The benzyl ester, alectorialic acid, is a relatively uncommon lichen substance but it has been reported from Alectoria nigricans (Ach.) Nyl., Bryoria capillaris (Ach.) Brodo & D. Hawksw. and B. nadvornikiana (Gyeln.) Brodo & D. Hawksw., as well as from the apothecia of Usnea florida (L.) F. H.Wigg. and the medulla of the lichen currently known as a chemotype of Usnea dasopoga (Ach.) Nyl. (=U. diplotypus auct. non Vain. p.p., Clerc Reference Clerc2011; Wirth et al. Reference Wirth, Hauck and Schultz2013), and in Anamylopsora pulcherrima (Vain.) Timdal (Huneck & Elix Reference Huneck and Elix1993). In nature, alectorialic acid is usually accompanied by various quantities of other substances which have been assumed to be degradation products of alectorialic acid (Solberg Reference Solberg1970).
Relatively unstable lichen substances may be degraded continuously, both in the lichen itself (in situ) or in their extracts (in vitro) at room temperature (Posner Reference Posner1990; Beckett et al. Reference Beckett, Ntombela, Scott, Gurjanov, Minibayeva and Liers2015). It has been shown that alectorialic acid is a relatively unstable substance and is slowly converted into other compounds at room temperature. Degradation products have been reported to include 5,7-dihydroxy-6-methylphthalide and alectorialin (Elix & Jayanthi Reference Elix and Jayanthi1987; Huneck & Elix Reference Huneck and Elix1993).
However, we have identified an alternative set of degradation products derived from alectorialic acid following our recent studies using thin-layer chromatography (TLC) and high performance liquid chromatography-mass spectrometry (HPLC-MS). The possible chemical reactions and characteristics of intermediate substances and final products are presented and an alternative pathway for the degradation of alectorialic acid is suggested.
This study was performed using specimens from GLM: Usnea diplotypus auct. non Vain., nr: L-32090 (Russia: North Caucasus: Adygheya, leg. 2011), Usnea florida nr: L-29074 (Russia: North Caucasus: Adygheya, leg. 2009) and as a standard, Alectoria nigricans nr: L-22131. TLC was performed using solvents B and C according to the method of Culberson & Ammann (Reference Culberson and Ammann1979) (only acetone used for extraction).
HPLC analyses were performed with a 1200 Series Agilent chromatograph (Waldbronn, Germany) with diode-array UV-VIS detection. For reverse phase chromatographic separation, a Kinetex 2.6 microns PFP, 100 Å column (150×2·10 mm, Phenomenex, Aschaffenburg, Germany) was used. The mobile phase consisted of (A) aqueous formic acid buffer (0·1%), and (B) acetonitrile. Analyses were performed at 45°C and a flow rate of 0·4 ml min−1. The gradient started with 30% (B) for 1 min, increasing to 85% (B) within 24 min. This mixture was held for 1 min to elute strongly hydrophobic analytes (Karich et al. Reference Karich, Kluge, Ullrich and Hofrichter2013). Spectra of eluting substances were recorded at 210, 215, 230, 250, 280, 310 nm. After separation, the samples were also analyzed with an ion trap mass spectrometer (6300 Series, Agilent, Waldbronn, Germany). Ionization was achieved by atmospheric pressure chemical ionization-electrospray ionization multimode (APCI/ESI) in the negative and positive mode. Mass spectra were recorded in the range 100–600 m/z. The substances were identified based on their chromatographic properties (Yoshimura et al. Reference Yoshimura, Kinoshita, Yamamoto, Huneck and Yamada1994; Huneck & Yoshimura Reference Huneck and Yoshimura1996; Mietzsch-Lumbsch et al. Reference Mietzsch-Lumbsch, Lumbsch and Elix1996; Orange et al. Reference Orange, James and White2001) and molecular masses (Huneck & Yoshimura Reference Huneck and Yoshimura1996). The sample of Alectoria nigricans (GLM-L-22131) was used as a reference for alectorialin, alectorialic acid and 5,7-dihydroxy-6-methylphthalide. For haematommic acid, the pure substance from the reference collection of J. Elix was used.
In the HPLC analysis of a fresh acetone extract of Usnea diplotypus, we identified usnic acid, alectorialin, alectorialic acid, 5,7-dihydroxy-6-methylphthalide and haematommic acid (Fig. 1A). A second HPLC scan of the same acetone extract of Usnea diplotypus, which had been stored for 24 h at room temperature, revealed that the concentration of alectorialic acid had decreased while the concentration of haematommic acid and 5,7-dihydroxy-6-methylphalide had increased; relative concentrations of alectorialin and usnic acid remained the same (Fig. 1B).

Fig. 1 HPLC chromatograms at 250 nm of acetone extracts (10 mg ml–1) of Usnea diplotypus thalli (A & B) and Usnea florida apothecia (C & D). A & C, analysis immediately after extraction; B & D, analysis after 24 h storage of the extract. 1=alectorialic acid; 2=alectorialin; 3=haematommic acid; 4=5,7-dihydroxy-6-methylphthalide; 5=squamatic acid.
Our analysis of apothecia of Usnea florida revealed 5,7-dihydroxy-6-methylphthalide and haematommic acid, as well as alectorialic, usnic and squamatic acids (Fig. 1 C & D). This extract showed similar decomposition phenomena after 24 h storage as were observed with the U. diplotypus extract (cf. Fig. 1 C & D). Chemical structures, UV-Vis spectra and MS data of the substances identified in U. diplotypus and U. florida are shown in Figs 2 and 3.

Fig. 2 Chemical structures of substances identified in U. diplotypus and U. florida. A, squamatic acid; B, usnic acid; C, haematommic acid; D, alectorialin; E, alectorialic acid; F, 5,7-dihydroxy-6-methylphthalide.

Fig. 3 UV-Vis spectra and mass spectroscopy (MS) molecular mass data for substances identified in U. diplotypus and U. florida. A, squamatic acid; B, usnic acid; C, haematommic acid; D, alectorialin; E, alectorialic acid; F, 5,7-dihydroxy-6-methylphthalide.
Observed HPLC peaks correspond with spots observed in TLC analyses of both species (Fig. 4). In Fig. 5 we outline the probable steps involved in the degradation of alectorialic acid. The formation of haematommic acid, which was not reported earlier, is plausible in the course of hydrolysis of alectorialic acid.
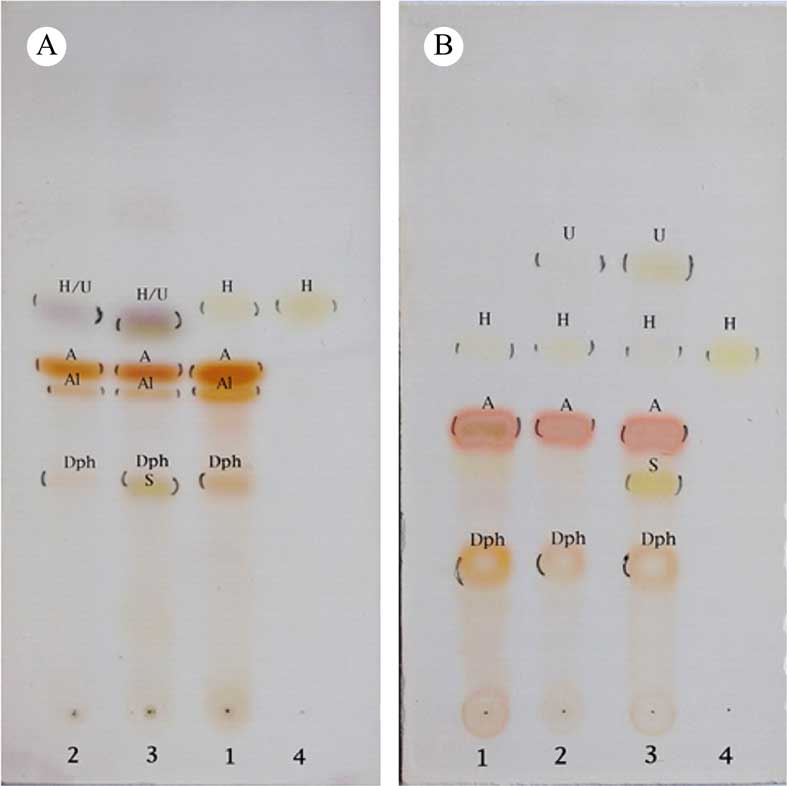
Fig. 4 TLC analyses of: 1, Alectoria nigricans; 2, Usnea diplotypus; 3, Usnea florida; 4, reference haematommic acid. Chromatograms were run in solvent B (n-hexane: 120 ml, diethyl ether: 90 ml, formic acid: 20 ml) and solvent C (toluene: 200 ml, acetic acid: 30 ml); the solvent front is at 10 cm (at the uppermost edge of the image). Key to components: A=alectorialic acid; A1=alectorialin; Dph=5,7-dihydroxy-6-methylphthalide; H=haematommic acid; S=squamatic acid; U=usnic acid. In colour online.

Fig. 5 Possible steps involved in the degradation of alectorialic acid to haematommic acid and 2,4-dihydroxy-6-hydroxymethyl-3-methylbenzoic acid and then to 5,7-dihydroxy-6-methylphthalide. Molecular mass in g mol–1; molecular mass in the negative mode of MS, (M-H)–.
The first author acknowledges financial support from the DAAD (German Academic Exchange Service) during a study period at TU-Dresden. She also gratefully thanks Dr René Ulrich for helpful discussions and acknowledges the use of services and facilities of the laboratory at the International Institute Zittau (IHI) and Senckenberg Museum of Natural History Görlitz.







