Introduction
Research on light emission of gas mixture plasma created in the active zone of a nuclear reactor is of interest from the perspective of producing ionizing radiation detectors, devices of direct transformation of the energy of nuclear reactions into light, and also diagnostics of high-temperature plasma in thermonuclear reactors (Apruzzese et al., Reference Apruzzese, Apicella, Maddaluno, Mazzitelli and Viola2017). Kinetics of processes at the excitation of gases by electron and ion beams or products of nuclear reaction will be the same that allow modeling processes at nuclear pumping by using particle accelerators (Ulrich et al., Reference Ulrich, Busch, Krötz, Ribitzki, Wieser and Murnick1993). The advantage of the experiments at nuclear reactors is ability, due to the lack of separating foil, to conduct research at high temperature, including research with vapors of metals. Radiation heating of the construction of experimental device also facilitates the conditions of conducting experiments at high temperature.
Although such research was started approximately 40 years ago (Dmitriev et al., Reference Dmitriev, Il'yashenko, Mis'kevich and Salamakha1979; De Young and Weaver, Reference De Young and Weaver1980), the number of works performed at nuclear reactors is small. The luminescence spectra of mixtures of noble gases with mercury and cadmium upon excitation by products of 3He(n,p)3H nuclear reactions were investigated in Batyrbekov et al. (Reference Batyrbekov, Batyrbekov, Dolgikh, Khasenov, Rudoi, Soroka and Tleuzhanov1988) and Khasenov (Reference Khasenov2004). Mis'kevich (Reference Mis'kevich1991) investigated the emission spectra of 3He mixtures with cadmium and a series of other elements. Luminescent emission spectra of mixtures comprised of several kilopascals of Ar, Kr, or Xe and 100–106 kPa of helium-3 were studied in (Boody and Prelas, Reference Boody and Prelas1993); the authors concluded that there are no perceptible atomic lines in the Nd:YAG or Nd:glass absorption band in such mixtures. Luminescence spectra of noble gases and their binary mixtures in the ranges of 350–875 and 740–1100 nm upon excitation of uranium fission fragments were studied in Gorbunov et al. (Reference Gorbunov, Grigor'ev, Dovbysh, Mel'nikov, Sinitsyn, Sinjanskii and Tsvetkov2004) and Abramov et al. (Reference Abramov, Gorbunov, Melnikov, Mukhamatullin, Pikulev, Sinitsyn, Sinyanskii and Tsvetkov2006).
The goal of the present work was the research of capability of using lithium layers as the source of the excitation of gas medium in the core of the nuclear reactor. Lithium is more accessible material in comparison with helium-3 or uranium-235. At the same time, melting temperature of lithium is small (453.7 K) that can be the obstacle for using at high temperature. The luminescence spectra of noble gases and their mixtures with lithium vapors were investigated in the range of 300–970 nm upon the excitation by products of 6Li(n,α)3H nuclear reaction in the core of stationary nuclear reactor. Emission of noble gases’ atoms and heteronuclear ionic molecules can be used to remove energy from a nuclear reactor in the form of a light.
Experimental setup
The investigations were performed at IVG1.M nuclear reactor with a flux density of thermal neutrons of 0.87·1014 n/cm2s (Sadvakassova et al., Reference Sadvakassova, Tazhibayeva, Kenzhin, Zaurbekova, Kulsartov, Gordiyenko and Chikhray2011). Ionization and excitation of gaseous medium was produced via nuclear reaction products:
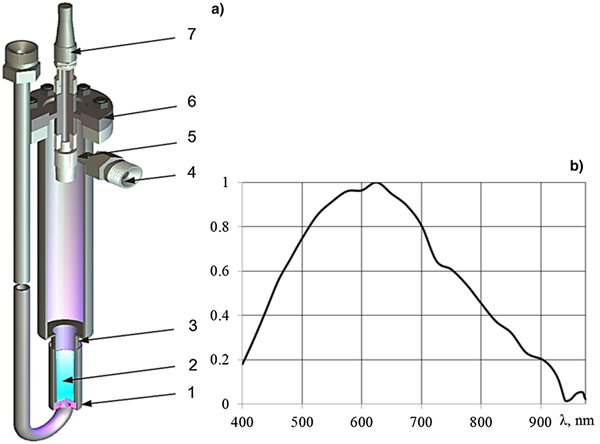
The scheme of the in-reactor ampoule device is shown in Figure 1a. The layer of lithium with a thickness of approximately 50 µm was deposited at a 150 mm length on the inner surface of a 12 mm-diameter pipe. Natural compound lithium (7.5% of 6Li) with impurity content <0.1% was used. The pipe, with a layer of lithium connected to the pipe of 20 mm inner diameter and 600 mm length, was sealed at the end with a flange with optical vacuum inlet. The ampoule device was placed in a dry experimental channel of the reactor; the pipe with lithium was positioned at the center of the reactor core, and the height of the core was 800 mm. The output of light radiation was observed via a collimator with quartz lens located inside the ampoule device and connected with a fiber by the optical vacuum inlet with an optical fiber. Light from the ampoule device was directed to the input of the QE65Pro (Ocean Optics) spectrometer through the 10 m-long fiber. The spectral sensitivity of the setup in the wavelength range 400–970 nm (Fig. 1b) was calibrated using a HL-2000CAL halogen lamp before uploading it into the reactor core. The intensities of the atomic lines and continuous spectra are given here in relative units, without correction for the spectral sensitivity of experimental facility.
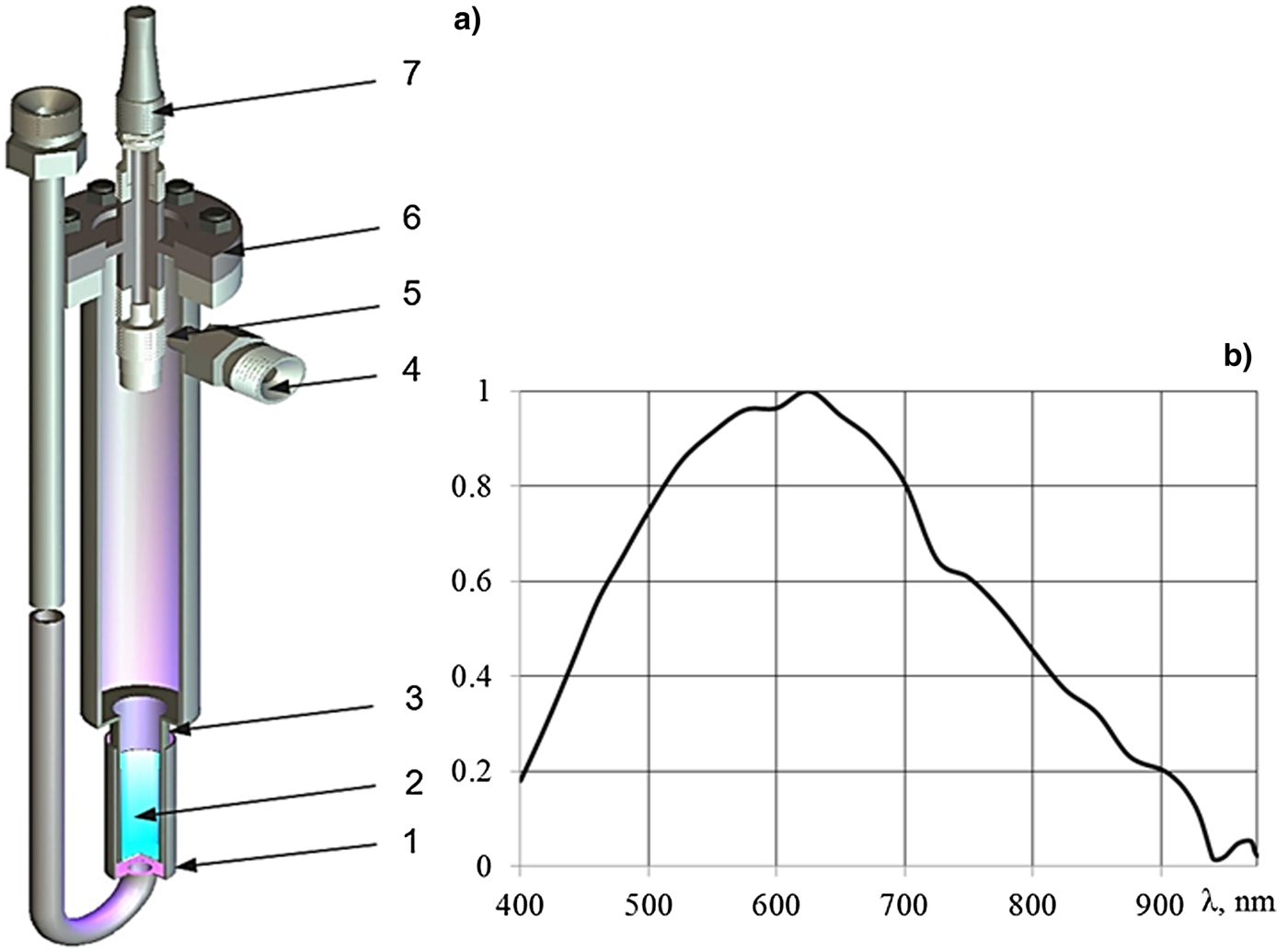
Fig. 1. Scheme of the ampoule device (a) and the relative spectral sensitivity of the apparatus (b). 1, cooling cover; 2, layer of lithium; 3, the body of the ampoule; 4, gas inlet and outlet connection; 5, collimator; 6, optical vacuum input; 7, fiber.
The measurements were conducted at a constant thermal power of the reactor of 6 MW. Heating of the ampoule device was implemented by means of radiation heating of the steel pipe and energy release in the lithium layer. The temperature of ampoule device was controlled by changing the flow rate of nitrogen gas in the external blowing of a tube with a layer of lithium. The temperature of the outer surface of the pipe with lithium was measured in the experiment. The volume of the ampoule device was degassed in the core of reactor at 410 K temperature and then flushed several times with the tested gas before filling; gases with content of impurities <0.001% were used. The vacuum system of “Liana” experimental stand (Sadvakassova et al., Reference Sadvakassova, Tazhibayeva, Kenzhin, Zaurbekova, Kulsartov, Gordiyenko and Chikhray2011) was used for pumping and filling of gas. To exclude of impurity lines of noble gases, the measurements were performed starting with high potential ionization gases. The temperature of 310 K was maintained during the measurements of noble gases’ emission, further increase in temperature to 523 K led to the appearance of lithium lines. Functioning at temperatures higher than melting point of lithium did not affect the reproducibility of the luminescence spectra during four cycles of the reactor, each with duration of 3 h. Molten lithium was retained on the walls due to wetting of the tube surface; lithium has a high value of surface tension (395 mN/m at 473 K) at low specific gravity. Coating of the tube surface is produced at vacuum, uniform layer of liquid lithium spreads over the entire surface both in vertical and horizontal planes.
Results and discussion
Noble gases
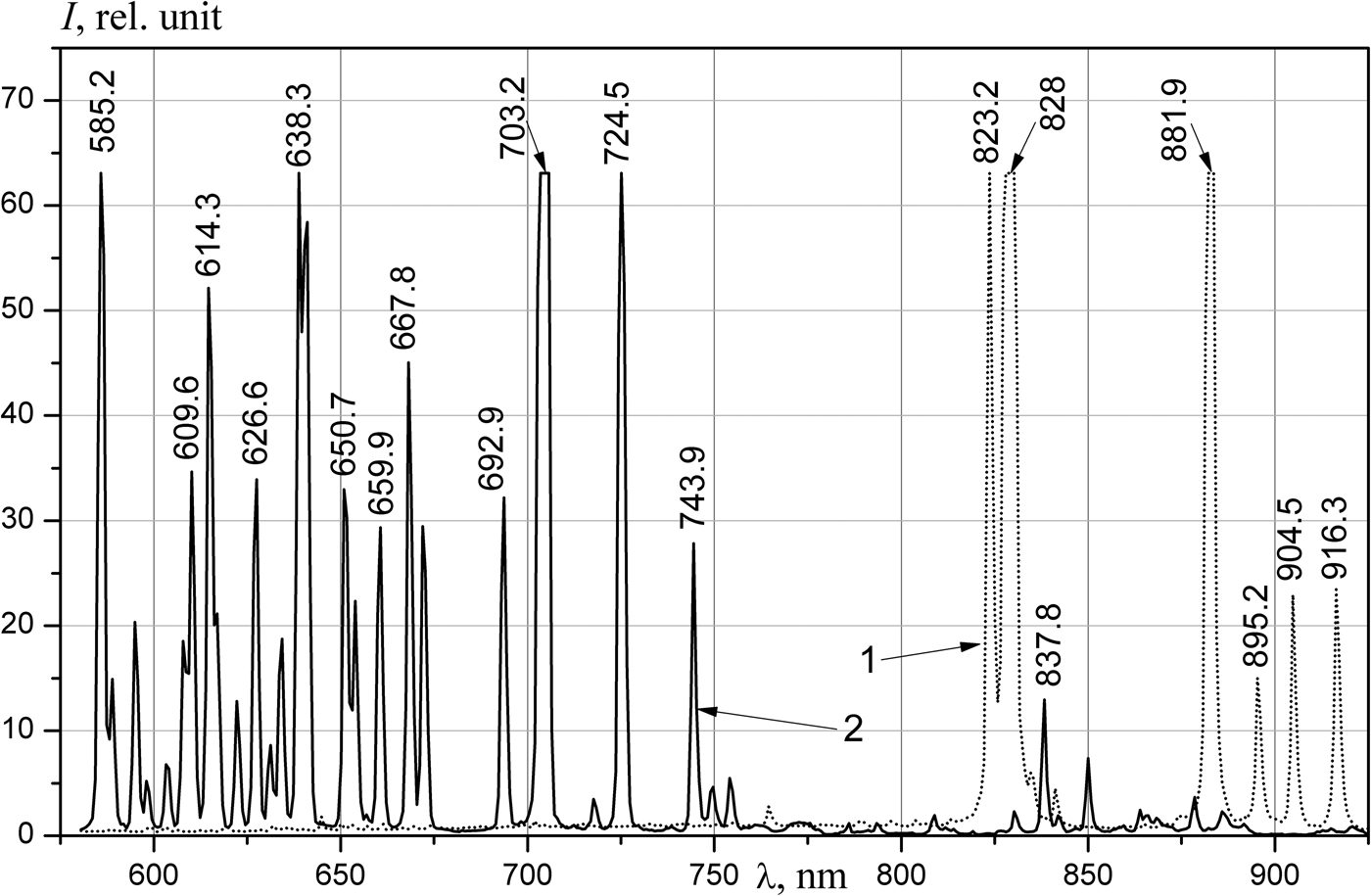
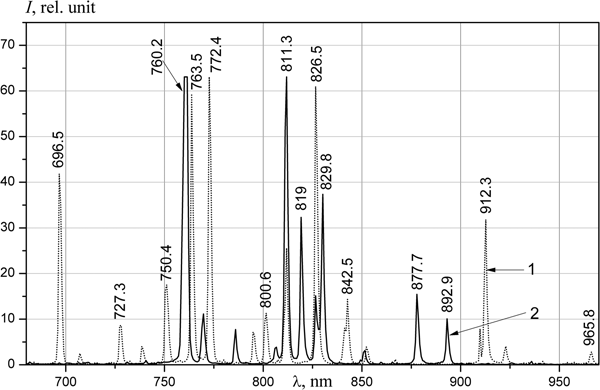
The spectra of the emission of noble gases (Figs 2 and 3) are similar to the measured spectrum obtained at the DC-60 heavy ion accelerator (Khasenov, Reference Khasenov2016). The relative intensities of lines near 725 nm slightly differ; these differences are related to absorption in the 10 m-long optical fiber. In helium, the lines of impurities of neon and the first negative nitrogen bands prevailed; weak helium lines were also present at 501.6, 706.5, and 728.1 nm. Lines of 3p–3s neon atom transitions prevail in neon, lines of 3d–3p transitions dominated in IR region, and the bands of first negative system of nitrogen (391, 428, and 470 nm) and weak bands of nitrogen molecule (337 and 358 nm) prevail in shortwave region. Only lines of the 4p–4s transitions of the argon atom were observed in argon. Notably, different from the results of Gorbunov et al. (Reference Gorbunov, Grigor'ev, Dovbysh, Mel'nikov, Sinitsyn, Sinjanskii and Tsvetkov2004) and Khasenov (Reference Khasenov2016), lines of atomic oxygen (777, 844, and 925 nm) were absent in helium, neon and argon. Only 5p–5s lines of krypton atomic transitions were in the krypton spectra; in addition to the 6p–6s transition lines, the 6d–6p transition line (951.3 nm) of xenon atom was also observed in xenon. The continuum of krypton and xenon in the UV-region was limited by the transmission of the fiber.
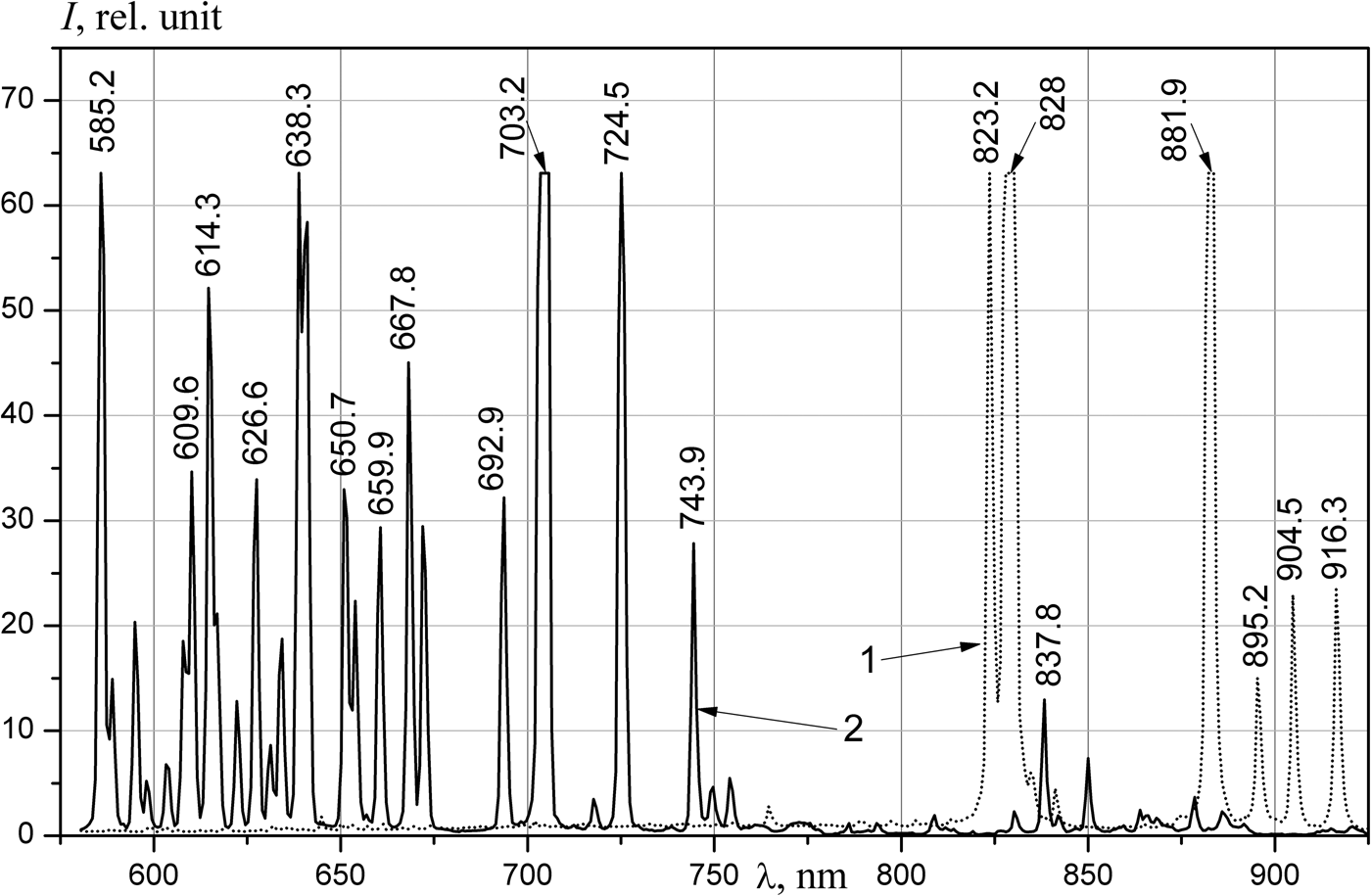
Fig. 2. The emission spectra of xenon at a pressure of 100 kPa (1) and neon at a pressure of 104 kPa (2), the integration time is 1 s. The intensity of the lines (rel. units): Ne 585.2 nm – 83, 638.3 nm – 67, 703.2 nm – 308, 724.5–110; Xe 823.2 nm – 75, 828 nm – 248, 881.9 nm – 155.

Fig. 3. The emission spectra of argon (1) and krypton (2) at a pressure of 101 kPa, the integration time is 1 s. The intensity of the lines (rel. units): Kr 760.2 nm – 106, 811.3 nm – 73; Ar 772.4 nm – 76.
Molecular bands, observed in the emission spectra of binary mixtures of noble gases upon excitation by an electron beam (Friedl, Reference Friedl1959; Kugler, Reference Kugler1964), were identified (Tanaka et al., Reference Tanaka, Yoshino and Freeman1975) as transitions between the states of heteronuclear ion molecules:
where X +Y molecular states asymptotically correspond to X + + Y states, and XY + correspond to X + Y + states; here, X, Y – atoms of noble gases, with Y being the heavier atom.
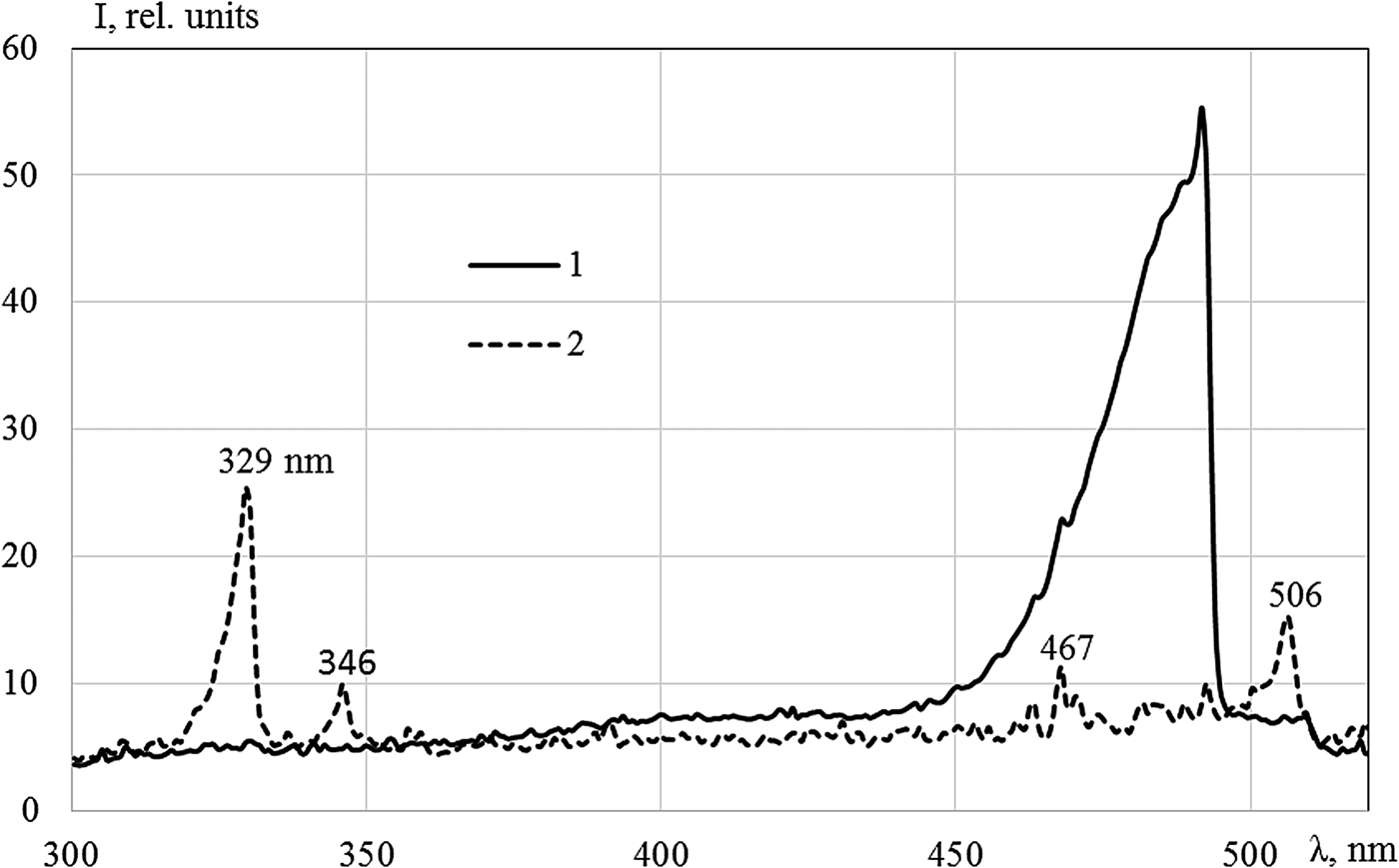
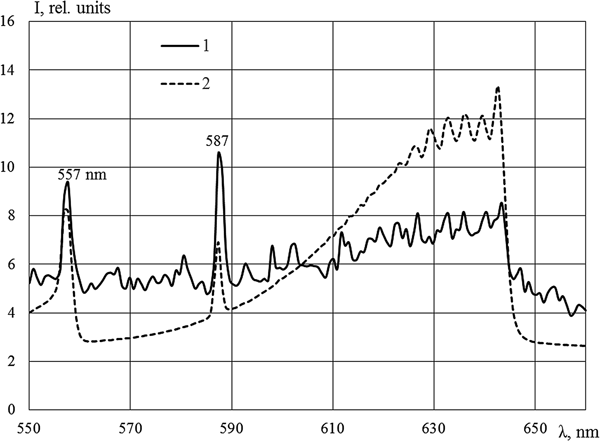
A powerful band with a maximum at the wavelength of 491 nm was observed in Kr–Xe (Fig. 4). Two mixtures were explored: Kr (50.5 kPa)–Xe (50.5 kPa) and Kr (1.07 kPa)–Xe (101 kPa). Only lines of xenon were presented in both mixtures; the intensities of lines were 10–12% higher in the mixture with small content of xenon. Considering the inequality in the magnitude of energy input in the mixtures and the measurements were conducted in different cycles of reactor, this difference is within the margin of error. The intensity of the 491 nm band in the mixture with 50% of xenon was slightly lower; this behavior is apparently caused by the long period of the measurements along with the large production of tritium in this case. As shown in Khasenov (Reference Khasenov2005), the isotopes of hydrogen effectively quench the level of Kr+Xe, from which the 491 nm band is emitted. The intensive radiation observed in the 491 nm band at 1% krypton content in mixture, with almost all energy of nuclear reaction products going to xenon, has yet to be explained (Khasenov, Reference Khasenov2005). Bands with 329 and 506 nm maxima, which are radiated from common Ar+Xe level, are observed in the Ar (50.5 kPa)–Xe (50.5 kPa) mixture (see Fig. 4). The intensity of the band with the maximum at 642 nm (in relation to atomic lines of krypton) in Ar–Kr is significantly less than that under the excitation by an argon ion beam at the DC-60 accelerator (Fig. 5).

Fig. 4. The emission spectra of the mixtures: Kr(1.07 kPa)–Xe (101 kPa) (1) and Ar (50.5 kPa)–Xe (50.5 kPa) (2), the integration time is 3 s (1) and 10 s (2). The line of xenon at 467 nm is indicated.
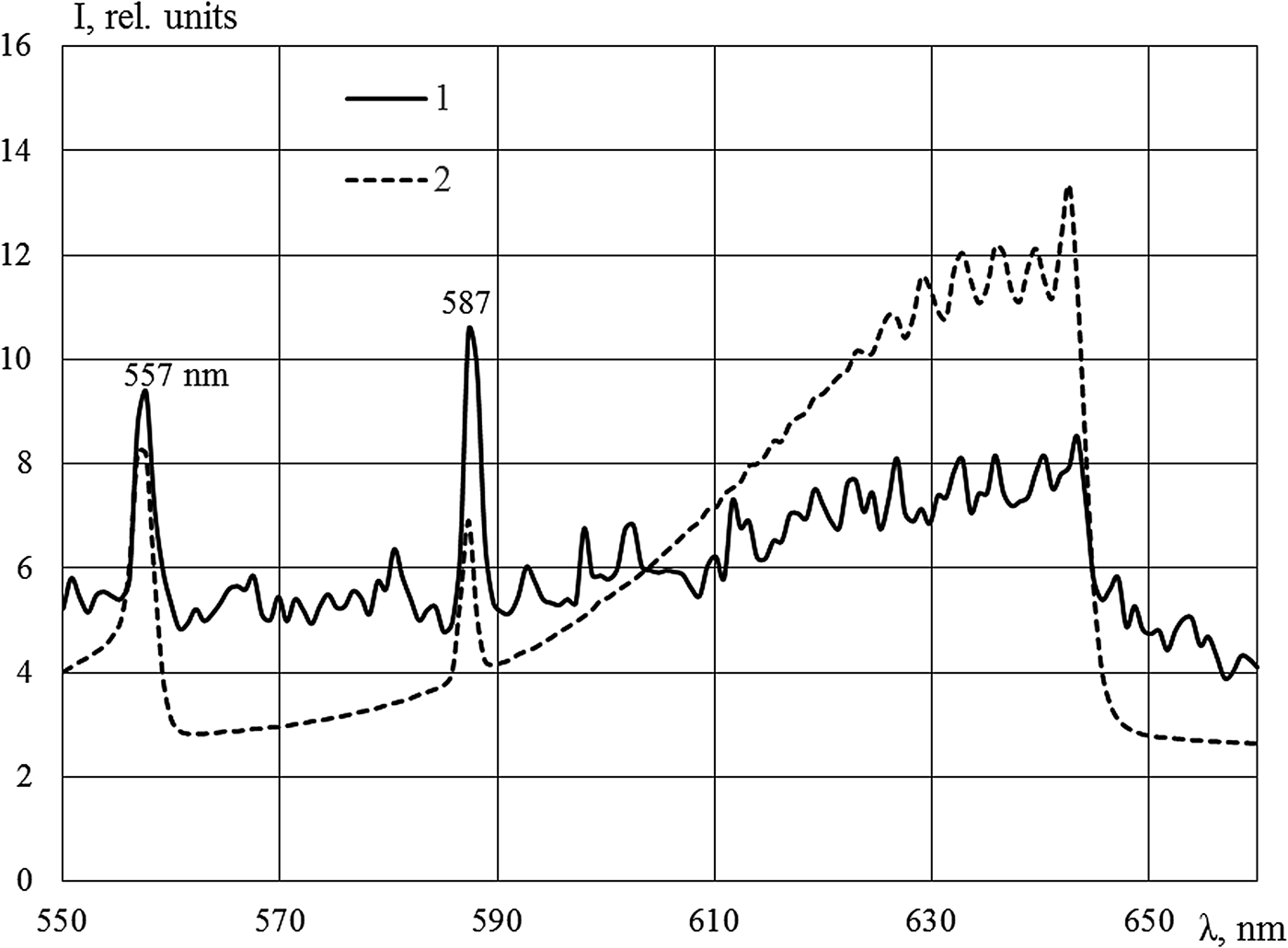
Fig. 5. The emission spectra of the Ar (50.5 kPa)–Kr (50.5 kPa) mixture (1) and the Ar (40.5 kPa)–Kr (40.5 kPa) mixture (2), the integration time is 10 s (1). Sources of pumping: IVG1.M reactor (1) and DC-60 accelerator (2). Krypton lines at 557 and 587 nm are indicated.
The bands of heteronuclear ion molecules described above are radiated from X +Y(IV(1/2)) levels, asymptotically corresponding to the states of atomic ions 2P1/2. The 663 nm band and the 705 nm band are absent in the Kr–Xe mixture and the Ar–Kr mixture, respectively, both starting from the levels corresponding to the 2P3/2 atomic state. The wavelengths of other possible transitions from these levels lie outside the region of spectral sensitivity of the installation (Tanaka et al., Reference Tanaka, Yoshino and Freeman1975). Week bands with maxima at 346, 349, and 545 nm, associated with transitions from Ar(2P3/2)+Xe, are present in the Ar–Xe mixture.
Mixtures of noble gases with lithium
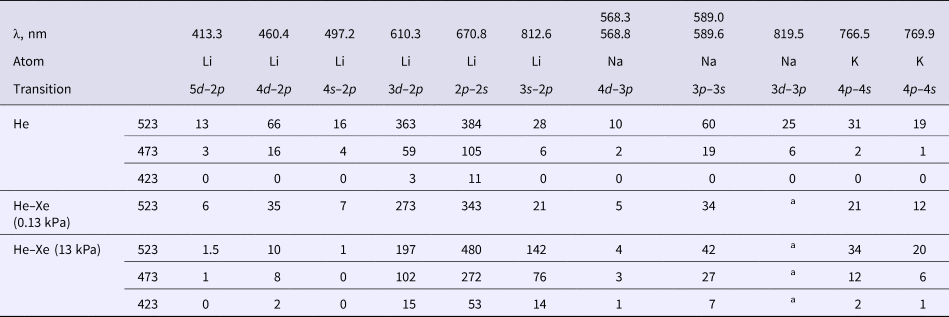
The atomic lines of alkali metals arising with the increase of ampoule temperature are given in Table 1. Along with lithium being illuminated, both sodium and potassium, contained in the form of impurities in lithium, are illuminated. The resonance lines at 670.8 nm were observed in the lithium spectrum, as well as transition lines from the 5d, 4d, 3d, 4s, and 3s levels to the resonance 2p level. The resonance line of lithium was slightly broadened with shading in the longwave side. Similarly, the resonance lines at 589.0 and 589.6 nm (they are also not resolved by spectrometer) and the transition lines from the 4d and 3d level to the 3p-level are present in the sodium spectrum. In the potassium spectrum, only 766.5 and 769.9 nm resonance lines had adequate intensity for registration. The intensities of xenon lines in He–Xe are given for comparison in Table 2. Considering the relative sensitivity of the installation (see Fig. 1b), the intensity of the lithium resonance lines in He–Xe (13 kPa) at 523 K is five times less than the intensity of 881.9 nm xenon line.
Table 1. Intensities (in relative units without correction on spectral sensitivity) of alkali metal lines in helium and He–Xe mixtures at full pressure of 100 kPa and different temperatures of the ampoule (423–523 K)

a The line was not resolved due to proximity to the 820.6 nm xenon line.
Table 2. Intensities (in relative units without correction on spectral sensitivity) of xenon lines in a He–Xe mixture at full pressure of 100 kPa and different temperatures of the ampoule (323–523 K)

The pressure of lithium-saturated vapors is 6.5 × 10−6 Pa at 523 K temperature and is <10−8 Pa at 423 K (Grigor'ev and Meilikhov, Reference Grigor'ev and Meilikhov1991). At such extremely low pressure of the vapor, effective transfer of excitation from ions and excited states of the buffer gas atoms to lithium atoms is impossible. Apparently, emission at lithium atom transitions is associated with sputtering of lithium under the effect of products of nuclear reaction. A fraction of the sputtered flux emerges from the solid surface in an electronically excited state and gives rise to the emission of light in a volume outside the surface (Thomas, Reference Thomas1979). The spectral compound of light emission during metal sputtering differs under the same conditions of excitation from the luminescence spectra of similar gaseous mixtures with vapors of the same metals that are formed by thermal evaporation. Beutler lines of CdII and ZnII ions and resonance lines of CdI and ZnI were anomalously highly excited under the sputtering of cadmium foil by 238Pu α-particles (Mis'kevich and Xiaolin, Reference Mis'kevich and Xiaolin2002) and sputtering of the zinc layer via products of the 3He(n,p)3H nuclear reaction (Miskevich and Tao, Reference Mis'kevich and Tao2008).
Cadmium and zinc lines intensity rapidly rose under the sputtering at temperature higher than 430 and 460 K, respectively. Mechanism of ejection of excited atoms from metal was studied in Mis'kevich and Xiaolin (Reference Mis'kevich and Xiaolin2002); temperature dependence of luminescence intensity is explained on the basis of the drop model. During braking in the metal nuclear particle knocks out some of the atoms of crystal lattice to interstitial sites, part of displaced atoms can be in excited state. Due to kinetic energy of a particle, fast heating and ejection of the microdroplet of heated metal occur. Displaced atoms diffuse inside the drop in the search of vacancies. At cadmium temperatures of 430–530 K, the coefficient of self-diffusion rapidly rises and this contributes to the ejection of displaced atoms from a drop.
It should be noted that temperature of the metal in drop will be near or higher than melting temperature and is weakly connected with the initial. Apparently, self-diffusion of displaced atoms from the base metal should be considered.
Ion lines are absent in the measured spectra of alkali metals. Lithium atoms in nd and ns states, the transitions from which the lithium resonance level is populated, are mainly created in a gas volume during the sputtering of lithium as well as the subsequent plasma chemical reactions. Considering the relative spectral sensitivity evaluation shows that the summary intensity of the transitions to the 2p-level is equal to the intensity of the resonance lines, the contribution of the direct population of the resonance level is not essential. The length of the path of α-particles with the energy of 2 MeV is 19 µm in lithium (Nemez and Hofman, Reference Nemez and Hofman1975); the frequency of nuclear reactions in such layer is 7.1·1011/cm2/s, and approximately 10% of the reaction products reach a surface of a layer. The power contributed to the gas is 50 mW/cm3 for helium and 100 mW/cm3 for the He (87 kPa)–Xe (13 kPa) mixture, corresponding to 7 × 1015/cm3/s rate of formation of electron–ion pairs for helium and 2·1016/cm3/s for the He–Xe mixture, respectively. To achieve comparable intensity of radiation of alkali metal and buffer gas atoms, it is necessary that one nuclear particle emerging from the layer produces ≈105 lithium ions and excited lithium atoms.
Apparently, the emission of ions and excited atoms from the surface occurs under the bombardment of not only nuclear particles but also ionized or excited particles of the buffer gas. If the intensity of lithium lines falls sharply at temperatures lower than 470 K in helium, then notable radiation below 420 K is observed in the He–Xe mixture. The intensity of the xenon lines in the He–Xe mixture monotonically decreases by 15–25% with temperature rising from 323 to 523 K (see Table 2). The decrease in the intensity of xenon lines with temperature is most likely associated with a decrease in the density of the gas in the excitation region and, accordingly, with a decrease in the energy deposited in the gas mixture. Another reason for the decrease in the intensity of xenon lines is the loss of energy of particles when sputtering lithium. An exception is the 828.0 nm line of the 6p[1/2]0–6s[3/2]1 transition, the intensity of which in this range of temperature decreases threefold. It is likely that this behavior is connected with the proximity of the 6p[1/2]0-level to the 5d-levels of xenon.
The processes of sodium lines radiation are similar to the processes in lithium, except the pressure of saturated vapors of sodium is 106 times higher that of lithium vapors at the same temperature. In sodium, d-levels of NaI are also populated, transitions from which populate the resonant 3p-levels. In potassium, only resonance lines of potassium atom are registered; the possible transitions from 5s and 3d-levels to 4p-level are outside the spectral range of installation sensitivity.
Population of atomic levels of alkali metals, apparently, occurs in processes of sputtering of lithium surface, as well as during recombination of alkali metal ions with electrons. Ions of alkali metals can be produced both under the sputtering of lithium layer and in plasma–chemical reactions in gas volume. Let us outline the most essential processes in He + Xe + M mixture (M – atom of alkali metal, N – third particle):
Under the volume (evaporation) mechanism of alkali metal excitation M + ions are produced in processes (1–9), and in Penning process:
Dimer formation process competing with (14):
The conditions of effective transfer of excitation to M atom under evaporation mechanism:
At the gas ionization rate S, the electron density: ![]() $n_{\rm e} = \sqrt {S/k_{13}} $. Substituting the characteristic values of the rate coefficients of processes (k 9 ≈ 5 × 10−10 cm3/s, k 13 ≈ 2 × 10−6 cm3/s, k 14~10−10 cm3/s, k 15 ≈ 5 × 10−32 cm6/s), and S = 2 × 1016/cm3/s we get: [M] > 5 × 1016/cm3 ~200 Pa for condition (16) and [M] > 4 × 1014/cm3 ~1 Pa for (17).
$n_{\rm e} = \sqrt {S/k_{13}} $. Substituting the characteristic values of the rate coefficients of processes (k 9 ≈ 5 × 10−10 cm3/s, k 13 ≈ 2 × 10−6 cm3/s, k 14~10−10 cm3/s, k 15 ≈ 5 × 10−32 cm6/s), and S = 2 × 1016/cm3/s we get: [M] > 5 × 1016/cm3 ~200 Pa for condition (16) and [M] > 4 × 1014/cm3 ~1 Pa for (17).
Vapors’ pressure of several Pascal is obtained in potassium at the temperature higher than 477 K, in sodium at 555 K. Thus, contribution of evaporation mechanism can be noticeable in potassium and, to a less degree in sodium. Apparently, sharper temperature dependence of the intensity lines of potassium in comparison with lithium and sodium (see Table 1) connected with predominance of evaporating mechanism of emission, content of potassium in lithium was small (~5 × 10−3%) for sputtering mechanism.
Conclusions
Products of the nuclear reaction 6Li(n,α)3H were used for excitation of gas mixtures in the core of a nuclear reactor, instead of the fission fragments of uranium, products of 3He(n,p)3H and 10B(n,α)7Li nuclear reactions widely used in earlier studies. Lines of alkali metal atoms emerge in the emission spectra with the increase of the gas mixture temperature. The presence of these lines in the spectra is connected with sputtering of the lithium layer via products of the nuclear reaction as well as with bombardment of the lithium layer via ionized and excited buffer gas particles. Lithium layers can be used to excite and ionize gas mixtures in the core of a nuclear reactor at temperatures up to ~400 K. At higher temperatures, part of the energy of ions and excited atoms of the gas plasma will be spent on sputtering of lithium.
The radiation spectra of noble gases and Ar–Xe, Ar–Kr, and Kr–Xe mixtures excited by products of a nuclear reaction in the core of stationary nuclear reactor were investigated. The luminescence spectra of noble gases were found to be similar to that obtained under the excitation by ion beam at the DC-60 heavy ion accelerator (Khasenov, Reference Khasenov2016), with np–ns transition lines predominant in the spectra, where n = 3, 4, 5, 6 for neon, argon, krypton, xenon, respectively. A powerful molecular band with maximum at 491 nm was found to be present in Kr–Xe, and a weak band with maximum at 642 nm was found in Ar–Kr. Bands at 329 and 506 nm were observed in Ar–Xe, week bands at 346, 349, and 545 nm are also present in this mix. The 491 nm band in Kr–Xe is of interest from the point of view of development of ionizing radiation detectors as well as outputting of light energy from a nuclear reactor core.
Author ORCIDs
M. U. Khasenov, 0000-0001-5823-2785
Acknowledgements
The work was performed within the Programs: “Development of nuclear energy in Kazakhstan” and “NU-Berkeley Program: 2014–2018 Strategic Research Program on critical state of substance, non-conventional materials and energy sources”.