Published online by Cambridge University Press: 07 June 2005
The dynamics of the expansion plasma produced by excimer laser ablation of a silicon target into oxygen and mixed O2/Ar atmosphere were studied by means of time-resolved imaging of the expanding plume. Experiments were performed in pure oxygen, ranging between 0.13 and 13.33 Pa, and at different O2/Ar ratios at a fixed total pressure of 13.33 Pa. The occurrence of a shock wave (SW) generated by the supersonic expansion of the plasma was observed at high pressure values. The presence of the SW had a strong influence on the structure of SiOx thin films. In fact, silicon dioxide thin films were always obtained in presence of the SW, irrespective of the oxygen content in the gaseous mixture. On the contrary, suboxide thin films were obtained when the expansion occurred at lower pressure values (no SW presence). The temperature rise following the developing of the SW, is supposed to enhance the oxygen molecules dissociation by increasing the efficiency of the silicon oxidation reaction.
Interaction phenomena of intense laser pulses with matter have a wide range of applications from inertial fusion energy, astrophysical phenomena, to medical applications (Desai, 2003; Schilling, 2003; Batani & Wooton, 2004; Honrubia & Tikhonchuk, 2004). Laser ablated slab acceleration was studied recently. Silicon nanocrystals embedded in silicon-rich oxide thin films present several potential applications in the optoelectronics field based on silicon technology. As a consequence, many efforts were devoted toward their production with different experimental techniques. Among these, pulsed laser ablation of silicon in a controlled inert or reactive atmosphere was successfully employed (Desbiens et al., 2002; Lackner et al., 2003; Gamaly et al., 2000). Following this approach, in a recent work (Fazio et al., 2004), we investigated the formation of silicon nanocrystals in thin SiOx films (x being the material compositional parameter) obtained by ablating a silicon target in pure oxygen. From the analysis of both infrared absorption and Raman spectroscopy results, it turned out that the nanocrystals formation is strictly related to both the oxygen pressure PO2 and to the substrate temperature TS. In particular, we found that, since the nanocrystals formation occurs through a silicon thermal diffusion mechanism, it is of paramount importance the value of the compositional parameter x. Then in the present work, we present a fast photography investigation of the plasma expansion dynamics, following the ablation process, which turns to be a key feature in determining the structural properties of the SiOx thin films.
The plasma was generated by a KrF excimer laser (wavelength 248 nm, pulse duration 25 ns) focused onto the surface of a poly-crystalline silicon target mounted on a rotating holder. The estimated energy density was 7.0 Jcm−2. The SiOx thin films were deposited onto silicon and germanium substrates positioned 45 mm away from the target. The substrates were kept at room temperature. Fast photography measurements were carried out in presence of pure oxygen gas at pressure values up to 13.33 Pa. When an O2/Ar gas mixture was used, the total pressure was fixed at 13.33 Pa. The images of the expanding plasma were acquired at different time delays with respect to the laser pulse using a fast intensified charge coupled device (Andor Technology + iStar iCCD) with a variable gate. The iCCD was triggered by a fast photodiode using a portion of the laser beam reflected by glass slide. The gate width (τG) was varied between 2 ns, in the initial stage of the expansion, and increased up to 50 ns. Each image was averaged on 10 successive laser pulses. Structural properties of the SiOx thin film were investigated by infrared transmission spectroscopy measurements carried out on a Perkin Elmer FTIR GX1000. The films thickness, measured with a stylus profilometer, ranged between 100 and 200 nm.
Two sets of samples were deposited: the first one in pure oxygen with pressure values PO2 increasing from 0.13 Pa up to 13.33 Pa; the latter in a mixed O2/Ar atmosphere with the total pressure kept fixed at 13.33 Pa, and controlling the O2/Ar ratio from 1/99 up to 80/20. Figures 1a and 1b show the FTIR absorption spectra of all the samples. The spectra are characterized by a broad band in the 900–1300 cm−1 region. This vibration band, typical of the Si-O bond, is the result of the superposition of several components: (1) the TO1 stretching mode, peaked at about 1030–1070 cm−1, (2) a less intense broad feature, in the high frequency side of the spectra, due to the overlapping of the two longitudinal LO1 and LO2 modes at 1150 and 1250 cm−1, and (3) the transverse TO2 mode at 1200 cm−1. These modes are normally infrared inactive but, under specific polarization conditions, they become IR active. This occurs in SiO2 systems where there is a disorder-induced mechanical coupling between the two TO modes (Montero et al., 1994). We reproduced the experimental absorption profiles using a superposition of Gaussian line shapes, one for each of the above reported vibrational modes. Moreover, as widely accepted in the literature (Pai et al., 1986), from the position of the TO1 mode peak the compositional parameter x, that is, the stoichiometry of the SiOx films, can be estimated. The results of such an analysis are summarized in Table 1 and in Figure 1c. There is a clear trend as a function of the oxygen pressure. Increasing the pressure from 0.13 Pa up to 10.67, the TO1 mode peak position shifted from 1020 cm−1 up to 1075 cm−1, and the x parameter increased from 1.20 up to 2.01. Thus, increasing PO2 the stoichiometry of the SiOx films approaches the SiO2 one. It's worth noting that, keeping fixed all the other experimental parameters, PO2 values above 6.67 Pa are needed to obtain stoichiometric films. On the contrary, for the samples deposited in a mixed atmosphere at a fixed pressure of 13.33 Pa and different O2/Ar partial pressure ratios, the results show that all of them present x values around 2.0. Thus, stoichiometric SiO2 samples can be obtained in presence of only 0.13 Pa of oxygen partial pressure. This finding clearly points out that the films stoichiometry does not depend trivially on the oxygen partial pressure, but the total pressure plays a role too. Taking into account that all the samples were deposited under the same laser fluence, by using the same laser spot and laser pulse energy, the mass removed from the silicon target by each pulse can be considered constant for all the samples. Then, the different behavior of the x parameter should be related to an increased efficiency of the oxidation reaction under high total pressures conditions. Let's consider the following well-known silicon oxidation reaction:
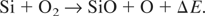
Deposition conditions and results of the FTIR analysis for the samples deposited in pure O2 and mixed O2/Ar atmosphere. ν1 and FWHM refer to the TO1 vibrational mode band of the Si-O bond


FTIR spectra of the samples deposited in pure O2 atmosphere (a) and in a mixed aqtmosphere with different O2/Ar partial pressure ratios (b); the spectra are shifted for sake of clarity. Compositional parameter x as obtained from the TO1 vibrational mode absorption peak position (c); line is a guide for the eye.
Taking into account that the dissociation energy of an oxygen molecule is 5.2eV, while the binding energy of SiO is 7.4 eV, the reaction (1) is exothermic with ΔE = 2.2 eV. Oxygen molecules can be dissociated by means of electron-molecule collisions, a mechanism that is more effective near the target surface where the electron density is higher. Dissociated oxygen atoms can then react with the silicon ones forming SiO complexes that upon reaching the substrates are incorporated in the growing film. Collisions between the species present in the plasma and oxygen molecules can also lead to SiO formation. Both these mechanisms become more and more effective as the oxygen molecules density increases. Nevertheless, such a requirement cannot explain the nearly stoichiometric samples deposited in presence of an O2/Ar gas mixture, with PO2 values as low as 0.13 Pa. In such a case a mechanism able to increase the rate of Eq. (1), independently of the overall number of oxygen molecules present, is needed. Such a mechanism should depend on the total ambient pressure rather than on its chemical composition, that is, on the plasma expansion regime, which strongly depends on the ambient pressure. In a recent work (Trusso et al., 2004), we observed the formation of a shock wave (SW) due to the supersonic expansion of a plasma, generated by the laser ablation of a silicon carbide target in a controlled nitrogen atmosphere. In the early stage of the plasma expansion, almost all the energy is kinetic and it will be soon converted into thermal energy as the plasma interacts with the background gas. Then, the temperature of the shocked region, at the contact front between the expanding plasma and the gas, raises reaching values of the order of several thousand of Kelvin degrees. The temperature rise produces an enhancement of emission from excited species in the plasma which can be observed in fast photography measurement or optical emission spectroscopy. In Figures 2a and 2b are reported spots of the expanding plasma in pure oxygen at 0.13 and 10.67 Pa. At low pressure, two distinct components can be observed: the first one show a nearly spherical expansion, the second one is faster and can be observed at a different time delay with respect to the laser pulse arrival. At high oxygen pressure (10.67 Pa) the effect of the ambient gas is clearly visible. Confinement of the plume and the bright edge in correspondence with the contact front between the plasma and the ambient gas is evident. The observation of such a bright edge is indicative of the developing of a SW as a result of the plasma expansion at velocity higher than the sound velocity in the ambient gas. The formation of the SW was observed for all the experiment performed at the pressures higher than 6.67 Pa. Figures 2c and 2d show images of the expanding plasma in the mixed O2/Ar atmosphere for different time delays. The developing of the bright edge of the SW can be clearly seen also in this case. According to the point blast-wave theory (Zel'dowich & Raizer, 1966), the position R of the moving front as a function of the time t is given by the following relation:
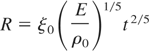
where ξ0 (≈1) is a factor related to both geometrical and thermodynamic quantities, E is the plume energy, and ρ0 the density of the undisturbed gas. Anyway, a SW can develop only if the mass removed from the target by the laser pulse is lower than the mass of the gas surrounding the SW, and when the pressure driving the moving front of the plasma SW is greater than the ambient pressure. The experimental R-t data, as obtained from the analysis of the time resolved images (see Fig. 2), show a good agreement with Eq. (2) in the early stage of the expansion. For longer times, the measured R values were systematically smaller than the ones predicted by Eq. (2). Recently, an analytical model was proposed to describe the expansion of laser generated plasma in presence of a background gas (Arnold et al., 1999). The model provides a description of the expansion in terms of three regimes: (1) free expansion characterized by a linear behavior (R ∝ t); (2) SW formation and expansion as R ∝ t2/5; and (3) the final stage when the plasma expansion stops. In an experiment, one or more of these regimes can be observed depending on the pressure, and the chemical nature of the background atmosphere, the energy of the plasma, etc. On the other hand, a family of R-t curves is usually obtained. Nevertheless, when the R-t data are reported in terms of the following dimensionless variables, all the data should lie on a single curve:
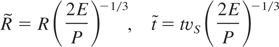
where P is the ambient pressure, E is the energy of the expanding plasma (estimated from Eq. (2) to be ≈ 4 mJ in the initial expansion stage), and vs is the sound velocity in the ambient gas. As reported in Figure 3, the experimental data describing the expansion in pure O2, fall onto a single curve which shows two different slopes as a function
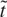
: a linear behavior in the initial stage, typical of free expansion, followed by the SW regime. It can be seen that the linear behavior holds for ambient pressure up to 2.67 Pa, while at higher pressure the SW develops. On the contrary, the measurements carried out in the mixed O2/Ar atmosphere (see the inset in Fig. 3) show only the SW regime for all the mixtures, with the total pressure being 13.33 Pa. A clear correlation between the x parameter and plasma expansion regime emerges from these results. The stoichiometry of the samples, in fact, is correlated more directly to the expansion regime rather than to the oxygen gas pressure: in the free expansion regime sub-stoichiometric films are obtained, on the contrary, in the SW one stoichiometric film are obtained even at the lowest O2/Ar gas ratio. In the shocked region the temperature values can be estimated from the relation (Landau & Lifshitz, 1987):
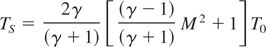
where γ is the specific heats ratio (≈ 1.2) (Zel'dowich & Raizer, 1966), M is the Mach number, and T0 is the ambient gas temperature. The Mach number can be evaluated from the V = R/t data, where M = V/vs and TS values in the 104 ÷ 105 K range are obtained. Since more oxygen molecules are likely to be dissociated, the rate of Eq. (1) increases, determining higher values of the film compositional parameter x. It can be concluded that, pulsed laser deposition processes are affected not only by the composition of the reactive gas atmosphere but also by the total pressure at which the deposition takes place, which, influencing the plasma expansion dynamics, determines significative modifications in the films structure.

CCD images of the emission of the expanding plasma for different time delays under different experimental conditions (see text).

Plot of the distance vs time in dimensionless variables. The solid lines refer to free expansion (R ∝ t) and to ideal shock wave (R ∝ t2/5) behaviours at different oxygen partial pressures. In the inset the same plot is reported for the O2/Ar gaseous mixture case.

Deposition conditions and results of the FTIR analysis for the samples deposited in pure O2 and mixed O2/Ar atmosphere. ν1 and FWHM refer to the TO1 vibrational mode band of the Si-O bond

FTIR spectra of the samples deposited in pure O2 atmosphere (a) and in a mixed aqtmosphere with different O2/Ar partial pressure ratios (b); the spectra are shifted for sake of clarity. Compositional parameter x as obtained from the TO1 vibrational mode absorption peak position (c); line is a guide for the eye.

CCD images of the emission of the expanding plasma for different time delays under different experimental conditions (see text).

Plot of the distance vs time in dimensionless variables. The solid lines refer to free expansion (R ∝ t) and to ideal shock wave (R ∝ t2/5) behaviours at different oxygen partial pressures. In the inset the same plot is reported for the O2/Ar gaseous mixture case.