1. INTRODUCTION
Compared with the traditional mechanical initiation or electric initiation, laser initiation of explosives has the characteristics of high safety and high reliability (Myers et al., Reference Myers, Bjorgaard, Brown, Chavez, Hanson, Scharff, Tretiak and Veauthier2016). In the past decades, research on laser initiation of energetic materials has been carried out world widely, including experimental (Ahmad et al., Reference Ahmad, Russell and Golding2009; Aluker et al., Reference Aluker, Belokurov, Krechetov, Mitrofanov and Nurmukhametov2010; Damm & Maiorov, Reference Damm and Maiorov2010; Abdulazeem et al., Reference Abdulazeem, Alhasan and Abdulrahmann2011; Zhang et al., Reference Zhang, Shen, Ye, Wu, Hu and Zhu2014, Reference Zhang, Shen, Ye, Wu, Hu and Zhu2015) and theoretical studies (Babushok et al., Reference Babushok, DeLucia, Dagdigian, Gottfried, Munson, Nusca and Miziolek2007; Cohen et al., Reference Cohen, Zeiri, Wurzberg and Kosloff2007; Lee et al., Reference Lee, Kim and Yoh2008; Civiš et al., Reference Civiš, Civiš, Sovová, Dryahina, Španěl and Kyncl2011; Aluker et al., Reference Aluker, Krechetov, Mitrofanov, Zverev and Kuklja2012). Up to present, there were two explanations for the mechanism of laser initiation: Thermochemical mechanism (Bourne, Reference Bourne2001; Shui et al., Reference Shui, Sun, Zhao, Cheng, Xiong, Wu, Fan, Yu, Yan, Yang, Gu, Zhong and Xu2013), and photochemical mechanism (Kuklja et al., Reference Kuklja, Aduev, Aluker, Krasheninin, Krechetov and Mitrofanov2001; Kuklja, Reference Kuklja2003; Bhattacharya et al., Reference Bhattacharya, Guo and Bernstein2010, Reference Bhattacharya, Guo and Bernstein2012; Aluker et al., Reference Aluker, Aluker, Krechetov, Mitrofanov, Nurmukhametov and Shvaiko2011).
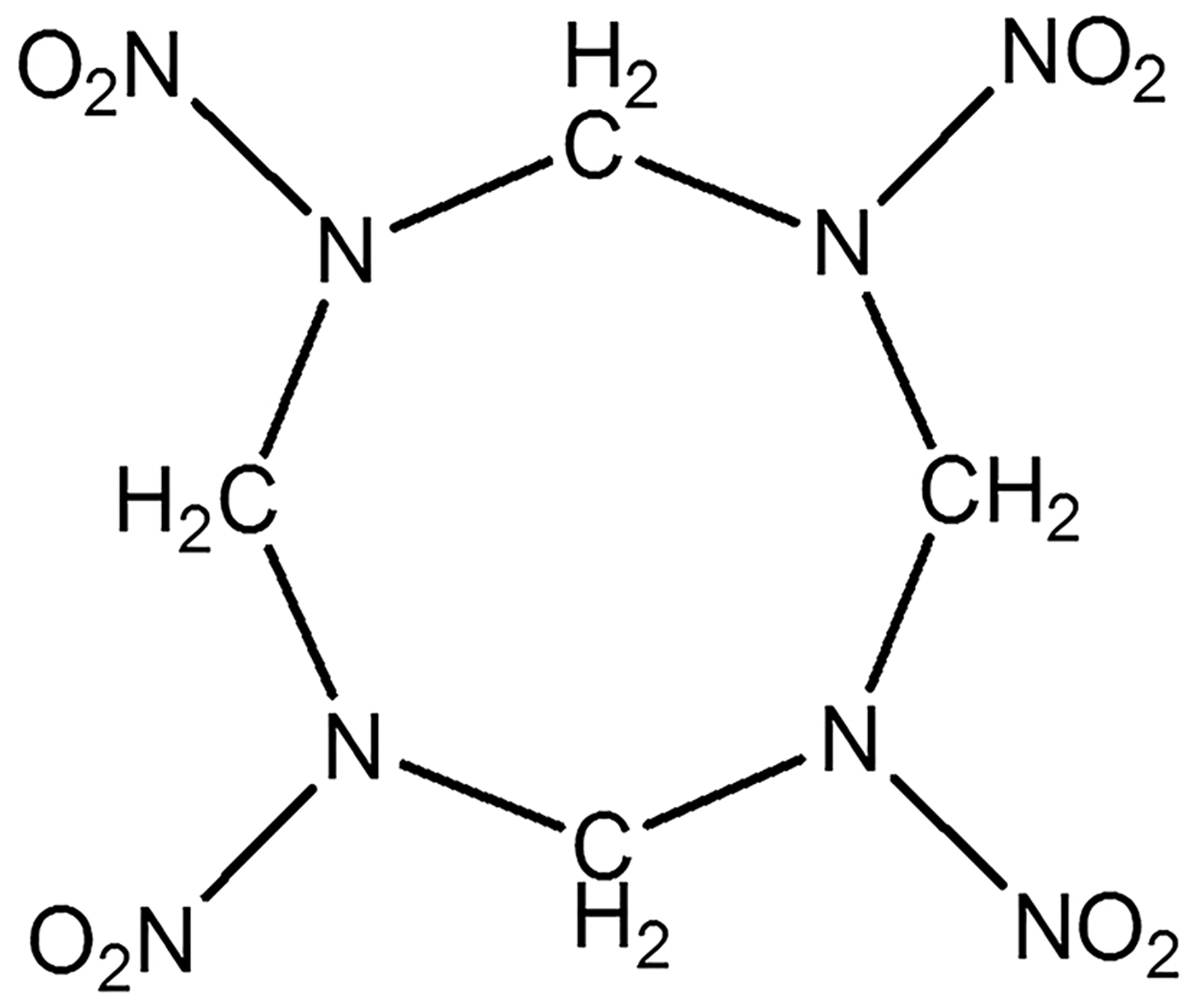
Cyclotetramethylene tetranitramine (HMX) is a powerful and relatively insensitive nitroamine high explosive, which is commonly used in military (Pravica et al., Reference Pravica, Galley, Kim, Weck and Liu2010; Sharia et al., Reference Sharia, Tsyshevsky and Kuklja2013). Typical structure of HMX is shown in Figure 1.

Fig. 1. Typical molecular structure of HMX.
A few number of studies were conducted to establish the numerical model of laser initiation of HMX (Dik et al., Reference Dik, Sazhenova and Selikhovkin1991; Meredith et al., Reference Meredith, Gross and Beckstead2015). CO2 laser ignition experiments of HMX were performed by Ali et al. (Reference Ali, Son, Asay, Decroix and Brewster2003) to illustrate the reactive mechanism during deflagration-to-detonation transitions. Laser-assisted combustion of HMX at heat fluxes of 100 and 300 W/cm2 was investigated, the flame and surface structures were observed by using a high-magnification video system, the major species at the surface were H2O, CH2O, HCN, NO2, N2O, N2, CO, and NO (Tang et al., Reference Tang, Lee, Kudva and Litzinger1999). Greenfield employed femtosecond laser pump–probe experiments at three wavelengths (226, 228, and 230 nm) to investigate the mechanisms and dynamics of the photodissociation of HMX, the results indicated that the decomposition dynamics of HMX was in the time scale of the femtosecond laser pulse duration (180 fs) (Greenfield et al., Reference Greenfield, Guo and Bernstein2006). Sunku et al. (Reference Sunku, Gundawar, Myakalwar, Kiran, Tewari and Rao2013) reported laser induced breakdown spectroscopy spectra of HMX by using ns and fs pulses, C, CN peaks, and other atomic peaks were recorded.
Fundamental understanding of mechanisms of interaction between laser beam and materials is far from complete (Yazdani et al., Reference Yazdani, Cang, Sadighi-Bonabi, Hora and Osman2009), and ways to control chemical reactions in energetic materials with laser excitation are yet to be established (Aluker et al., Reference Aluker, Krechetov, Mitrofanov, Zverev and Kuklja2012; Wang et al., Reference Wang, Tsyshevsky, Zverev, Mitrofanov and Kuklja2017). To gain a better understanding of the various reactive transient species and intermediates and the processes involved in ion formation and occurrence after 1064 nm laser irradiation, herein, we present some of our experimental results of HMX by using time-of-flight mass spectroscopy (TOFMS). A pulse laser at 1064 nm was used to ablate the solid HMX sample, mass spectra of both the negative and positive ions were recorded.
2. EXPERIMENTAL SECTION
The schematic of homemade reflection TOFMS has been given in previous publication (Zhang et al., Reference Zhang, Shen, Wu, Ye, Hu and Zhu2013). The ablation was produced by a Nd:YAG solid-state laser source (LOTIS TII, LS-2147, Belarus) delivering pulses of 15 ns with energy up to 800 mJ at the wavelength of 1064 nm. The laser beam was focused onto the sample surface by using a focus lens. The HMX sample (about 50 mg) was compressed in a small aluminum cylindrical cup with 80 MPa pressure. The spectrometer consists of three basic regions: The ion source (also known as the extraction/acceleration region), the flight tube (also known as the field-free, or drift region), reflector, and the detector. The ion source is the region where ions are either introduced to the spectrometer or created. The flight tube is the region where ions are directed toward the detector and, most importantly, where mass separation takes place. Ions are accelerated out of the ion source towards the flight tube. The detector is a pair of microchannel plates (MCPs). The ions entering the extraction field were accelerated and travelled into the field-free tube, after being reflected by the reflector, these ions were finally detected by MCPs. A digital storage oscilloscope synchronized with the laser pulse and the TOFMS system recorded the collected mass spectra. Irradiation of the sample was performed in a vacuum chamber at a pressure of about 4 × 10−4 Pa. Laser energy was measured with an energy meter (Ophir, Model 30A, Israel).
3. RESULTS AND DISCUSSION
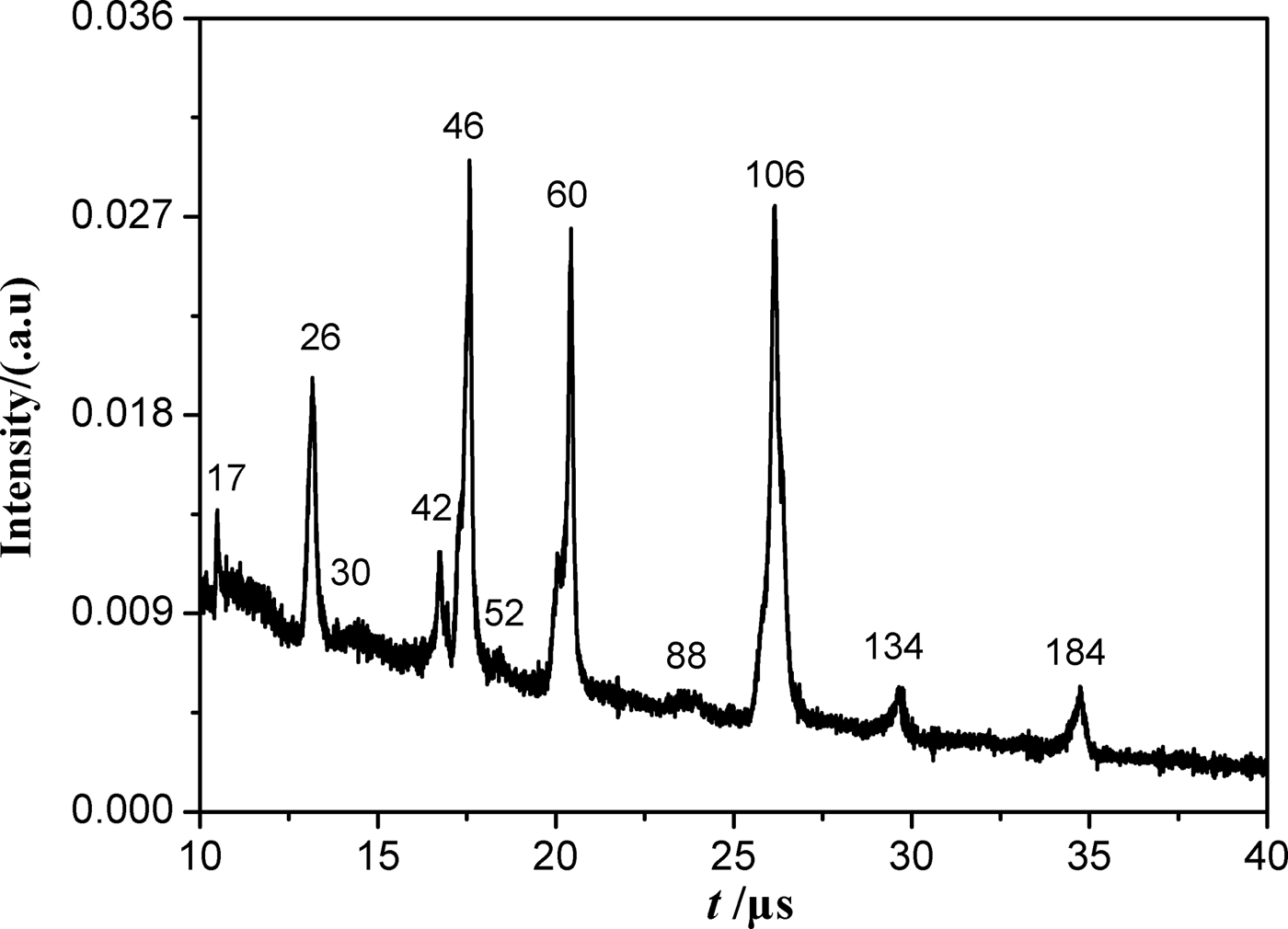
For the negative ions (Fig. 2), three strong peaks appear at mass charge ratio (m/z) of 46, 60, and 106, according to the molecular structure and decomposition characteristics of HMX, these ions can be corresponded to NO2, CH2NO2/N2O2, and N(NO2)2/CH2(NO2)2, respectively. It may be pointed out that CH2NO2/N2O2, as well as N(NO2)2/CH2(NO2)2, cannot be clearly distinguished due to the limited mass resolution of the spectrometer. This limited mass resolution is caused mainly by the large broadening of the kinetic energy distributions of the species produced in the ablation process. A relative strong peak at m/z = 26 can be ascribed to CN. Some other peaks can be seen at m/z = 17, 30, 42, 52, 88, 134, and 184, which may be due to OH, NO/CH2O, CH2N2/C2H2O/CNO, C2N2, (CH2)2NNO2, CH2(NNO2)2, and CNN(CNNO2)2, respectively.

Fig. 2. Typical TOF mass spectrum of negative ions for HMX produced by 1064 nm laser ablation.
From the TOF mass spectrum of the positive ions (Fig. 3), it can be noticed that three series of peaks are recorded. The first series are observed at m/z = 18 and 19, corresponding to H2O and H3O. The second series appear at m/z = 24, 26, 27, 28, 29, 30, and 32, possible assignments for these peaks are C2, CN, HCN, CO/N2/H2CN, HCO, NO/CH2O and O2, respectively. Peaks at m/z values of 40, 42, 44, 45, 46, and 47 can be ascribed to CN2/C2H2N, CH2N2/C2H2O/CNO, N2O/CO2/CH2NO, HN2O, NO2, and HONO ions, respectively. Compared with our previous research, the mass spectra of HMX have certain similarities to that of cyclotrimethylenetrinitramine (RDX), this may be due to the similar molecular structure between RDX and HMX (Zhang et al., Reference Zhang, Shen, Ye, Wu, Hu and Zhu2014).

Fig. 3. Typical TOF mass spectrum of positive ions for HMX produced by 1064 nm laser ablation.
By varying the laser fluence, the energy dependence of the relative intensity for the primary atom lines was obtained, as shown in Figures 4 and 5. For the negative ions (Fig. 4), with the increasing of the laser fluence, the relative intensity of m/z = 42 gradually increased. Otherwise, the intensity of m/z = 106 showed the opposite tendency. The m/z = 42 particles perhaps produced by decomposing of m/z = 106. Moreover, the relative intensities of m/z = 26 and m/z = 60 also displayed an opposite tendency with the increasing of laser fluence, which indicated that CN may be product of CH2NO2 by losing two OH particles. The majority of particles lied in m/z = 46 under our experimental conditions, the relative intensity increased first and then decreased with the variation of laser fluence.

Fig. 4. Relative intensity of negative ions for HMX as a function of the laser fluence.

Fig. 5. Relative intensity of positive ions for HMX as a function of the laser fluence.
For the positive ions (Fig. 5), it was observed that the major positive ions appeared at m/z = 18 in the experimental fluence range, the relative intensity slightly decreased first, while the laser fluence was above 41.4 J/cm2; the proportion increased sharply, above 50% of the positive ions are H2O. The other positive ions could be found at m/z = 19, 27, 28, 30, and 40, the percentage of these ionic particles was under 15%. Most of the positive ions are not stable, they are easy to obtain the electron and change into neutral particles or negative ions.
According to the molecular structure, the main particles obtained by laser ablation of HMX can be considered to arise from three processes: HONO elimination, rupture of the N–NO2 bond, and concerted symmetric ring fission of HMX into four CH2N2O2. The possible steps were proposed on the basis of TOFMS results and the structure of HMX, which were similar to the initial steps of theoretical calculation results (Lewis et al., Reference Lewis, Glaesemann, VanOpdorp and Voth2000).
Figure 6 shows laser-induced HONO elimination pathways of HMX. HONO particles were produced through cleavage of C–H bond of the ring and the adjacent N–N bond. N–N bond of HMX molecule was gradually stretched, and a hydrogen atom of its adjacent CH2 got close to the oxygen atom of the nitro group, then transition state TS1 was formed, followed by the fracture of N–N and C–H bond, intermediate INT1 was produced by removing of a HONO (m/z = 47) fragment. The remaining nitro groups of INT1 would continue to experience a similar reaction to eliminate three HONO particles to generate intermediate INT4. Four C–N bonds of INT4 ring gradually extended to generate the transition state TS5, HCN (m/z = 27) particles were formed by cleavage of C–N bonds.

Fig. 6. Laser-induced HONO elimination pathways of HMX.
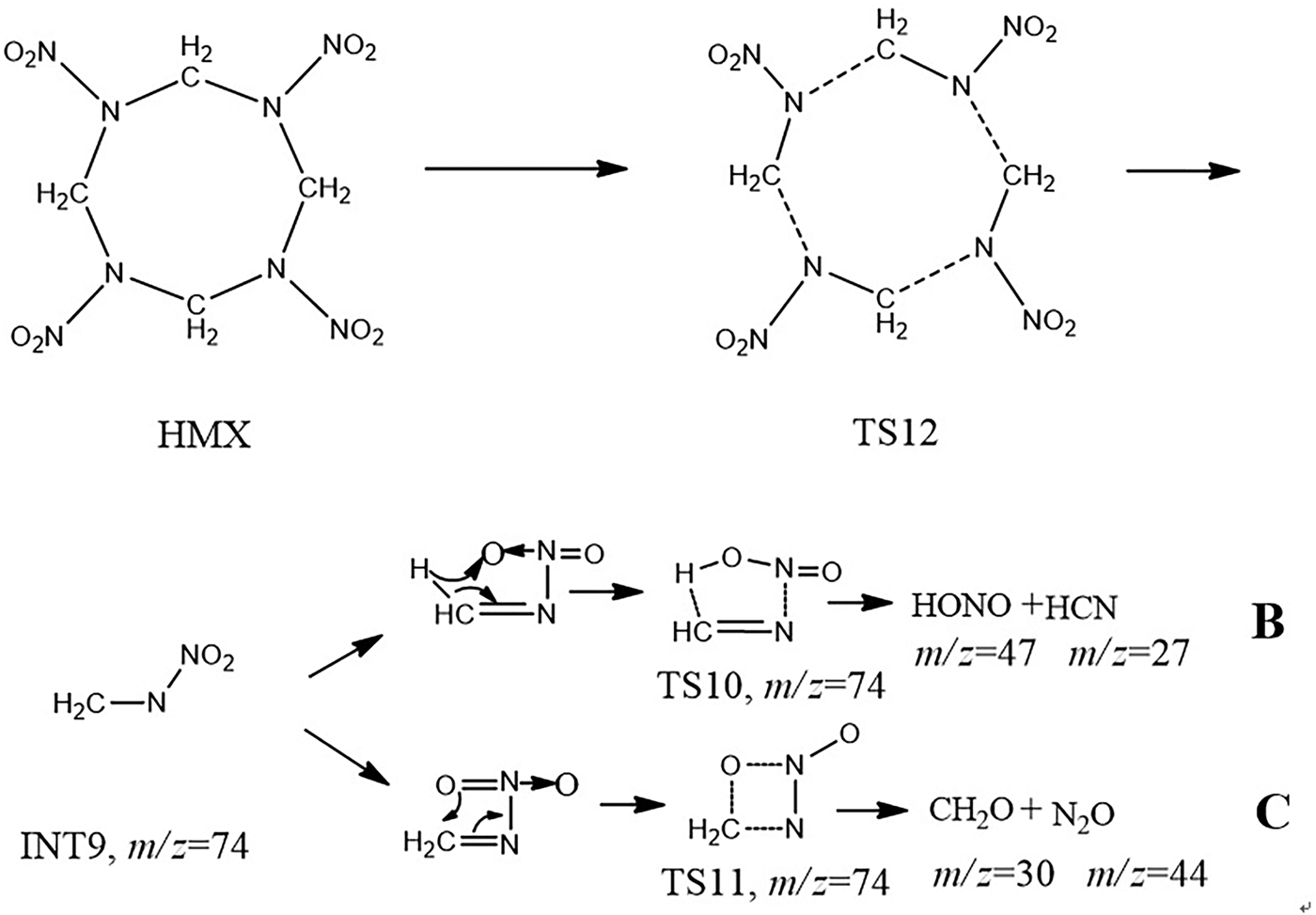
Figure 7 is laser-induced rupture of the N–NO2 bond pathways of HMX. Possible peak assignments, together with a mechanism of formation of small particles, were proposed as: Loss of a nitro group (m/z = 46) from HMX molecular led to intermediate INT5, through a subsequent elongation of C–N bond, intermediate INT6 was formed via transition state TS6. This intermediate chain gave fragment INT7 at m/z = 176 via elimination of intermediate INT9 though transition state TS7. Thus, the same fragmentation process led to intermediate INT8 at m/z = 102 producing by transition state TS8. INT7 arose from transition state TS9 through loss of CH2N fragment. INT9 could generate smaller particles through two different paths, HONO (m/z = 47) and HCN (m/z = 27) could be generated via the transition state TS10 (Path B), CH2O (m/z = 30) and N2O (m/z = 44) could also be generated via transition state TS11 (path C).

Fig. 7. Laser-induced rupture of the N–NO2 bond pathways of HMX.
Another possible laser-induced decomposition pathway is shown in Figure 8. The four non-adjacent C–N bonds on the eight-membered ring were gradually elongated to form the transition state TS12, followed by the simultaneous fracture of the C-N bonds, INT9 was produced, INT9 might undergo the similar reaction with the reaction shown in Figure 7 to form HONO, HCN, CH2O, and N2O.
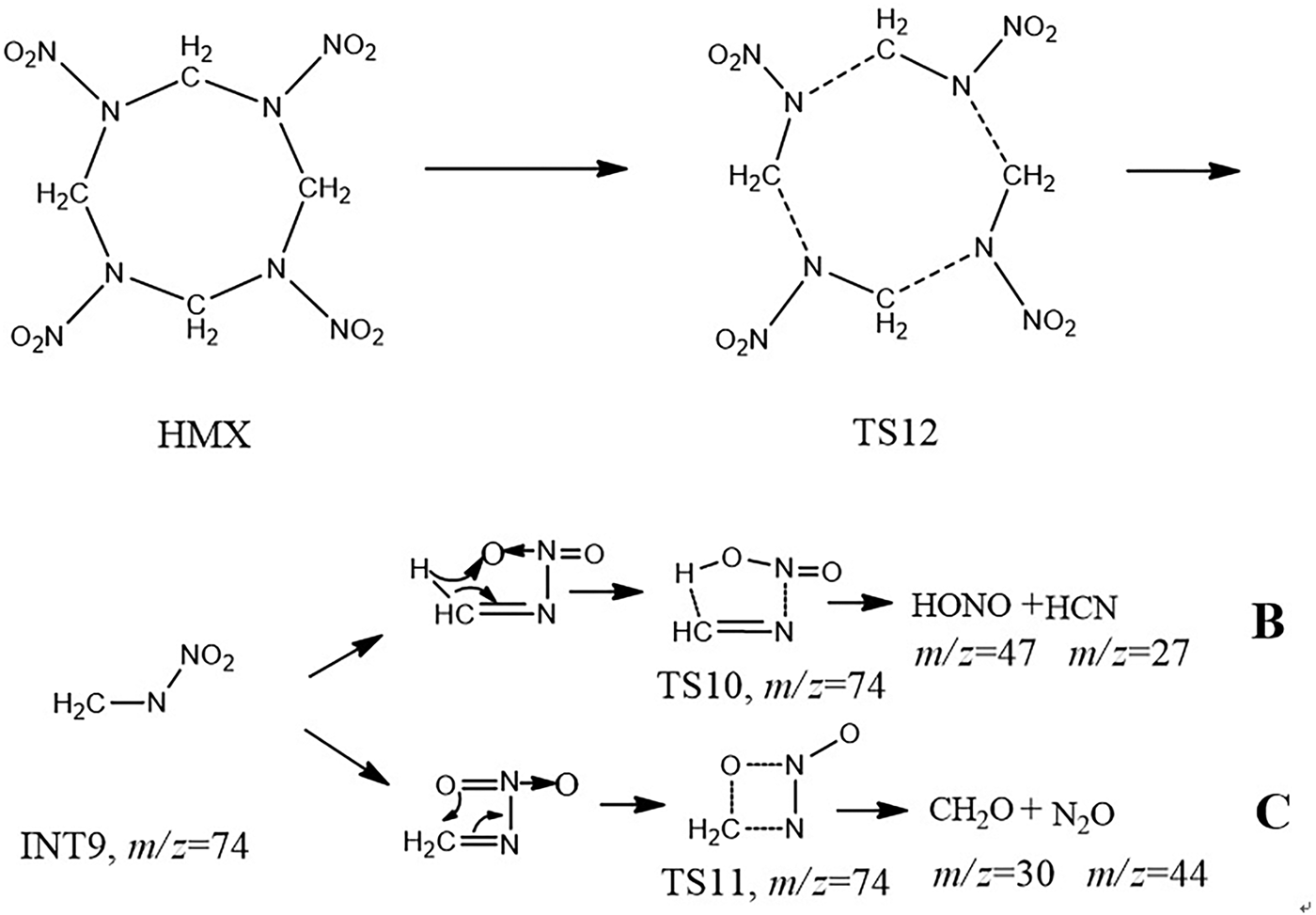
Fig. 8. Laser-induced concerted symmetric ring fission pathways of HMX.
4. CONCLUSIONS
We have presented an experimental investigation of the ions produced during 1064 nm laser ablation of HMX in vacuum by TOF mass spectrometry. Important negative and positive ions were observed, consisting of mostly low-molecular-mass species. Most of these products resulted mainly from HONO elimination, N–NO2 bond breaking, and concerted symmetric ring fission pathways. Some other reactions may exist to account for the undetermined ions hence, it is necessary to develop other experimental and theoretical methods to confirm the process of these reactions, which will help to understand the laser initiation of HMX.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 11604149) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).