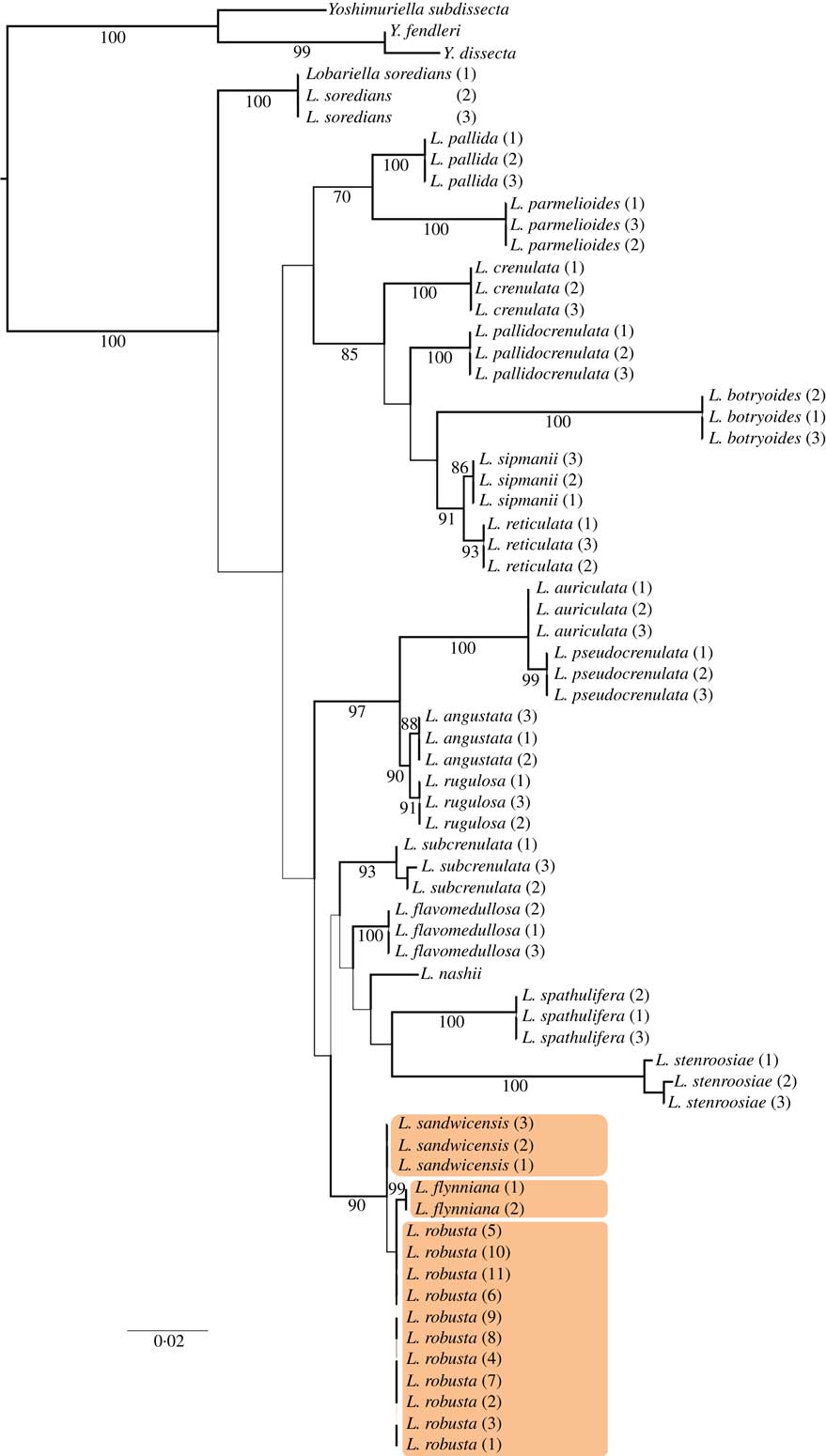
Fig. 2 Best-scoring maximum likelihood tree based on an expanded, single-locus ITS data set of Lobariella highlighting the position of the Hawaiian species. Branch thickness is proportional to bootstrap support and bootstrap values of ≥70% are indicated below branches. For voucher information see Table 1.
The relaxed, uncorrelated, lognormal molecular clock analysis estimated the stem node age of the genus Lobariella versus its sister genus, Yoshimuriella, at (18–)26(–42) mya, and the crown node age at (11–)17(–28) mya (Fig. 3). In this analysis, the Hawaiian L. flynniana-sandwicensis clade diverged from the neotropical L. flavomedullosa-subcrenulata clade (1–)8 mya, and the two Hawaiian species L. flynniana and L. sandwicensis separated from each other (0–)2(–6) mya.

Fig. 3 Relaxed log-normal molecular clock tree of Lobariaceae showing origin and diversification of Lobariella including two Hawaiian species. Bars indicate 95% posterior density intervals of the node age estimates. The relative placement of the Lobaria fossil (Peterson Reference Peterson2000) is indicated. For voucher information see Table 1.
Discussion
For a long time, the neotropical genus Lobariella was believed to contain just five species, with only one of them, L. crenulata, occurring outside the Neotropics on the Hawaiian Islands (Yoshimura Reference Yoshimura1984, Reference Yoshimura1998; Yoshimura & Arvidsson Reference Yoshimura and Arvidsson1994). Molecular phylogenetic revision, in combination with a revised morphological and chemical concept, revealed the existence of more than 25 species in the Neotropics, a five-fold increase (Lumbsch et al. Reference Lumbsch, Ahti, Altermann, Amo de Paz, Aptroot, Arup, Bárcenas Peña, Bawingan, Benatti and Betancourt2011; Moncada et al. Reference Moncada, Lücking and Betancourt2013).
Based on this new concept, material reported from Hawaii under the name Lobariella crenulata seemed to represent two species, L. crenulata s. str. and L. subcrenulata, suggesting that the archipelago had been colonized at least twice independently from the Neotropics, with the trade winds as the likely dispersal agent (Geiger et al. Reference Geiger, Ranker, Neale and Klimas2007). However, apart from unsequenced herbarium collections fitting the concept of L. crenulata sensu Moncada et al. (Reference Moncada, Lücking and Betancourt2013), the present study revealed three species new to science for Hawaii, namely L. flynniana, L. robusta and L. sandwicensis (see below). These three species form a well-supported clade of closely related taxa, with a comparatively long stem branch, suggesting ‘micro-radiation’ after initial colonization by an ancestor within the group formed by the neotropical species L. flavomedullosa, L. nashii, L. subcrenulata and relatives.
The molecular clock analysis suggests that initial colonization leading to this clade took place between 1–8 mya, with crown divergence at around 2 mya, much later than, for example, in the vascular plant family Campanulaceae, estimated at c. 13 mya (Givnish et al. Reference Givnish, Millam, Theim, Mast, Patterson, Hipp, Henss, Smith, Wood and Sytsma2009). The close relationship between other Hawaiian taxa of Lobariaceae, in the genera Crocodia, Podostictina and Pseudocyphellaria, and lineages from the Palaeotropics (Moncada et al. Reference Moncada, Reidy and Lücking2014) supports the notion that colonization of the archipelago by these macrolichens occurred comparatively late, which might explain the absence of large radiations as observed for vascular plant lineages. The Hawaiian lobeliads, with 126 species evolving from a single colonization c. 13 mya, display a diversification rate of 0·37 species per million years (calculated from Givnish et al. Reference Givnish, Millam, Theim, Mast, Patterson, Hipp, Henss, Smith, Wood and Sytsma2009); this rate would produce two species within two million years, suggesting that the diversification rate in Hawaiian Lobariella is comparable to that of vascular plants. This view is supported by a recent study of the related genus Sticta in Madagascar and the Masquarenes, with a radiation into more than a dozen to c. 30 species dating back to the Late Miocene, c. 11–5 mya (Simon et al. Reference Simon, Magain, Goffinet and Sérusiaux2016). Hence time of colonization, more than evolutionary constraints, might determine the level of radiation in these lichen fungi. Apart from the fact that successful establishment of epiphytic lichens depends on the prior development of woody vegetation, the reason for late colonization of Hawaii by Lobariella and other Lobariaceae could be the relatively young age of these lineages: the crown node age estimate for Lobariella, with a mean value of 17 mya, is rather close to the initial colonization by Hawaiian lobeliads (13 mya). Thus, at the time when Lobariella diversified in the Neotropics, the Hawaiian lobeliads had already colonized the archipelago.
The younger evolutionary age of lichenized lineages could also be a reason why lichen fungi are much less diverse than vascular plants in general. There are 15 131 species currently recognized in the class Lecanoromycetes (Lücking et al. Reference Lücking, Hodkinson and Leavitt2017) compared to 335 498 species of vascular plants (Roskov et al. Reference Roskov, Abucay, Orrell, Nicolson, Flann, Bailly, Kirk, Bourgoin, DeWalt and Decock2016), a ratio of 22:1. However, while Lecanoromycetes date back to c. (283–)320(–353) mya (Lücking et al. Reference Lücking, Huhndorf, Pfister, Rivas Plata and Lumbsch2009; Amo de Paz et al. Reference Amo de Paz, Cubas, Divakar, Lumbsch and Crespo2011; Rivas Plata Reference Rivas Plata2011; Prieto & Wedin Reference Prieto and Wedin2013; Beimforde et al. Reference Beimforde, Feldberg, Nylinder, Rikkinen, Tuovila, Dörfelt, Grube, Jackson, Reitner and Seyfullah2014; Pérez-Ortega et al. Reference Pérez-Ortega, Garrido-Benavent, Grube, Olmo and de los Ríos2016), vascular plants date back to between 433–513 mya based on the fossil record and molecular clock studies (Edwards & Feehan Reference Edwards and Feehan1980; Lücking et al. Reference Lücking, Huhndorf, Pfister, Rivas Plata and Lumbsch2009; Willis & McElwain Reference Willis and McElwain2014; Silvestro et al. Reference Silvestro, Cascales‐Miñana, Bacon and Antonelli2015), an age difference of c. 50%. Under such a time difference, comparable diversification rates would partially explain the extant species richness ratio of 22:1. On the other hand, global differences in species richness do not seem to hold at a smaller scale. Thus, while 1386 native vascular plant species are known from Hawaii, our recent phylogenetic studies suggest that the current number of 880 lichenized species listed for the archipelago (Smith Reference Smith2013) may be a substantial underestimation and the total might well be comparable to that of vascular plants; in Pseudocyphellaria the number of species recognized for Hawaii has increased by 70% (Moncada et al. Reference Moncada, Reidy and Lücking2014), and now in Lobariella by 300%. It therefore appears that such comparable species richness in vascular plants and lichens in the archipelago is the result of different evolutionary processes: early and infrequent colonization by vascular plant lineages, with strongly isolated evolutionary histories leading to substantial radiations, versus later and more frequent colonization by lichen fungal lineages, with subsequent divergence but lower levels of radiation. Notably, this was recently shown for Galapagos basidiolichens, which are now recognized as ten endemic species, each derived from an independent, comparatively recent colonization event (Dal Forno et al. Reference Dal Forno, Bungartz, Yánez-Ayabaca, Lücking and Lawrey2017).
Of the three Hawaiian species of Lobariella described as new below, the most remarkable is L. flynniana. It forms numerous lateral, much branched and ascending lobules, giving the species a fruticose rather than foliose appearance. Such a phenotype is unknown in any other species of the genus. A comparable situation is found in the Hawaiian Phaeophyscia laciniata Essl. (Esslinger Reference Esslinger1978) and in the supposedly endemic genus Ramalinopsis (Follmann Reference Follmann1974) which represents a foliose species nested within the fruticose genus, Ramalina. The phenomenon of developing novel morphotypes on islands is well documented for vascular plants, including the aforementioned genus Geranium (Fosberg Reference Fosberg1936; Pax et al. Reference Pax, Price and Michaels1997; Kidd Reference Kidd2005). The very close relationship between the three sequenced Hawaiian Lobariella species, in spite of their morphological differences, is also in line with findings for vascular plants. For instance, the species of the silversword alliance, although recognized in three separate genera, are all closely related genetically with differences that, in continental taxa, are usually found within populations of a single species (Baldwin et al. Reference Baldwin, Kyhos, Dvorak and Carr1991; Baldwin & Sanderson Reference Baldwin and Sanderson1998; Carlquist et al. Reference Carlquist, Baldwin and Carr2003). Thus, not only evolutionary rates but also other evolutionary phenomena found in vascular plant island biota appear to be expressed in lichen fungi, including levels of endemism.
Comparison of the newly collected and sequenced samples with specimens revised from the US National Herbarium (US), the University of Hawaii at Manoa (HAW), and the National Tropical Botanical Garden (PTBG) revealed that the material we had first identified as Lobariella subcrenulata represents the newly described L. sandwicensis. Thus, it is questionable whether the material currently identified as L. crenulata, which is characterized by a different medullary chemistry dominated by gyrophoric acid (Moncada et al. Reference Moncada, Lücking and Betancourt2013), actually represents that species since morphological similarity in the absence of molecular data can be misleading. If future data show that it is an undescribed species, the different chemistry suggests that this taxon is not related to the L. flynniana clade (Moncada et al. Reference Moncada, Lücking and Betancourt2013), which indicates a second, independent colonization of the islands by Lobariella. At present, inferred endemism for Hawaiian Lobariella is estimated at 75%, compared to zero based on previous, traditional taxonomy. This percentage is exactly the same as inferred for Pseudocyphellaria s. lat. (Moncada et al. Reference Moncada, Reidy and Lücking2014) and is in the same range as for vascular plants (Wagner et al. Reference Wagner, Herbst and Sohmer1999; Eldredge & Evenhuis & Reference Eldredge and Evenhuis2002; Wagner & Herbst Reference Wagner and Herbst2002).
Currently, Lobariella appears to be a rare genus in Hawaii. In spite of an intensive search, during our fieldwork in 2013 we were able to collect only five specimens during eight days at 14 sites on three of the four largest islands, whereas for Pseudocyphellaria s. lat. and Sticta we gathered close to 200 samples (Moncada et al. Reference Moncada, Reidy and Lücking2014). On the Big Island (Hawaii), L. robusta has so far been found only at the type locality. It appears that the mostly cyanobacterial species in Pseudocyphellaria and Sticta can cope better with habitat threats than the green-algal Lobariella species, which is supported by the rarity of other green-algal Lobariaceae in Hawaii (Magnusson & Zahlbruckner Reference Magnusson and Zahlbruckner1943). One of these reported taxa, Pseudocyphellaria dissimulata, in reality represents L. sandwicensis (see below). Whether the rarity of green-algal, lobarioid lichens is a recent trend or goes further back is difficult to assess. In herbarium collections of Lobariaceae from Hawaii revised by us in HAW, PTBG and US, the proportion of cyanobacterial Pseudocyphellaria and Sticta specimens (193) was also substantially higher than Lobariella (13) but field observations from the late 1970s suggest that Lobariella was more abundant at that time. In the light of the results presented here, it appears necessary to raise awareness of Hawaiian cryptogams in terms of conservation. If we assume that many other lichen groups show similar evolutionary histories, with high inferred endemism, conservation of Hawaiian lichens (Smith Reference Smith1991) takes on an entirely new dimension. Much research is needed to understand the evolution of Hawaiian cryptogams so the rare and endemic species can be conserved where necessary.
Taxonomic Treatment
Lobariella crenulata (Hook. f.) Yoshim.
Notes. This species was reported from Hawaii by Yoshimura (Reference Yoshimura1984, Reference Yoshimura1998) and Yoshimura & Arvidsson (Reference Yoshimura and Arvidsson1994) but many collections revised by us belong to the newly described Lobariella sandwicensis (see below). Only five collections have the morphology and chemistry characteristic of L. crenulata (Moncada et al. Reference Moncada, Lücking and Betancourt2013) but fresh material is required to confirm this identification with molecular data. Given that L. sandwicensis is similar to the neotropical L. subcrenulata in morphology and chemistry, but represents a phylogenetically distinct species, it is not unlikely that the Hawaiian material of L. crenulata represents a distinct lineage as well.
Specimens examined. USA: Hawaii: Hawaii, Waimea, Keck Observation Office, roadside tree, 30 ix 2008, C. W. Smith s. n. (HAW); Kauai, Kokee Park, Kalalau Lookout area, 1200 m, dry upland scrub forest, 1965, M. E. Hale 31684 (US); Kauai, Waimea, Na Pali-Kona Forest Reserve, Koaie Canyon, 900 m, mixed forest, on exfoliating bark of Dodonaea, 1996, T. Flynn et al. 5933 (PTBG); Kauai, Waimea, Kokee State Park, Kalalau Valley, T. Flynn 3890 (PTBG); Maui, East Maui, Makawao Forest Reserve, below Puu Nianiau, 1800 m, over moss on Myrsine trunk, 1975, C. W. Smith 1962 (HAW).
Lobariella flynniana Lücking, Moncada & C. W. Sm. sp. nov.
MycoBank No.: MB 820526
ITS barcoding sequence: KY769446, KY769447 (isotype)
Differing from Lobariella subcrenulata in the delicate thallus with numerous, richly branched, lateral lobules.
Type: USA, Hawaii, Kauai, West Kauai, western slopes of Mount Waialeale, Kokee State Park, 20 km ENE of Waimea and 35 km NW of Lihue, at end of Kokee Road, Pihea Trail, 22°08'51''N, 159°37'53''E, 1250–1350 m, mostly undisturbed montane mesic forest, 15 June 2013, B. Moncada, R. Lücking & T. Flynn 7028 (F—holotype; PTBG—isotype).

Fig. 4 A–C, Lobariella flynniana (holotype); A, thallus in situ; B & C, marginal lobules enlarged. D & E, L. robusta (holotype); D, thallus lobes; E, phyllidia enlarged. F, L. sandwicensis, thallus in situ. Scales: A & D=5 mm; B, C & E=1 mm; F=10 mm. In colour online.
Thallus growing on tree branches, up to 5 cm diam., except for the primary lobes loosely attached and ascending, appearing subfruticose; photobiont green (presumably Dictyochloropsis s. lat. clade 2 according to Dal Grande et al. (Reference Dal Grande, Beck, Cornejo, Singh, Cheenacharoen, Nelsen and Scheidegger2014)). Primary lobes up to 1 cm long, with irregular, crenulate to incised apices, 1–3 mm wide, much branched laterally and dividing into numerous, ascending, irregularly oriented lobules 0·2–0·5 mm wide giving the thallus a subfruticose appearance. Upper surface light green when hydrated, pale green-grey to yellowish grey when dry, smooth; maculae absent; pseudocyphellae present, initially (at lobe margins) visible as irregular, white lines or dots up to 0·10×0·05 mm in size, becoming elongate-linear up to 0·3×0·1 mm. Genuine isidia or phyllidia absent, except for the much branched, ascending lateral lobules. Lower surface cream-coloured to white; primary lobes except near the margin with a short, dense, cream-coloured tomentum formed of up to 20 µm long hyphae composed of globose cells up to 5 µm diam., and discrete, up to 0·5 mm long and 0·1 mm wide, cream-coloured rhizines composed of strongly agglutinated, parallel hyphae; rhizines unbranched to sparsely branched at the tip and covered with tomentum in the thallus centre; lower surface of lateral lobules glabrous or with short, white tomentum formed of up to 15 µm long hyphae composed of globose cells up to 5 µm diam. Upper cortex paraplectenchymatous, 15–20 µm thick with 3–5 µm thick epicortex, formed of 2–3 cell layers; algal layer 15–20 µm thick; medulla 50–80 µm thick; lower cortex paraplectenchymatous, 7–10 µm thick, formed of 2–3 cell layers.
Apothecia and pycnidia not observed.
Secondary chemistry. Chemosyndrome B (according to Moncada et al. Reference Moncada, Lücking and Betancourt2013): cortex with pseudocyphellarin A (minor to trace), K+ yellow; medulla with Lobariella unidentified 3 (major), 4-O-methyl-gyrophoric acid (major), gyrophoric acid (minor or trace), and Lobariella unidentified 2 (minor), K+ emerald green to sordid yellow, C−.
Etymology. With great pleasure, we dedicate this new species to Timothy Flynn, Herbarium Collections Manager at the National Tropical Botanical Garden on Kauai, for his invaluable assistance and his keen eye in finding the rarest Hawaiian lichens.
Ecology. Lobariella flynniana is thus far known only from the type collection in montane mesic forest on West Kauai. It was found growing exposed to frequent fog on the stem of a small tree in a semi-exposed situation.
Notes. This species is readily distinguished from all other species of the genus by the small, delicate thallus producing numerous, richly branched, lateral lobules giving the lichen a subfruticose appearance, a unique morphology among Lobariella species.
Lobariella robusta Lücking, Moncada & C. W. Sm. sp. nov.
MycoBank No.: MB 820527
ITS barcoding sequences: KY769461, KY769462 (isotype)
Differing from Lobariella nashii and L. stenroosiae in the broad, squamiform, mostly unbranched phyllidia and the robust, rather thick thallus.
Type: USA, Hawaii, Hawaii, Waimea, Keck Observation Headquarters, 20°01'25''N, 155°39'55''E, 822 m, corticolous on Metrosideros polymorpha, 27 September 2016, C. W. Smith CWS05 (HAW—holotype; B, F—isotypes).
Thallus growing on tree trunks, up to 5 cm diam., rather closely attached; photobiont green (presumably Dictyochloropsis s. lat. clade 2 according to Dal Grande et al. (Reference Dal Grande, Beck, Cornejo, Singh, Cheenacharoen, Nelsen and Scheidegger2014)). Primary lobes up to 2 cm long, with rounded, incised apices, 5–10 mm wide. Upper surface light green when hydrated, pale green-grey to yellowish grey when dry, smooth; maculae absent; pseudocyphellae present, initially (at lobe margins) visible as irregular, white lines or dots up to 0·10×0·05 mm in size, becoming elongate-linear up to 0·5×0·1 mm. Phyllidia present and abundant, laminal, squamiform, obliquely arranged or bent down to the surface in more or less the same direction, dorsiventral, with both sides corticate but the underside darker, 0·3–1·0 mm long and broad, rounded to flabellate or fan-shaped when larger, unbranched or apically crenulate or shallowly branched. Lower surface cream-coloured to light brown; primary lobes with a short, dense, cream-coloured tomentum formed of up to 30 µm long hyphae composed of globose cells up to 5 µm diam., and discrete, up to 1 mm long and 0·15 mm wide, white to cream-coloured rhizines composed of strongly agglutinated, parallel hyphae; rhizines unbranched to sparsely branched at the tip and covered with tomentum in the thallus centre. Upper cortex paraplectenchymatous, 15–25 µm thick with 3–5 µm thick epicortex, formed of 2–3 cell layers; algal layer 20–30 µm thick; medulla 50–100 µm thick; lower cortex paraplectenchymatous, 10–15 µm thick, formed of 2–3 cell layers.
Apothecia frequent, cup-shaped, up to 2 mm diam., with thick, strongly prominent, lobulate, grey to cream-coloured margins; lobules 5–10 per apothecium, more or less regular with rounded or rarely bifurcate tips; disc concave, dark red-brown. Excipulum composed of more or less parallel, partly branched hyphae with wide lumina resembling a paraplectenchyma, 30–40 µm wide, hyaline; hypothecium formed of densely intricate hyphae partially resembling a paraplectenchyma, 15–25 µm high, pale yellowish. Hymenium 100–120 µm high, clear, with orange, strongly conglutinated, 10–20 µm high epithecium; asci narrowly clavate, 100–120×10–13 µm. Ascospores narrowly fusiform, 70–80×3–4 µm, 7-septate, hyaline.
Pycnidia not observed.
Secondary chemistry. Chemosyndrome B (according to Moncada et al. Reference Moncada, Lücking and Betancourt2013): cortex with pseudocyphellarin A (minor to trace), K+ yellow; medulla with Lobariella unidentified 3 (major), 4-O-methyl-gyrophoric acid (major), gyrophoric acid (minor or trace) and Lobariella unidentified 2 (minor), K+ emerald green, C−.
Etymology. The epithet refers to the rather robust, leathery thallus of this species.
Ecology. This species is known from a single, well-developed population on the island of Hawaii, in mesic, open-landscaped parkland where it grows together with Lobariella crenulata.
Notes. Lobariella robusta agrees with the neotropical, related species L. nashii and L. stenroosiae (Moncada et al. Reference Moncada, Lücking and Betancourt2013) in producing dorsiventral phyllidia and in the secondary chemistry. However, in the last two species the phyllidia are laciniate and sparsely to frequently branched, whereas in L. robusta they are broadly squamiform and mostly unbranched. Also, the thallus is more robust and leathery in L. robusta. In spite of the morphological differences, the species is very closely related to the marginally lobed L. flynniana, differing only in two consistent indels in the ITS.
Additional specimens examined. USA: Hawaii: Hawaii, Waimea, Keck Observation Office, roadside tree, 30 ix 2008, C. W. Smith s. n. (F, HAW); Hawaii, Waimea, Keck Observation Headquarters, 20°01'25''N, 155°39'55''E, 822 m, corticolous on Metrosideros polymorpha, 2016, C. W. Smith CWS06, CWS10 (B, HAW), CWS07, CWS08, CWS09, CWS11, CWS12 (B).
Lobariella sandwicensis Lücking, Moncada & C. W. Sm. sp. nov.
MycoBank No.: MB 820528
ITS barcoding sequence: KY769448 (isotype)
Differing from Lobariella subcrenulata in the smaller thallus with narrower lobes.
Type: USA, Hawaii, Kauai, West Kauai, western slopes of Mount Waialeale, Kokee State Park, 20 km ENE of Waimea and 35 km NW of Lihue, at end of Kokee Road, Pihea Trail, 22°08'51''N, 159°37'53''E, 1250–1350 m, mostly undisturbed montane mesic forest, 15 June 2013, B. Moncada, R. Lücking & T. Flynn 7029 (F—holotype; PTBG—isotype).
(Fig. 4F)
Thallus growing on tree trunks, up to 10 cm diam., more or less closely attached; photobiont green (presumably Dictyochloropsis s. lat. clade 2 according to Dal Grande et al. (Reference Dal Grande, Beck, Cornejo, Singh, Cheenacharoen, Nelsen and Scheidegger2014)). Individual lobes up to 5 cm long, with irregular to almost rounded apices and irregular to crenate-lobate margins, 3–8 mm wide, irregularly branched, forming more or less rounded thallus rosettes. Upper surface bright green when hydrated, pale green-grey when dry, smooth except for the erumpent pseudocyphellae; maculae absent; pseudocyphellae present, initially (at lobe margins) visible as irregular, white lines or dots up to 0·10×0·05 mm in size, becoming elongate-linear up to 0·5×0·1 mm. Isidia or phyllidia absent. Lower surface cream-coloured, becoming grey-brown towards the centre, with a short, dense, cream-coloured to pale brown tomentum formed of up to 30 µm long hyphae composed of globose cells up to 5 µm diam., and discrete, up to 1 mm long and 0·1 mm wide, cream-coloured to pale brown rhizines composed of strongly agglutinated, parallel hyphae; rhizines unbranched to sparsely branched at the tip and covered with tomentum in the thallus centre. Upper cortex paraplectenchymatous, 15–25 µm thick with a 3–5 µm thick epicortex, formed of 3–4 cell layers; algal layer 15–25 µm thick; medulla 60–100 µm thick; lower cortex paraplectenchymatous, 7–15 µm thick, formed of 2–3 cell layers.
Apothecia rare, cup-shaped, up to 3 mm diam., with thick, strongly prominent, lobulate, grey to cream-coloured margins; lobules 5–10 per apothecium, more or less regular with rounded or rarely bifurcate tips; disc concave, orange-brown. Excipulum composed of more or less parallel, partly branched hyphae with wide lumina resembling a paraplectenchyma, 30–50 µm wide, hyaline; hypothecium formed of densely intricate hyphae partially resembling a paraplectenchyma, 15–25 µm high, pale yellowish. Hymenium 100–120 µm high, clear, with a yellow-orange, strongly conglutinated, 10–20 µm high epithecium; asci narrowly clavate, 100–120×10–13 µm. Ascospores narrowly fusiform, 50–70×3·5–4·5 µm, 7-septate, hyaline.
Pycnidia not observed.
Secondary chemistry. Chemosyndrome B (according to Moncada et al. Reference Moncada, Lücking and Betancourt2013): cortex with pseudocyphellarin A (major to trace), K+ yellow; medulla with Lobariella unidentified 3 (major), 4-O-methyl-gyrophoric acid (major), gyrophoric acid (minor or trace) and Lobariella unidentified 2 (minor), K+ emerald green, C−.
Etymology. The epithet points out the inferred endemism of this new species, using the historical name of the Sandwich Islands for Hawaii.
Ecology. Lobariella sandwicensis appears to be the most common species of the genus in Hawaii. It occurs in mesic montane forest in shaded to semi-exposed situations and is known, at least based on earlier and historical collections, from the islands of Kauai, Maui and Oahu. It has not been found on the island of Hawaii, where L. robusta occurs.
Notes. Lobariella sandwicensis is very similar in morphology to the neotropical, related species L. subcrenulata and it shares the same chemistry. It tends to produce smaller thalli with narrower lobes but without molecular data such a difference would be considered infraspecific or ontogenetic variation. While both species belong in the same clade within the genus, the differences in the ITS barcoding locus are substantial, as shown by the long branch leading to the Hawaiian Lobariella clade (Fig. 2).
Additional specimens examined. USA: Hawaii: unknown locality, H. Mann & W. T. Brigham s. n. (US); Kauai, on ridge W of Hanapepe River, 1895, A. A. Heller 2754 (US); Kauai, boundary of Hanalei and Waimea, Awaawapuhi Trail, 1200 m, T. Flynn 4774b (PTBG); Kauai, Waimea, 1905, Hochreutiner 3514 (G; as Sticta dissimulata Nyl.); Kauai, Waimea, Kokee State Park, Ditch Trail, 1100 m, summer dry forest, epiphytic on small branches of Dodonaea viscosa, 1991, T. Flynn & M. Chapin 4640 (PTBG); Kauai, Waimea District, Kokee State Park, from the yard of John H. R. Plews, Mohilhi Road, 1109 m, epiphytic on dead stems of Dubautia latifolia, 1996, T. Flynn, M. Thom & J. H. R. Plews 5971 (PTBG); Kauai, West Kauai, western slopes of Mount Waialeale, Kokee State Park, 20 km ENE of Waimea and 35 km NW of Lihue, at end of Kokee Road, Pihea Trail, 22°08'51''N, 159°37'53''E, 1250–1350 m, mostly undisturbed montane mesic forest, 2013, B. Moncada, R. Lücking & T. Flynn 7027 (F); Kauai, West Kauai, western slopes of Mount Waialeale, Kokee State Park, Awaawapuhi Trail, bottom of trail, corticolous on Dodonaea, 1981, C. W. Smith 6188 (HAW); Kauai, Waimea District, Na Pali-Kona Forest Reserve, “Pig Ridge”, SE of Waialae Stream, 3300–3600 ft., Acacia koa forest with Metrosideros, Panicum, Bidens, badly damaged by pig rooting, epiphytic on Metrosideros polymorpha, 1993, T. Flynn & G. Kawakami 5456, 5456a (PTBG); Kauai, Waimea, Na Pali-Kona Forest Reserve, Kohua Ridge, 1140 m, Metrosideros dominated forest, with Cheirodendron, Melicope, Myrsine, Elaeocarpus, Dianella, Dryopteris, Athyrium, and Asplenium, on Vaccinium calycinum, 1995, T. Flynn et al. 5695 (PTBG); Maui, East Maui, Haleakalā Volcano, Lower Waikamoi Preserve (The Nature Conservancy), 5 km SE of Pulakani and 18 km SE of Kahului, lower access trail to preserve off Olinda Road, 20°48'23''N, 156°15'19''E, 1200–1300 m, disturbed primary forest dominated by Acacia koa and Campanulaceae, with invasive Hedychium gardnerianum in lower portions, 2013, B. Moncada, R. Lücking & P. Bily 6940a (F); Maui, East Maui, Haleakalā Volcano, Haleakalā Crater, Paliku, 2120 m, on Sophora chrysophylla, C. W. Smith 2971 (HAW); Maui, West Maui, Pu’u Kukui, trail from cabin to summit, 1300–1700 m, mossy forest to alpine bog, 1965, M. E. Hale 31259, 31287, 31290 (US); Oahu, Waianae Range, Land of 10 000 Snails, just about “snail jail”, 730 m, on Pisonia, 30 vi 2011, C. W. Smith s. n. (HAW); Oahu, Waianae Range, Pu’u Kaua, on Schinus terebinthifolius, C. W. Smith 1559 (HAW); Oahu, unknown locality, E. Bailey s. n. (US); Oahu, unknown locality, H. Mann & W. T. Brigham s. n. (US).

Funding for field and laboratory work was provided by two grants from the National Science Foundation (NSF) to The Field Museum: DEB-1025861 “ATM – Assembling a Taxonomic Monograph: The Lichen Family Graphidaceae”; PI Thorsten Lumbsch, CoPI Robert Lücking, and DEB-1354884 “Collaborative Research: Evolution, Diversification, and Conservation of a Megadiverse Flagship Lichen Genus”; PI Thorsten Lumbsch, CoPI Robert Lücking. Additional funding was obtained from the Field Museum’s Women’s Board Field Dreams program 2011, through gifts by the Robert Thomas Bobins Foundation through Mrs Virginia Bobins (Chicago), Mr and Mrs John Borland Jr. (Chicago), Mrs Peggy Carr (Chicago) and Mrs Sue Dickes (Winnetka). Patrick Bily (The Nature Conservancy Hawaii), Timothy Flynn (National Tropical Botanical Garden), Daniel Pomaika’i (Maui Soil and Water Conservation Districts, Maunalai Arboretum) and Philip Thomas (Research Corporation of the University of Hawaii and the Hawaiian Ecosystems at Risk Project) provided invaluable field assistance and shared their profound knowledge of Hawaiian ecosystems and fauna and flora. The aforementioned institutions, as well as Jill Kajikawa-kent (University of Hawaii) and Rae Matthews and the Clark family (National Tropical Botanical Garden), assisted with logistics. The Hawaii Department of Land & Natural Resources, Divisions of Forestry and Wildlife and Division of State Parks, kindly provided collecting and research permits and Chelsea Carineo, Wendee Kokubun, Ryan Peralta, Patrick Porter, Matthew Rittenhouse and Lance de Silva are thanked for processing permit requests. Peter Buol helped to trace Darwin’s communication to Joseph Dalton Hooker about Hawaii.







