Introduction
General explosives, such as 1, 3, 5-trinitroperhydro-1, 3, 5-triazine (RDX), cyclotetramethylene tetranitramine (HMX), and 2, 4, 6-trinitrotoluene (TNT), are considered to be applicable to all weapons. However, ammunition loaded with these explosives may explode after accidental shock or impact, resulting in casualties (Madeira et al., Reference Madeira, Speet, Nieto, Abrell, Chorover, Sierra-Alvarez and Field2017; Todde et al., Reference Todde, Jha, Subramanian and Shukla2018). The synthesis and application of insensitive munitions explosives with high-energy density and low sensitivity attract the enthusiasm of researchers from various countries (Badgujar et al., Reference Badgujar, Talawar and Mahulikar2017; Pagoria et al., Reference Pagoria, Zhang, Zuckerman, Lee, Mitchell, DeHope, Gash, Coon and Gallagher2018; Viswanath et al., Reference Viswanath, Ghosh and Boddu2018). Much of the current research on energetic materials is focused on the development of insensitive compositions to avoid the occurrence of inadvertent detonations (Hunter et al., Reference Hunter, Coster, Davidson, Millar, Parker, Marshall, Smith, Morrison and Pulham2015). 1, 1-diamino-2, 2-dinitroethylene (FOX-7, C2H4N4O4, m/z = 148) is a novel high-energy and low sensitivity energetic compound (Gao et al., Reference Gao, Wu, Huang, Wang, Qiao, Yang and Nie2014; Zeng and Bernstein, Reference Zeng and Bernstein2016; Wuxi et al., Reference Wuxi, Yu, Wei, Yunfei, Xuezhong, Bozhou, Wei and Qi-Long2018); the chemical structure of FOX-7 is shown in Figure 1. Compared with the presently most widely used and powerful energetic compounds, excellent performances make FOX-7 be expected to be one of the potential candidate component in modern insensitive ammunitions and solid propellants in the future (Dreger et al., Reference Dreger, Tao and Gupta2016; Gao and Shreeve, Reference Gao and Shreeve2016; Zhang et al., Reference Zhang, Sun, Xu, Song and Zhao2016).
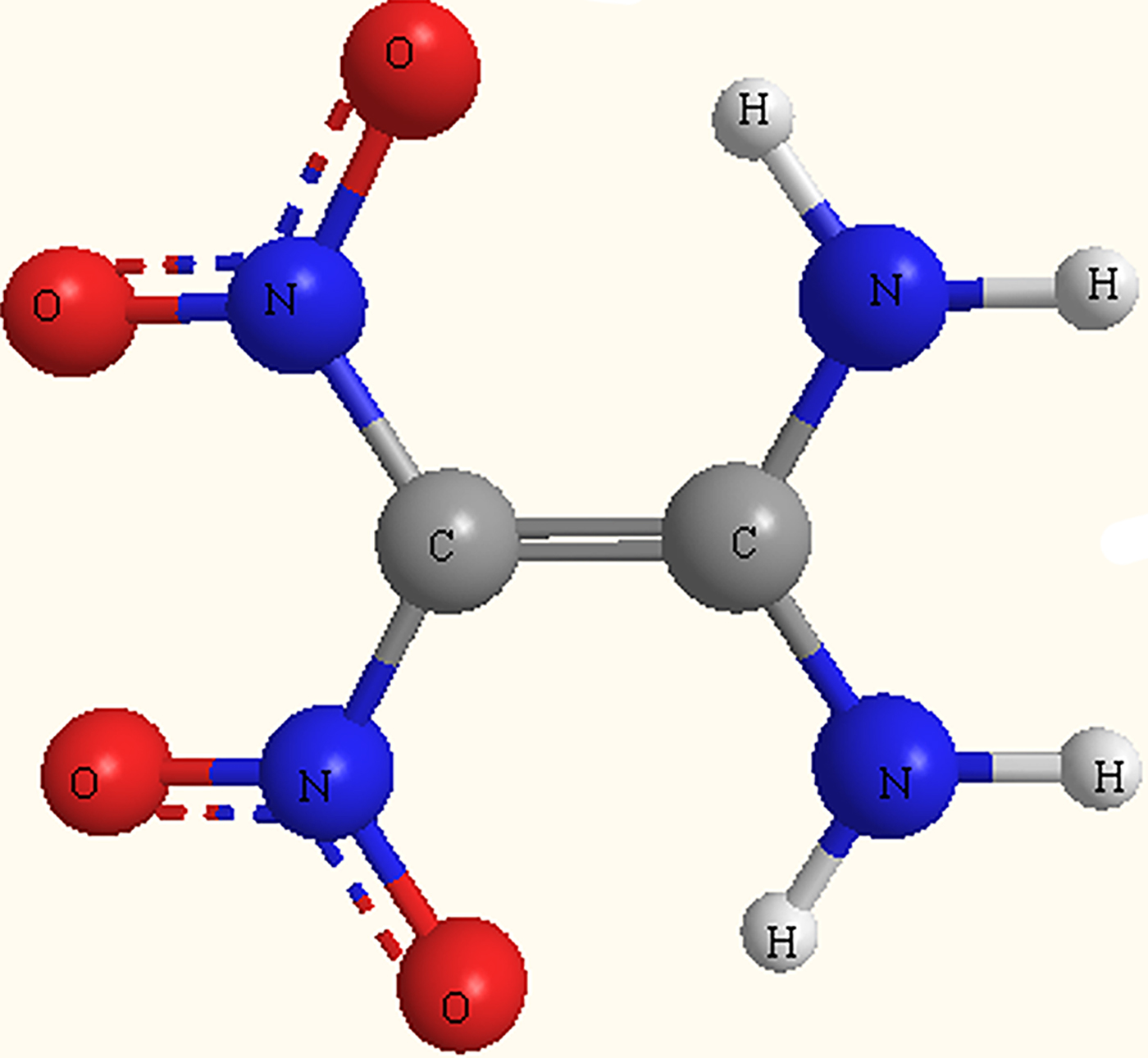
Fig. 1. Molecular structure of FOX-7.
Laser initiation is one of the most promising methods in initiating combustion, deflagration, or detonation of energetic materials because of its several advantages, such as precisely timed release, high safety, multipoint ignition, and more efficient energy coupling, relatively insensitive energetic materials can be ignited directly by laser irradiation (Myers et al., Reference Myers, Brown, Chavez, Scharff and Veauthier2017; Szimhardt et al., Reference Szimhardt, Wurzenberger, Beringer, Daumann and Stierstorfer2017; Zhang et al., Reference Zhang, Shen, Ye, Wu, Zhu and Hu2017; Yan et al., Reference Yan, Liu, Jiang, Xie, Zhang, Wang, Zhou, Yang, Xiang, Li, Liao, Wang, Li, Tan, Huang, Yang, Li, Li, Li, Yuan and Zu2018). Although laser initiation of different energetic materials has been extensively investigated experimentally, the initiation mechanism is still not completely understood. The need to reveal the dissociation mechanisms of FOX-7 under laser irradiation is essential in order to develop safer and more reliable initiation system (Gottfried, Reference Gottfried2012; Civiš et al., Reference Civiš, Civiš, Sovová, Dryahina, Kubišta, Skřehot, Španěl and Kyncl2016; Dang et al., Reference Dang, Gottfried and De Lucia2017).
Few researches have been conducted on laser-induced initiation performance and mechanism of FOX-7. Fang and McLuckie (Reference Fang and McLuckie2015) ignited carbon black-doped FOX-7 by a low-power diode laser of continuous wave at the wavelength of 974 nm. The threshold ignition power was found to be 10 W and the minimum delay times of ignition could be achieved as 0.07 and 9 ms, respectively, at a laser power of 40 W. Laser beam width or power density had some effects on the ignition delay, and a smaller beam width allowed shorter ignition delay. Laser ignition reliability could be affected by the inhomogeneity of the FOX-7 sample (Fang and McLuckie, Reference Fang and McLuckie2015). Atomic spectrometry was considered to be an effective method for detecting laser-induced fragments (Carter et al., Reference Carter, Fisher, Goodall, Hinds, Lancaster and Shore2011; Zhang et al., Reference Zhang, Ma, Shen, Wu, Ye, Hu and Zhu2014). The combined experiments using laser-induced breakdown spectroscopy and selected ion flow tube mass spectrometry have been conducted to investigate the decomposition mechanism of FOX-7 under 193 nm ArF excimer laser ablation. NO, NO2, HCN, HONO, HCHO, CH3CH2OH, and C2H2 fragments and CN (388 nm), OH (308.4 nm), and NO (237.1 nm) radicals and C, N, and H atomic lines were identified (Civiš et al., Reference Civiš, Civiš, Sovová, Dryahina, Španěl and Kyncl2011).
The dissociation of FOX-7 laser irradiation has not been specifically addressed. Time-of-flight mass spectroscopy (TOFMS) is a promising method for the analysis of the transient dissociation products. In the present work, the fragmentation of FOX-7 induced by 532 nm laser pulses has been studied by using TOFMS. The TOFMS of both negative and positive ions were obtained. Detailed analysis of the relative abundance of different fragment species as a function of the incident laser fluence and delay time were presented. The possible laser-induced dissociation mechanisms of FOX-7 were purposed based on the distribution of fragments.
Experimental section
The basic physical and chemical processes involved in FOX-7 under laser irradiation are illustrated in Figure 2. The transient products consist of positive ions, negative ions, neutral molecules, clusters and particles mainly appear in the ablation and dissociation step which are of great significance to reveal the laser-induced reaction mechanism of FOX-7.
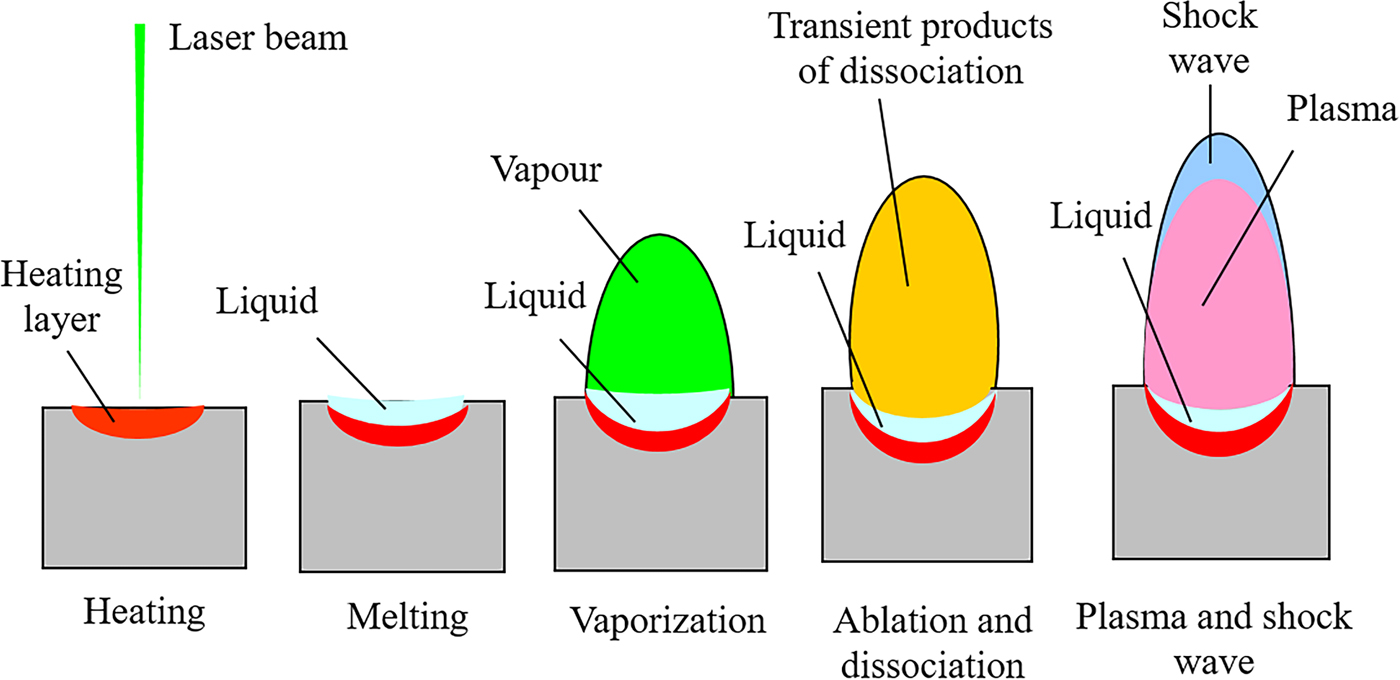
Fig. 2. Main steps of laser interaction with FOX-7.
A TOFMS were adopted to detect the ion products of FOX-7 under laser ablation, as shown in Figure 3, the experimental setup has been described in detail in our previous research (Zhang et al., Reference Zhang, Shen, Wu, Ye, Hu and Zhu2013), and only a brief overview is given in this section. The FOX-7 sample (synthesized by ourselves) was pressed into an aluminum holder by using a pressing tool with a pressure of 80 MPa to ensure a flat surface and a consistent filling density. The Nd:YAG 532 nm laser (LOTIS TII, LS-214, maximum energy of 480 mJ, pulse duration of 17 ns) was focused onto the solid FOX-7 target in a high-vacuum chamber (background pressure <4.0 × 10−4 Pa) by a 495 mm quartz lens to give a spot diameter of about 0.8 mm onto the sample surface. A total of two molecular pumps which connected with two mechanical pumps worked together to achieve the high vacuum. The generated ion fragments were accelerated by the electrodes and then reached the reflectron. After being reflected, the ions reached the micro-channel plates (MCPs) and were finally detected. The signals were recorded by an oscilloscope (Tektronix, DPO7140), which were accumulated 128 times to improve the signal-to-noise ratio. The measurement of beam fluence was realized by an energy meter (Ophir, Model 30A). The trigger sequences of laser beam, pulse high voltage, and oscilloscope were controlled and monitored via an adjustable time delay generator (GH024).

Fig. 3. Schematic representation of the experimental setup for the measurement of ablated ion fragments.
Results and discussion
Distribution of ionic fragments
Figure 4 shows the representative negative ion and positive ion TOFMS of various ion species in the FOX-7 plume generated by 532 nm laser with fluence of 11.5 J/cm2 and delay time of 80 µs. The ablation products with masses much lower than that of FOX-7 (m/z = 148) were detected, indicating that extended dissociation of the FOX-7 into smaller fragments occurred. For the negative mode, as shown in Figure 4(a), the strongest peak at m/z = 42 has two possible assignments: CNO and C2H4N. The second strongest can be found at m/z = 26, which may be assigned to CN. Similarly, the origin of the peak at m/z = 46 is most likely NO2. Another relative strong peak at m/z = 68 may be because of C2N2O fragment. In addition, two weak ion peaks were produced at m/z = 108 and 160; these peaks are probably attributable to NH2(NO2)2/NONO2(NH2)2 and (C2N4N4O4)C.

Fig. 4. TOF mass spectra for negative (a) and positive (b) ion species of FOX-7 generated by 532 nm laser ablation with fluence of 11.5 J/cm2 and delay time of 80 µs.
For the positive mode, as shown in Figure 4(b), some intense peaks caused by laser ablation of FOX-7 are readily observed. The peak with the highest intensity in this sequence of spectra is at m/z = 27 and corresponds to HCN; this indicates that HCN is a particularly stable species. Other relatively strong peaks appear at m/z = 40 and 39, which can be assigned to C2NH2 and C2NH. Finally, some low-intensity peaks are observed at m/z = 17, m/z = 18 and m/z = 24; possible assignments for these peaks are NH3/OH, NH4/H2O, and C2, respectively. Kinetic energy distribution broadening of the species produced in the excitation process has some effect on the mass resolution of instrument; thus, it is difficult to distinguish fragments with similar mass-to-charge ratio.
Influence of different laser fluence on ion distribution
The entire mass spectra of the 532 nm laser-induced reaction products of FOX-7 under different laser fluence are given in Figure 5, which was taken with a time delay of 80 µs. As shown in the figures, the mass distribution changes drastically as the fluence increases. This fluence dependence displays threshold-like behavior, where emission appears to “turn on” as the fluence increases above an apparent threshold. The broad distribution of m/z = 26 and m/z = 42 peaks at 15.8 J/cm2 indicates high kinetic energy of the laser-ablated CN and CNO/C2H4N fragments. As the laser fluence drops, most of the ion peaks disappear because of the weak chemical reaction. The energy threshold is about 3.6 J/cm2; two relative weak peaks can be found at m/z = 26 in the negative mass spectra and at m/z = 40 in the positive mass spectra near the thresholds. Below the energy thresholds, it produces no presence of ion peaks in the mass spectra. Some new ion species appear and the intensity is also increasing along with the increasing of laser fluence, the intensity of the main peaks reach the maximum at 11.5 J/cm2, indicating that there is a difference in the chemical reaction of the FOX-7 sample at different laser energy densities.
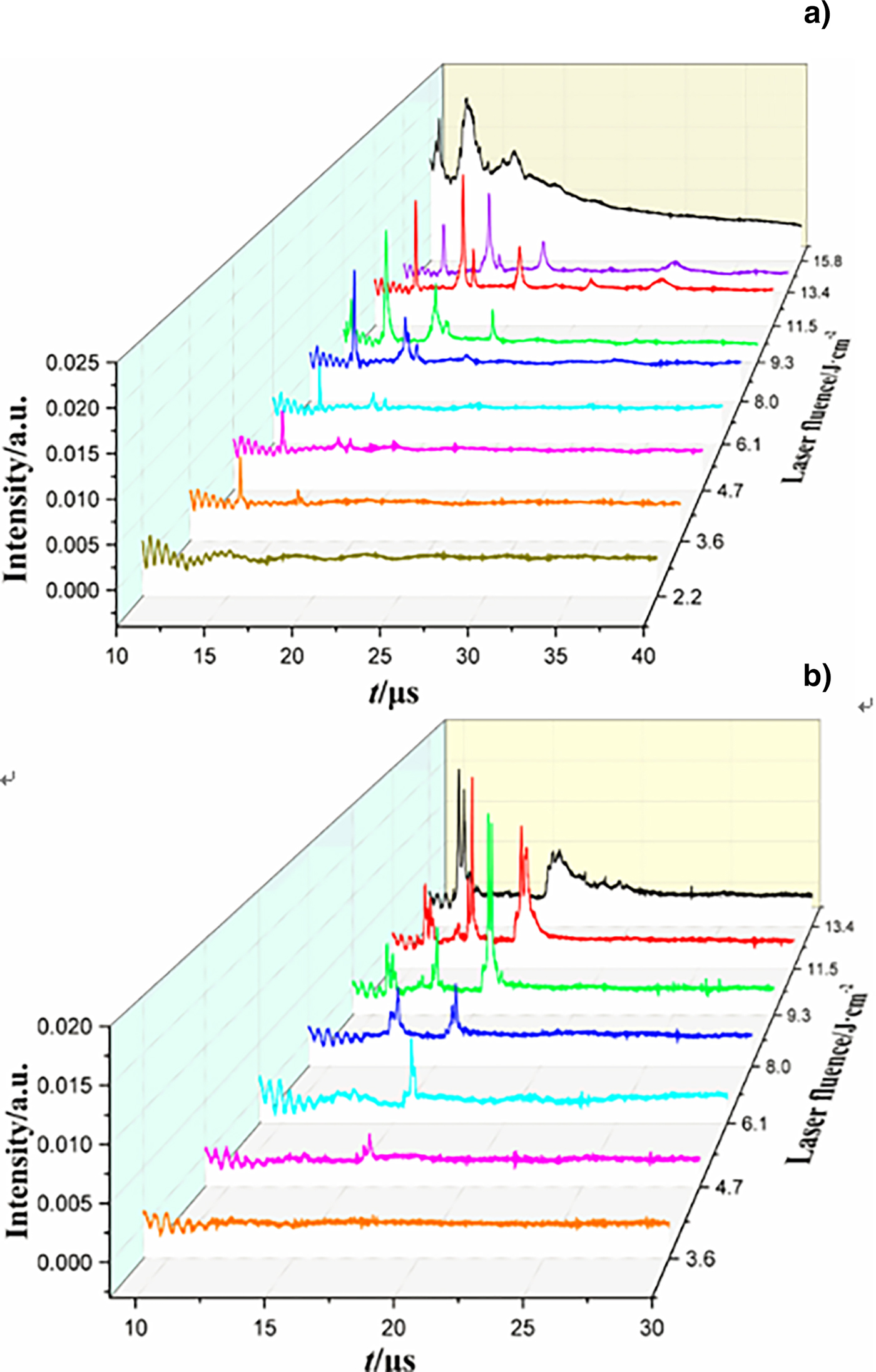
Fig. 5. TOF mass spectra for negative (a) and positive (b) ion species of FOX-7 generated by 532 nm ablation observed at different laser fluence with an extraction delay time of 80 µs.
Influence of delay time on ion distribution
Typical TOFMS of the negative and positive ion species observed for the 532 nm ablation of FOX-7 under different extraction delay time with the laser fluence of 11.5 J/cm2 are shown in Figure 6. Regarding to negative ions, at low delay time (55–65 µs), two dominant peaks appear at m/z = 42 and m/z = 68. Moreover, some relative weak peaks can be observed at m/z = 136, m/z = 160, and m/z = 224; these peaks decrease or disappear as the delay time increases, which indicate that larger fragments can easily decompose to small species, leading to the increase of dominant peaks at lower m/z. When the delay time is raised to 70 µs, a new peak can be found at m/z = 26 and the intensity of m/z = 68 has a sharp decrease; this change is an account of CN as a product of the decomposition of C2N2O/C2N2N3. As the delay time becomes 80 µs, the intensity of most ion peaks reaches the maximum. However, as the delay time increases further, the ion peaks broaden and the intensity decreases.
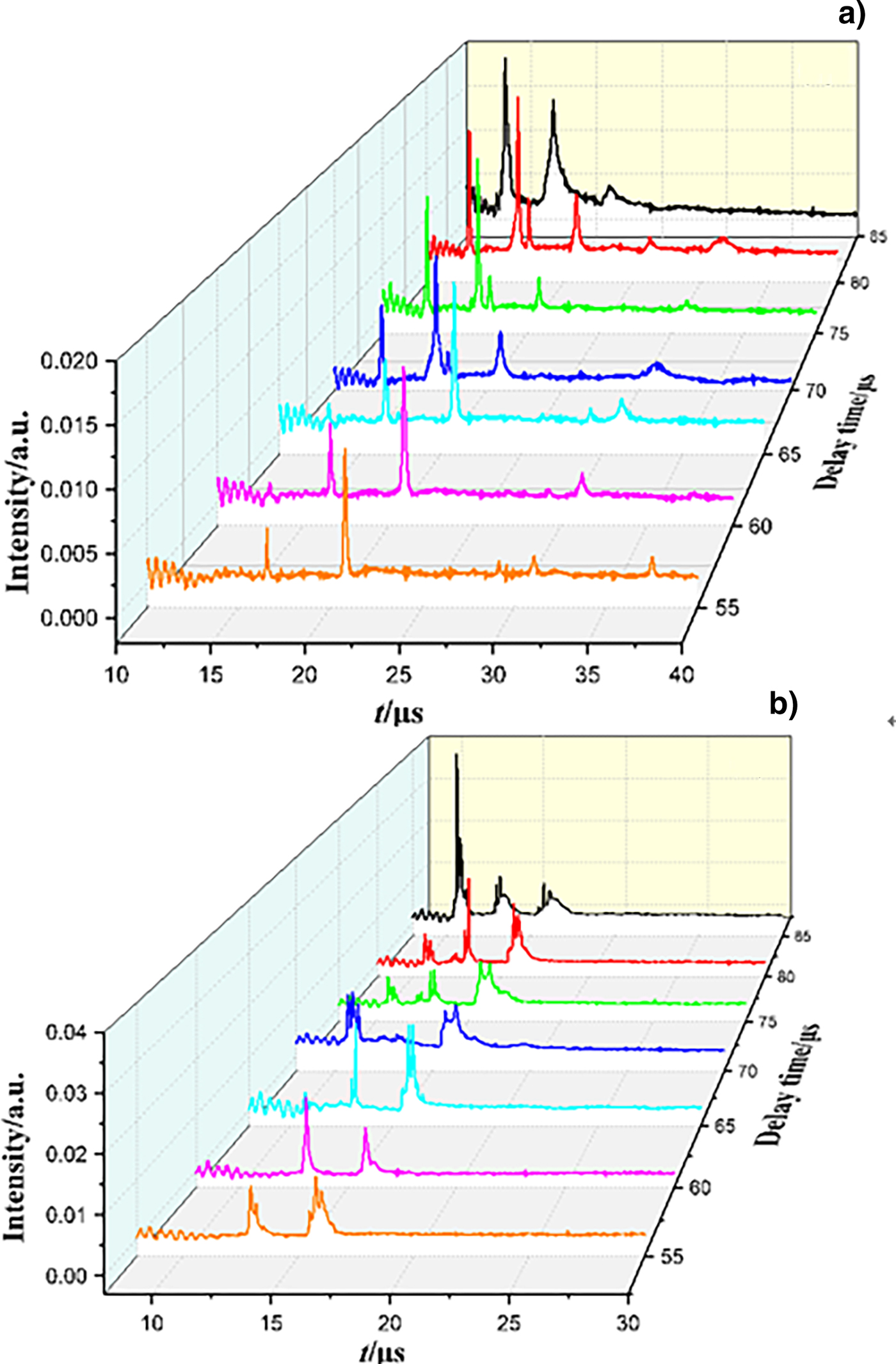
Fig. 6. TOF mass spectra for negative (a) and positive (b) ion species of FOX-7 generated by 532 nm ablation obtained with various delay times with the laser fluence of 11.5 J/cm2.
On the other hand, the diversity of the positive ion peaks is not as rich as the negative ions, as shown in Figure 6(b). At lower delay time (55–65 µs), only two peaks can be seen at m/z = 27 and m/z = 40, corresponding to HCN and C2NH2, and the intensity of these peaks increases slightly as the delay increases. Two new peaks are produced at m/z = 17 and m/z = 18 at 70 µs. As the delay time further increases, the ion peaks will broaden and overlap.
Possible dissociation reactions
Based on the observed ion fragments and the molecular structure of FOX-7, possible dissociation processes are proposed. The presence of NO2(m/z = 46) indicates that one possible decomposition mechanism is the C–NO2 bond breakage. Meanwhile, m/z = 24 (C2) can be explained by the rearrangement of FOX-7 after losing two carbon atoms. Similarity, the C2N2O(m/z = 68) fragment is the result of the rearrangement reaction; the H4N2O3(m/z = 80) may decompose to smaller species easily which cannot be found in the mass spectra. The formation of the m/z = 40 and m/z = 108 fragments observed in the mass spectra suggests that the dissociation of FOX-7 into C2NH2 ions and (NO2)2NH2 ions readily occurs in the ablation plume, as shown below:
The transient immediate products (NO2)2(NH2)2(m/z = 124) and C2H4N3O2(m/z = 102) are not stable and can easily lead to small fragments, which account for the (NH2)(NO2)2/NONO2(NH2)2(m/z = 108) and C2H4N(m/z = 42). Similarity, CNO(m/z = 42) and CN(m/z = 26) are the decomposition products of C2N2O(m/z = 68); C2(m/z = 24) and NH4(m/z = 18) are the decomposition products of C2H4N(m/z = 42). Furthermore, C2H4N(m/z = 42) can decompose into smaller fragments, as described below:
In addition, the fragments NH4/H2O(m/z = 18), HCN(m/z = 27), and C2NH2(m/z = 40) may also lose a hydrogen atom, ultimately forming NH3/OH(m/z = 17), CN(m/z = 26), and C2NH(m/z = 39). The peaks in the mass spectra indicate that the following reaction schemes may exist:
The m/z = 160 may be explained by the following multi-molecular rearrangement reaction, which means the FOX-7 molecular can obtain a carbon atom to form larger fragment:
Conclusions
To summarize, the laser-induced dissociation of solid FOX-7 at 532 nm has been investigated by TOFMS. Both the negative and positive ions mass spectra were recorded and the possible distributions of these ion fragments were confirmed. The major peaks can be ascribed to CN, CNO/C2H4N, NO2, C2N2O, HCN, and C2NH2. There is an energy threshold to trigger the chemical reactions which is about 3.6 J/cm2 in the current wavelength. On increasing the laser fluence, the degree of fragmentation increases and some new dissociation products are produced because of cleavage of the chemical bond and atomic rearrangement reaction. The relationship between ion intensity and delay time shows that the chemical reactions at different times after laser irradiation are different. On the basis of the observed spectral features, the possible dissociation mechanisms were proposed. The characterization of the laser-induced decomposition mechanism of FOX-7 may lead to an increased understanding of the relationship between the structure, sensitivity, and performance.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 11604149) and the Fundamental Research Funds for the Central Universities (No. 30918011315).








