1. INTRODUCTION
Nowadays, low-temperature plasmas formed in different types of gas discharge are widely used for surface modification of metals and dielectrics (Oh et al., Reference Oh, Olabanji, Hale, Mariani, Kontis and Bradley2011; Yeo et al., Reference Yeo, Choi, Chi, Lee and Jhon2013; Zhang et al., Reference Zhang, Zhou, Shao, Xie, Xu and Yang2014b). A number of methods have been developed, for example, plasma nitriding, arc spraying, and low-energy high-current electron beam treatment (Kaklamani et al., Reference Kaklamani, Bowen, Mehrban, Dong, Grover and Stamboulis2013; Li et al., Reference Li, Liu, Zhang, Wu, Zhang, Fu and Chu2014). However, most of these plasma sources require vacuum equipment. The use of nanosecond-pulse discharges, which can generate non-thermal plasma with high-power density, provides scientists another approach for surface modification with low cost, simple facilities, and easy operation (Mao et al., Reference Mao, Zou, Wang, Liu and Jiang2009). The fast rise-time of the nanosecond pulse limits the increase of the large current and suppresses the generation of spark channels at atmospheric pressure, so the surface modification by nanosecond-pulse discharges is able to be conducted without vacuum equipments in atmospheric pressure air, breaking the space limit of integrated production lines in industrial applications (Leonov et al., Reference Leonov, Petrishchev and Adamovich2014). Therefore, in recent years, technologies for surface modification by different types of nanosecond-pulse discharges have developed rapidly in the fields of microelectronics, nano-technology, and biomedicine (Shao et al., Reference Shao, Tarasenko, Zhang, Baksht, Yan and Shut'ko2012a; Correale et al., Reference Correale, Michelis, Ragni, Kotsonis and Scarano2014; Zhang et al., Reference Zhang, Tarasenko, Shao, Beloplotov, Lomaev, Sorokin and Yan2014a). For example, Voitsekhovskii et al., presented the preliminary study of modification of the CdHgTe epitaxial films with p-type conduction by a nanosecond-pulse volume discharge (Voitsekhovskii et al., Reference Voitsekhovskii, Grigor'Ev, Korotaev, Kokhanenko, Tarasenko and Shulepov2012). The results showed that a high-conductivity layer, which exhibited an n-type conduction, were formed in the near-surface region of the epitaxial films, which indicated the nanosecond-pulse volume discharge in atmospheric air was promising for modification of electro-physical properties.
Among nanosecond-pulse discharges, runaway electrons preionized diffuse discharges (REP DD) are considered as an efficient source for generating large-volume non-thermal plasmas at atmospheric pressure. Such REP DD could be formed in atmospheric air by applying nanosecond or sub-nanosecond high-voltage pulses (~100 kV) to the cathode of small curvature radius (Yang et al., Reference Yang, Wang, Li, Song and Nie2010; Shao et al., Reference Shao, Tarasenko, Zhang, Baksht, Zhang, Erofeev, Ren, Shutko and Yan2013). In such a case, the electric field near the cathode can reach critical values at which electrons may get chance to run away and gain more energy than they lose in collisions. These so-called runaway electrons preionized gas molecules give rise to the secondary electron avalanches. Many secondary electron avalanches overlap with the main electron avalanche and eventually ignite a volume discharge (Zhang et al., Reference Zhang, Tarasenko, Shao, Baksht, Burachenko, Yan and Kostyray2013). Furthermore, the runaway electrons in diffuse discharges, which could be accelerated to several or tens of keV, together with ultra violet (UV), vacuum ultra violet (VUV), and X rays, contribute to the surface modification (Zhang et al., Reference Zhang, Shao, Tarasenko, Ma, Ren, Kostyrya, Zhang and Yan2012; Baksht et al., Reference Baksht, Burachenko, Lomaev, Panchenko and Tarasenko2015).
In previous works, a volume discharge sustained by nanosecond-pulse generator was used to modify the near-surface layers of a copper (Cu) foil in atmospheric air (Shulepov et al., Reference Shulepov, Tarasenko, Goncharenko and Kostyrya2008). The results showed that carbon contaminations on the Cu with a depth of 50 nm were cleaned after the modification, while the oxygen penetrated to a depth of about 25 nm. Then, the effect of the atmospheric-pressure diffuse discharges in N2 and CO2 on the surface modification of Cu specimens was investigated, and the results showed that an increase in the hardness of the surface layer was achieved because of the oxidation (Shulepov et al., Reference Shulepov, Akhmadeev, Tarasenko, Kolubaeva, Krysina and Kostyrya2011). Nevertheless, the studies were not conducted in detail and were carried out in a narrow range of experimental conditions. The aim of this work is to study possibilities of REP DD, formed in atmospheric air in various conditions, for Cu surface modification. The experiments were carried out on two setups operated in pulsed-periodic mode and in single-pulse mode with different input power density, but in both cases, the diffuse discharge formed due to preionization by runaway electrons. The mechanical properties and microstructure of Cu surface specimens before and after the modification would be compared. Cu foil was chosen for our experiments because of its wide usage as an industrial material with high thermal and electrical conductivity and relatively low cost. The microhardness of near-surface layers were measured by the nanoindentation test, while the energy-dispersive X-ray analysis (EDX) and Auger electron spectroscopy (AES) were used to analyze the concentration changes of main chemical elements of the Cu surface.
2. EXPERIMENTAL SETUPS AND MODIFICATION CONDITIONS
2.1. Experimental Setup #1
A schematic diagram of the experimental setup #1 is depicted in Figure 1. A home-made generator based on magnetic pulse compression was used to excite repetitive REP DD. The output voltage varied from 0 to 40 kV, and it had a rise-time of ~150 ns and a full-width at half-maximum of ~300 ns. The polarity of the pulse was positive. REP DD was created in a tube-to-plane gap. The tube electrode, whose diameter was 12 mm, was made of Cu sheet with a thickness of 1 mm and a radius of curvature 0.5 mm. The plane electrode was a Cu foil with a diameter of 80 mm and a thickness of 50 µm, and it also served as the sample for plasma modification. The Cu foil was a commercial Cu foil (Shengzhuo, China), and its content was 99.7%. The voltage applied to the electrodes was measured via a high-voltage probe (Tektronix, P6015). The division ratio was 1000:1 and the bandwidth was 75 MHz. The current was measured by a Pearson Model 4100 current probe, which had a rise-time of 10 ns and a current-to-voltage ratio of 1 A to 1 V. The above two signals were recorded using an oscilloscope (Tektronix DPO2024, with a bandwidth of 200 MHz and a time resolution of 1 GS/s). The length of the signal cables was 3 m. The images were taken by a Canon EOS500D digital camera with a Tamron Lens (Model A001), which was parallel to the discharge area and was approximately 1 m away from it.

Fig. 1. Schematic view of the experimental setup #1 for Cu surface modification.
Before the experiments, Cu samples were cleaned twice in an ultrasonic bath (KQ2200DB, Kunshan Hechuang, China) with distilled water and alcohol at 40 °C. The microhardness of the Cu surface was measured by a microhardness instrument (HX-1000TM, Shanghai Taiming, China) with a measuring force of 9.807 N and a load time of 15 s, and its value was the average of 15 measured data on different locations. The contact angles were measured by an optical microscope (JGW-360a, Cheng De, China), and from the profile of about 2 µl liquid drops of distilled water and polyethyleneglycol placed on the Cu surface immediately after the modification at room temperature. The values of static contact angle were obtained by means of Laplace–Young curve fitting on the basis of images of the water drop profiles and were the average of eight measured data on different locations. Furthermore, surface energy is an important parameter that determines most of surface properties and can be characterized by contact angle measurements. In this work, surface energy including the polar and dispersive components was calculated using the measured average contact angles of these two liquids according to the Owens–Wendt method (Zhang et al., Reference Zhang, Zhou, Shao, Xie, Xu and Yang2014b). The concentration changes of the main chemical elements of the Cu surface were studied by EDX, which was carried out by TEAM EDS. We selected three points from the separated fields in each area. The distances between the selected points and the center were about 0.5, 1.2, and 2.4 cm, respectively. The values for different species in each field were the average of three measured data.
Figure 2 shows the typical discharge image and voltage–current waveform of REP DD used for the modification. The experimental conditions were as follows: the applied voltage was 26 kV, the pulse repetitive frequency (PRF) was 800 Hz, the gap was 2.5 cm, and the exposure time of the discharge image was 1 s. Figure 2a shows the discharge image from the side view. Homogeneous plasma channels started from the tube electrode and expanded forward the Cu plane and overlapped in the whole gap. In the experiments, in order to capture the discharge image from the front view, an indium–tin-oxide glass was used as the anode instead of the Cu plane, show in Figure 2b. Note that the plasma channels were non-constricted and diverging, and there were few plasma channels in the center of the plasma region because the tube was hollow. Furthermore, shown from Figure 2c, the current had an amplitude of 5 A and duration of 200 ns, which was in coherence with the characteristics of diffuse discharges. Based on the voltage–current waveform, the calculated peak of the instantaneous power reached 130 kW by integrating the measured voltage and current. Because the plasma region was approximately 11.717 cm3, the maximum specific input power in the air plasma could be calculated and was about 11.09 kW/cm3 per pulse.

Fig. 2. Typical discharge image from (a) the side view, (b) the front view, and (c) voltage–current waveform of REP DD for experimental setup #1.
2.2. Experimental Set-up #2
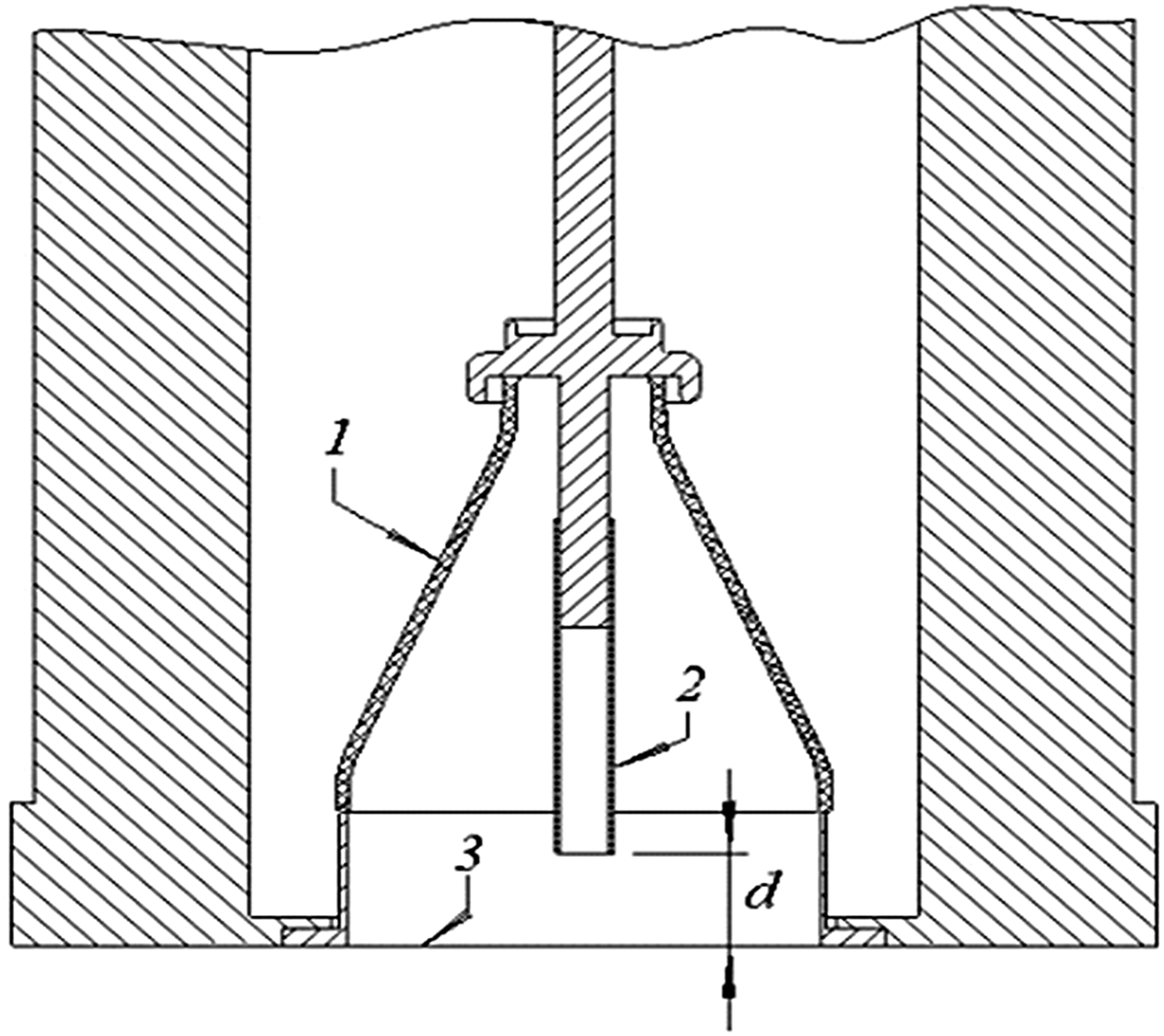
A high-voltage pulsed generator RADAN-220 was used for generating REP DD in ambient air at atmospheric pressure (Baksht et al., Reference Baksht, Burachenko, Kostyrya, Lomaev, Rybka, Shulepov and Tarasenko2009). Figure 3 shows the schematic diagram of the discharge chamber of the REP DD. Negative voltage pulses with an amplitude up to 350 kV (in the open-circuit regime) and a rise time of 0.5 ns (on a matching load) were applied to the tube cathode (2). The diameter of the tube cathode was 6 mm. The Cu foil M1 (American analogue C11000) with a dimension of 20 × 22 mm2 and a thickness of 200 µm was placed on the plane anode (3). The distance d from the plane anode to the cathode was 16 mm. Different from the pulsed generator for experimental setup #1, the REP DD was generated in single-pulse mode.

Fig. 3. Schematic view of the experimental setup #2 for Cu surface modification. 1 – insulator, 2 – tube cathode, 3 – plate anode. d – distance between tube cathode and plane copper sample (anode).
Before the experiments, Cu samples were cleaned twice in the ultrasonic unit (Elmasonic S 10H, Elma-Hans Schmidbauer GmbH & Co.) with distilled water and alcohol at 65°C during 15 min for each step. The microhardness of the Cu surface was measured by Berkovich diamond indenter on the system of NanoTest 600 (Micro Materials Ltd., UK) by Oliver–Pharr method at loads of 0.5, 1, and 2 mN (Oliver & Pharr, Reference Oliver and Pharr1992). After REP DD modification, concentration changes of the main chemical elements of the surface layers were analyzed by AES using a STIL-2 Auger-spectrometer (Shulepov, Reference Shulepov2004).
Figure 4 shows the typical discharge image and electrical waveforms of the REP DD for experimental setup #2. It could be seen that the REP DD had a cone-like shape, on which the discharge current density was maximal along the axis of the gap, and decreased substantially in the periphery. Because of the rapid rise of the applied voltage (~560 kV/ns), the high over-voltage made the streamers overlapping with each other, thus the REP DD was more homogeneous in the case of the setup #2 than that in the case of the setup #1 (Shao et al., Reference Shao, Tarasenko, Zhang, Lomaev, Sorokin, Yan, Kozyrev and Baksht2012b). In other words, the treatment on the Cu surface would be more homogeneous using experimental setup #2. Furthermore, the current for the setup #2 reached approximately 3 kA, which was larger than that for the setup #1. Under such experimental conditions, the specific input power in air plasma was calculated to be about 100 MW/cm3 per pulse. It should be pointed that pulse repetition rate and treatment time for the experimental setup #1 was chosen such that it would be equal to exposure dose (or input power density) for the experimental setup #2 during 6600 pulses with respect of current pulse duration.

Fig. 4. Typical discharge image (a) and voltage–current waveform (b) of REP DD for experimental setup #2.
3. RESULTS AND DISCUSSIONS
3.1. Modification Results for Experimental Setup #1
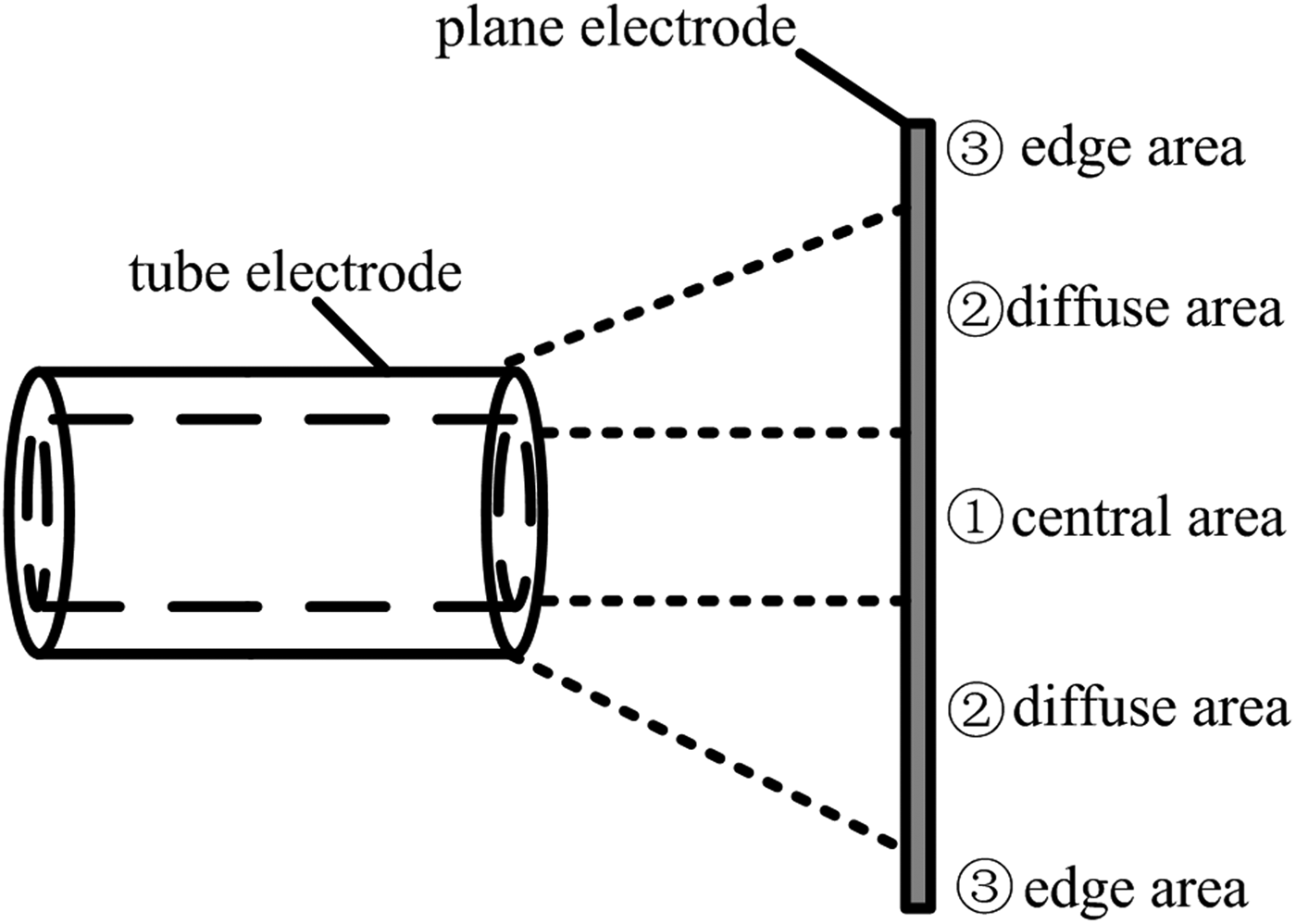
Shown from the discharge images in Figure 2, plasma channels were not evenly distributed in the gap. According to the density of plasma channels taken effect on the Cu surface, the whole area of the Cu surface could be divided into three different areas with their own modification result, including the central area, the diffuse area and the edge area. Figure 5 shows the schematic picture of these areas on the Cu surface modified by REP DD from a sectional view, as well as Figure 6 presents the corresponding photograph of the Cu surface after surface modification for 360 s under the same experimental condition as that in Figure 2. It could be seen that the Cu surface was obviously changed after the modification. There was a round area with the normal color of Cu in the center of the surface, and it was named the central area in Figure 5. There was also a light-colored annulus surrounding the said round area, and it was named the diffuse area. Outer the annulus, the color of the Cu surface turned back to its normal color, and it was named the edge area. Theoretically, the higher density of the plasma channels taken effect on the area, the better modification result could be achieved, so the optimal modification effect is most likely to be obtained on the diffuse area (Shao et al., Reference Shao, Sun, Yan, Wang, Yuan, Sun and Zhang2006). Furthermore, the sizes of these areas are closely related to the arrangement and parameters of the electrodes, so increasing the gap is an easy way to enlarge the diffuse area. However, the increase of the gap is limited by the requirement for generating diffuse discharges. Under our experiments, gaps ranged 2–3.5 cm guaranteed the formation of diffuse discharges when the PRF was 800 Hz.

Fig. 5. Schematic picture of different areas in the modification by REP DD.

Fig. 6. Image of Cu surface after it is modified by REP DD for 360 s for experimental setup #1.
Figure 7 presents the water contact angles and surface energy of the Cu surface at different areas before and after the modification. The water contact angle and surface energy of the Cu surface before the modification were 87°and 21.10 mJ/m2. After the modification by REP DD for 360 s, all the water contact angles of the Cu surface in the central, diffuse and edge areas were at least 20° lower than that of the untreated one, though there was slight difference in the standard deviations of water contact angles at different areas. Meanwhile, the surface energy in the central, diffuse, and edge area significantly increased to 62.48, 54.28, and 52.14 mJ/m2, respectively.

Fig. 7. Water contact angle and surface energy of Cu foil before and after the modification.
Furthermore, in order to further investigate the modification effects in different areas on the Cu surface, seven points in these areas were selected from the center to the edge area of the Cu foil, whose neighboring distance was 0.5 cm. Figure 8 shows images of the water contact angles at Points 1–7 shown in Figure 6 after the modification. It could be observed that all the water contact angles in the central, diffuse, and edge areas were lower than its original value and the lowest water contact angle was obtained at Point 4, which was located in the diffuse area. Therefore, a conclusion could be drawn that the modification could enhance the hydrophilicity of the Cu surface in all the central, diffuse, and edge areas, and the best modification effect on the hydrophilicity was taken place in the diffuse area.

Fig. 8. Images of the water contact angle at different areas after the modification.
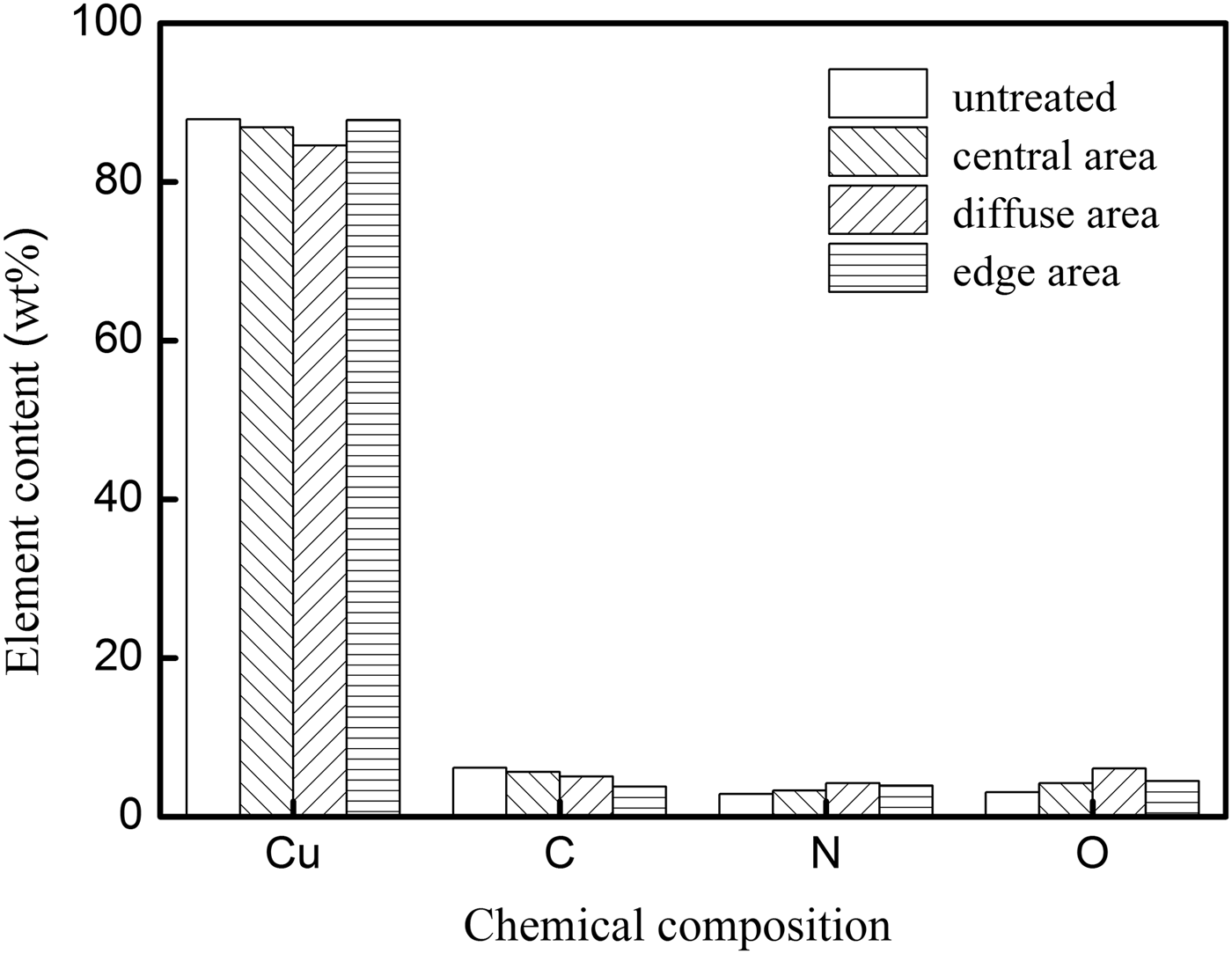
EDX was used to study the chemical ingredient change of the Cu surface. Figure 9 shows the weight percent of different chemical compositions before and after the modification. It could be seen that the concentrations of nitrogen and oxygen at all the three areas increased, while all the corresponding carbon concentrations decreased. For example, in the diffuse area, the oxygen concentration increased from 3.09 to 6.07% after the modification, while the carbon concentration decreased from 6.14 to 5.08%. Therefore, to some extent, the carbon contamination was cleaned and the Cu layer was oxidized.

Fig. 9. Weight percent of different chemical compositions before and after the modification.
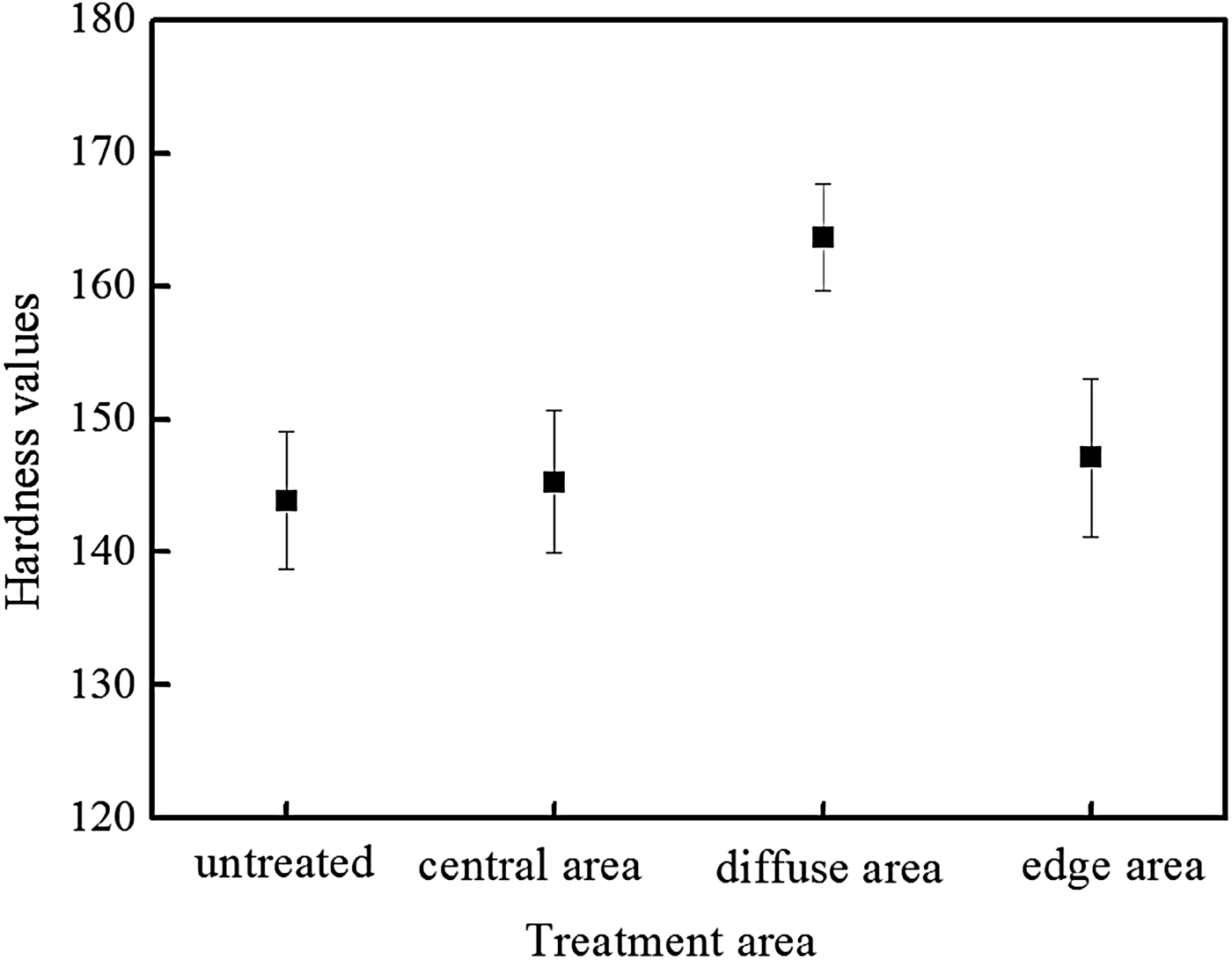
Measurement of the microhardness could reflect the mechanical property of Cu surface. Figure 10 shows the microhardness of Cu surface at different areas before and after the modification. It could be observed that the microhardness increased about 14% at the diffuse area, which was much larger than those at the central and edge areas. The microhardness only increased 1 and 2.2% at the central area and at the edge area, respectively. Therefore, the microhardness of Cu surface was slightly improved at both the central and edge areas; however, it could get an efficient enhancement at the diffuse area.

Fig. 10. Microhardness of Cu surface before and after the modification.
3.2. Modification Results for Experimental Setup #2
Figure 11 shows the schematic diagram (a) of the plasma treatment arrangement and the photograph of the Cu foil; (b) modified by REP DD for 6600 pulses. From the center to the outer, the color of the Cu surface was deepened, and it could be observed that there were three concentric zones. The center circular zone had a diameter of 1 cm, in which the color was the lightest. The second ring zone with an inner and outer diameter of 1 and 1.5 cm was more distant from the longitudinal axis of discharge, which could be considered as a transient zone between the first and the third zone. Beyond the boundary of the second ring zone, there was the edge zone, in which the color was dark and close to the normal color of the Cu foil. In order to analyze the modification effect for experimental setup #2, three points at different zones on the Cu surface were selected.

Fig. 11. Schematic diagram (a) of the plasma treatment arrangement for experimental setup #2 and photograph and (b) of the copper foil modified by REP DD for 6600 pulses.
Element composition of the near-surface Cu layers and its mechanical properties at the said three points were studied. Figure 12 shows carbon concentrations in different depths of the Cu foil, which were obtained using the AES. As can be seen, the carbon concentration at Points 1 and 2 had more significant changes, decreasing ~10 times in the nearest-surface layer after the modification. For Point 3, the carbon concentration in the nearest-surface layer was approximately half of its initial value; however, in the depths deeper than 40 nm its carbon concentration was almost the same as those of Points 1 and 2. Therefore, it could be drawn that a carbon clear effect could be achieved by the REP DD modification.

Fig. 12. Carbon concentrations in different depths before and after the modification.
The formation of oxide layer on the Cu surface could be achieved under the action of REP DD. Figure 13 presents the oxygen concentrations in different depths at the three points before and after the treatment by 6600 pulses. It could be observed that all the oxygen concentrations at Points 1–3 were increased in the nearest-surface layer, among which, the rise of oxygen concentration at Points 1 and 2 were more prominent. For example, the oxygen concentration at Point 1 was nearly two times higher than the untreated one, increasing from 17.6 to 45.2%. Moreover, not only the oxygen concentrations in the nearest-surface layer were affected, the action of REP DD could also influence the oxygen concentration of the Cu foil in the deeper layer. It could be seen that both the oxidation depths at Points 1 and 2 were longer than that at Point 3 and the maximal depth of oxidation in the Cu foil could reach 118 nm from the surface.

Fig. 13. Oxygen concentrations in different depths before and after the modification.
Figure 14 shows the change of the microhardness at Points 1–3 after the modification. In general, the microhardness decreased with the increase of the depth. After the expose to 6600 discharge pulses, the microhardness values at Points 1 and 2 were both increased. At a depth of 400 nm from the surface, the microhardness at Point 1 could be even 20% higher than that of the untreated one.

Fig. 14. Microhardness in different depths before and after the modification.
4. DISCUSSION
From the results of both experimental setups, bluish white layers could be observed on the Cu surface after modification using REP DD, indicating that chemical compound formed on the Cu surface. Only one Cu compounds, which could be colored white. Crystal structure of CuO, a different crystal lattice, is formed after the modification (Shemakhanskaya, Reference Shemakhanskaya1989). The interaction of an REP DD with air results in the formation of nitrogenous compounds containing-groups (NO and NH) and compounds containing-groups (OH). When the interaction is prolonged, nitric acid (HNO3) vapors are also formed (Lidin et al., Reference Lidin, Molochko and Andreeva2007). Generally, free electrons in the plasma channels accelerate in the gap and kinetic energy for these electrons provided by the REP DD with high over-voltage and high-power density is high enough for basic reactions such as excitation, ionization, and fragment of the gas species. The discharge provides chemical reactions resulting in the formation of Cu compounds colored blue or dark blue, such as
The copper nitrate Cu(NO3)2 is originally white; however, in the presence of water, it becomes blue crystalline hydrate (Shulepov, Reference Shulepov2004). In our previous work devoted to REP DD treatment of the stainless steel, the microhardness increased because of the increase in the scalar dislocation density and the formation of internal stress fields (Shulepov et al., Reference Shulepov, Erofeev, Ivanov, Oskomov and Tarasenko2015). Several factors may contribute to the appearance of the internal stress fields, such as dense discharge plasmas, a shock wave, optical radiations of different spectral regions, including UV, VUV, and X rays from the discharge plasma and a super-short avalanche electron beam with a wide energy range. Therefore, a combination of the above factors causes the increase of the microhardness of the Cu surface, as shown in Figures 10 and 14.
4. CONCLUSION
In this work, surface modification of Cu foil by REP DD sustained by nanosecond-pulse and sub-nanosecond pulse generator at atmospheric pressure is investigated. Our experimental results show that REP DD in both the repetitive and single modes could significantly change the mechanical properties and microstructure of the Cu surface. The higher the density of the diffuse discharge plasma treated the area, the better the modification effect of the Cu surface could be achieved. Using the experimental setup #1, the best modification results could be achieved as follows: the water contact angle decreased from 68° to 42.3°, the surface energy increased from 21.10 to 62.48 mJ/m2. In the nearest-surface layer, the carbon concentration decreased from 6.14 to 5.08%, the oxygen concentration increased from 3.09 to 6.07%, and the microhardness increased about 14%. Using the experimental setup #2, in the nearest-surface layer, the carbon concentration could decrease at most ~10 times and the oxygen concentration could increase to at most ~3 times after the modification. The maximal depth of oxidation could reach 118 nm from the surface. At a depth of 400 nm from the surface, the microhardness could be even 20% higher than that before the modification. To sum up, the modification by REP DD could enhance the hydrophilicity, improve the microhardness, clean the carbon contamination, and bring oxidation layer on the Cu surface.
ACKNOWLEDGEMENTS
The work on the experimental setup #1 was supported by the National Natural Science Foundation of China under Grants 51222701, 51477164, 51511130040, and the National Basic Research Program of China under Grant 2014CB239505-3. The work on the experimental setup #2 was performed in the framework of the Russian Science Foundation (the project #14-29-00052).