INTRODUCTION
Spatial heterogeneity has been considered determinant in shaping species richness and diversity in megadiverse, highly heterogeneous tropical forests (Condit et al. Reference CONDIT, PITMAN, LEIGH, CHAVE, TERBORGH, FOSTER, NÚÑEZ, AGUILAR, VALENCIA, VILLA, MULLER-LANDAU, LOSOS and HUBBELL2002, Erwin Reference ERWIN and Wilson1988, Givnish Reference GIVNISH1999, Ozanne et al. Reference OZANNE, ANHUF, BOULTER, KELLER, KITCHING, KÖRNER, MEINZER, MITCHELL, NAKASHIZUKA, SILVA DIAS, STORK, WRIGHT and YOSHIMURA2003, Pennington & Dick Reference PENNINGTON, DICK, Hoorn and Wesselingh2010, Sedio et al. Reference SEDIO, WRIGHT and DICK2012, Wright Reference WRIGHT2002). Such heterogeneity may be expressed both horizontally and vertically, considering that tree canopies may reach well above 50–70 m high in tropical forests, and contributes to the high diversity found in tropical forests (Barker & Pinard Reference BARKER and PINARD2001, Erwin Reference ERWIN and Wilson1988, Lowman & Nadkarni Reference LOWMAN and NADKARNI1995, Lowman & Wittman Reference LOWMAN and WITTMAN1996, Ozanne et al. Reference OZANNE, ANHUF, BOULTER, KELLER, KITCHING, KÖRNER, MEINZER, MITCHELL, NAKASHIZUKA, SILVA DIAS, STORK, WRIGHT and YOSHIMURA2003).
Canopies have unique conditions compared with other forest strata, including variation in temperature, light incidence and humidity (Nakamura et al. Reference NAKAMURA, ROGER, CAO, CREEDY, FAYLE, FREIBERG, HEWITT, ITIOKA, KOH, MA, MALHI, MITCHELL, NOVOTNY, OZANNE, SONG, WANG and ASHTON2017, Ozanne et al. Reference OZANNE, ANHUF, BOULTER, KELLER, KITCHING, KÖRNER, MEINZER, MITCHELL, NAKASHIZUKA, SILVA DIAS, STORK, WRIGHT and YOSHIMURA2003) and faunal composition. Vertical stratification in tropical forests has been observed for insects (Basset et al. Reference BASSET, CIZEK, CUÉNOUD, DIDHAM, GUILHAUMON, MISSA, ODEGAARD, ROSLIN, SCHMIDT, TISHECHKIN, WINCHESTER, ROUBIK, ABERLENC, BAIL, BARRIOS, BRIDLE, CASTAÑO-MENESES, CORBANA, CURLETTI, ROCHA, BAKKER, DELABIE, DEJEAN, FAGAN, FLOREN, KITCHING, MEDIANERO, MILLER, OLIVEIRA, ORIVEL, POLLET, RAPP, RIBEIRO, ROISIN, SCHMIDT, SORENSEN and LEPONCE2012, Schulze et al. Reference SCHULZE, LINSENMAIR and FIEDLER2001, Yanoviak & Kaspari Reference YANOVIAK and KASPARI2000), birds (Pearson Reference PEARSON1971, Walther Reference WALTHER2002) and small mammals (Bernard Reference BERNARD2001, Kalko & Handley Reference KALKO and HANDLEY2001, Vieira & Monteiro-Filho Reference VIEIRA and MONTEIRO-FILHO2003, Voss et al. Reference VOSS, LUND and SIMMONS2001).
Nonetheless, studies on the vertical distribution of the fauna in tropical communities are still scarce for many groups (Kays & Allison Reference KAYS and ALLISON2001, Ozanne et al. Reference OZANNE, ANHUF, BOULTER, KELLER, KITCHING, KÖRNER, MEINZER, MITCHELL, NAKASHIZUKA, SILVA DIAS, STORK, WRIGHT and YOSHIMURA2003) and often limited (Lowman & Wittman Reference LOWMAN and WITTMAN1996). There are a few exceptions for rodents and marsupials in the Atlantic Forest (Grelle Reference GRELLE2003, Passamani Reference PASSAMANI1995, Vieira & Monteiro-Filho Reference VIEIRA and MONTEIRO-FILHO2003) and some preliminary insights from studies of bats (Carvalho & Fabián Reference CARVALHO and FABIÁN2011, Pires & Fabián Reference PIRES and FABIÁN2013). Bats are relatively easy to sample, and are key components of tropical community dynamics because of their high species richness, high abundance and functional diversity (Kalko et al. Reference KALKO, HANDLEY, HANDLEY, Cody and Smallwood1996, Voss & Emmons Reference VOSS and EMMONS1996, Willig Reference WILLIG1986). Constraints related to the body size (mass can vary from 3 to 250 g – Bernard Reference BERNARD2001) and ecomorphology of bats affect the ways that they use the vertical space (Marinello & Bernard Reference MARINELLO and BERNARD2014) with direct implications for their ecology, and to the processes they are involved in, such as seed dispersal and forest regeneration (Melo et al. Reference MELO, DIRZO and TABARELLI2006, Mendes et al. Reference MENDES, ARROYO-RODRÍGUEZ, ALMEIDA, PINTO, PILLAR and TABARELLI2016, Santo-Silva et al. Reference SANTO-SILVA, ALMEIDA, MELO and TABARELLI2013, Reference SANTO-SILVA, WITHEY, ALMEIDA, MENDES, LOPES and TABARELLI2015; Tabarelli et al. Reference TABARELLI, AGUIAR, GIRÃO, PERES and LOPES2010). Clearly, studies to account for the diversity of tropical bat communities relying solely on captures using ground-level mist nets provide incomplete pictures of the structure of bat assemblages (Meyer et al. Reference MEYER, AGUIAR, AGUIRRE, BAUMGARTEN, CLARKE, COSSON, VILLEGAS, FAHR, FARIA, FUREY, HENRY, RODGKISON, JENKINS, JUNG, KINGSTON, KUNZ, GONZALEZ, MOYA, PATTERSON, PONS, RACEY, REX, SAMPAIO, SOLARI, STONER, VOIGT, STADEN, WEISE and KALKO2011, Rex et al. Reference REX, MICHENER, KUNZ and VOIGT2011, Tavares et al. Reference TAVARES, OLIVEIRA, PALMUTI, NOGUEIRA, GOMES, MARCOS, SILVA, FARIAS and BOBROWIEC2017). Studies in different strata of the Amazon forest indicated that some bat species might follow sharp vertical distributions (Bernard Reference BERNARD2001, Handley Reference HANDLEY1967, Kalko & Handley Reference KALKO and HANDLEY2001, Marques et al. Reference MARQUES, PEREIRA and PALMEIRIM2015, Pereira et al. Reference PEREIRA, MARQUES and PALMEIRIM2010, Sampaio et al. Reference SAMPAIO, KALKO, BERNARD, RODRÍGUEZ-HERRERA and HANDLEY2003, Simmons & Voss Reference SIMMONS and VOSS1998). General patterns include the prevalence of selected species of insect-feeding emballonurid bats and small fruit-eating stenodermatine bats in the canopy, while the Piper-feeding Carollia perspicillata, and the common blood-feeding Desmodus rotundus are understorey users in the Amazon.
Here we provide the first test of vertical stratification for Atlantic Forest bat communities based on a comprehensive inventory in south-eastern Brazil. We hypothesize that bat abundance, species richness and composition are distinct between canopy and understorey strata of Atlantic forests even for forests not as tall as Amazonian sites. We expected to find different bat ensembles and distinct patterns of relative abundance in different strata due the bat ecological requirements (e.g. food and roosts), and ecomorphological constraints to flight. We also hypothesize that trophic guilds of bats are probably not distinct between forest strata, but are driven by specific intra-guild competition, which can displace competing species to occupy different strata.
METHODS
Study area and sampling
The study site is located in the Rio Doce State Park (PERD), eastern state of Minas Gerais (19°48′18′′–19°29′24′′S, 42°38′30′′–42°28′18′′W; Nunes et al. Reference NUNES, GARCIA, LIMA and CARVALHO-OKANO2007). Altitude varies between 230 and 515 m asl (Lopes et al. Reference LOPES, SILVA, SOUZA and NETO2002) and climate is Aw (hot and wet – Lopes et al. Reference LOPES, SILVA, SOUZA and NETO2002), with well-defined rainy and dry seasons. With a total area of 35974 ha, PERD harbours a complex of 44 natural lakes (Bovini et al. Reference BOVINI, CARVALHO-OKANO and VIEIRA2001) and is one of the largest strictly preserved remnants of semi-deciduous Atlantic Forest in Brazil, bordered to the north by Piracicaba river and to the east by Doce river.
We captured bat in eight sites selected according to their vegetation structure and level of disturbance: four sites in secondary forest with different levels of succession (A1–A4), and four sites in a primary forest area (A5–A8 – Figure 1), and repeating some of the sites sampled in the late 1990s as reported by Tavares et al. (Reference TAVARES, PERINI and LOMBARDI2007). In each of the eight sampling sites two Ecotone© 12 × 3-m canopy nets were set forming a rectangle of 12 × 6-m with maximum heights varying from 11 to 19.5 m. Canopy nets were fixed using pulleys and supporting ropes (Humphrey et al. Reference HUMPHREY, BRIDGE and LOVEJOY1968). An additional eight ground-level mist-nets were erected to sample the understorey. Most canopy nets were set in natural tree fall gaps and some in man-made openings, and understorey nets were set along trails previously opened. All nets remained opened for a period of 6 h after sunset.
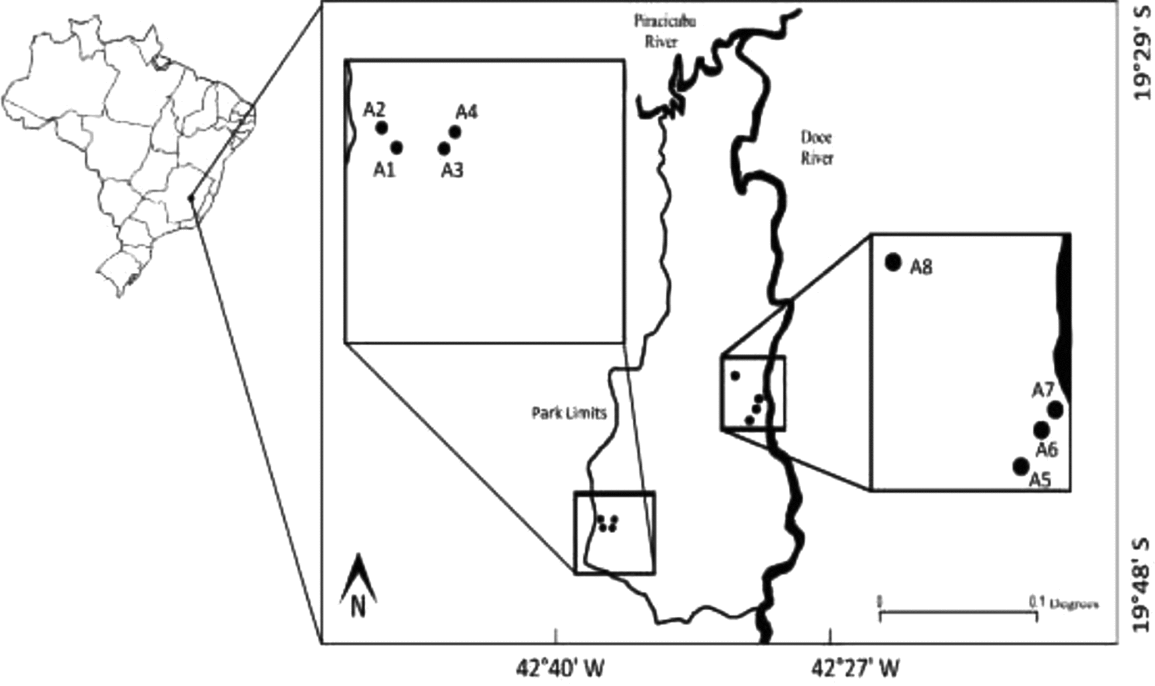
Figure 1. Sampling sites in the Atlantic Forest of Rio Doce State Park, eastern state of Minas Gerais, Brazil. In each site nets were set at ground and canopy level to access the vertical stratification of local bat faunas.
We conducted 10 field expeditions between April 2012 and September 2013 on a total of 80 nights of sampling and eight net-nights in each expedition. Sampling effort was calculated according to the methods described by Straube & Bianconi (Reference STRAUBE and BIANCONI2002) multiplying total area of nets (number of nets × 12 × 3 m) and total time (hours per night × number of nights). The canopy sampling effort by site was 4320m2h totalling 34560m2h for that stratum, the understorey sampling effort per site was 17280m2h, totalling 138240m2h, and the total effort for this study including both canopy and understorey stratum was 172800m2h.
We followed the guidelines for animal research as described in Sikes et al. (Reference SIKES2016). Collection permits were granted by the Instituto Chico Mendes – ICMBio (18528-3) and by Instituto Estadual de Florestas de Minas Gerais (IEF COL 003/12).
Analyses
We ran non-metric multidimensional scaling (NMDS – Clarke Reference CLARKE1993) for the ordination of bat assemblages along the vertical space of PERD employing the Bray–Curtis index which quantifies the compositional dissimilarity between two different sites using previously standardized data (number of captures/sampling effort). We also tested the similarities of strata by using a one-way analysis of similarity (ANOSIM – Clarke Reference CLARKE1993) of the results produced by our NMDS analyses. ANOSIM is a non-parametric statistical test used to test whether the similarity between groups is greater or equal to the similarity within the groups. The NMDS and ANOSIM were performed using the Primer and Permanova software (Anderson et al. Reference ANDERSON, GORLEY and CLARKE2008). We identified species that had dissimilar distribution among the strata by the Indicator Value test (INDVAL test – Dufrene & Legendre Reference DUFRENE and LEGENDRE1997). The INDVAL quantifies the fidelity and specificity of species in relation to groups of sites in a user-specified classification of sites, and tests for the statistical significance of the associations by permutation tests. Only species with ≥ 10 captures were considered for the ordinations. INDVAL associated with the Monte Carlo test were performed in PC-ORD.
We classified all species into trophic guilds as proposed by Kalko et al. (Reference KALKO, HANDLEY, HANDLEY, Cody and Smallwood1996). In such classification, trophic guilds are defined by a combination of predominant diet (based on most consumed food items), foraging strategies, and main habitat used (cluttered and uncluttered space). We also used t-test to verify the significance among trophic guilds by strata (canopy versus understorey) based on bat captures with the help of the software Bioestat 5.0 (Ayres et al. Reference AYRES, AYRES, AYRES and SANTOS2007).
RESULTS
We captured a total of 429 bats in the understorey and 189 bats in the canopy. We recorded 31 bat species distributed in the families Emballonuridae, Phyllostomidae, Vespertilionidae and Molossidae. Carollia perspicillata, Artibeus lituratus and Desmodus rotundus accounted for >70% of the captures (265, 154 and 37 bats, respectively). We captured representatives of a total of eight out of the 10 guilds expected to occur in tropical bat communities (Table 1).
Table 1. Bat species, strata of capture and assigned guild for bat assemblages of eight Atlantic Forest sampling sites at Rio Doce State Park, eastern state of Minas Gerais, Brazil. Species marked with (S) were used in an Indicator Value test. Guilds are (following Kalko et al. Reference KALKO, HANDLEY, HANDLEY, Cody and Smallwood1996): uncluttered space/aerial Insectivores (USAI), background cluttered space/aerial insectivores (BSAI), highly cluttered space/gleaning insectivores (HSGI), highly cluttered space/gleaning carnivores (HSGC), highly cluttered space/gleaning sanguinivores (HSGS), highly cluttered space/gleaning frugivores (HSGF), highly cluttered space/gleaning omnivores (HSGO) and highly cluttered space/gleaning nectarivores (HSGN).

The NMDS ordination indicated that the bat assemblages of PERD were different between canopy/sub-canopy and understorey sets (P<0.001; R global = 0.768) (Figure 2), and site A1 appears as an outlier (Figure 2). A total of 16 species were predominantly or exclusively captured in the canopy, and eight in the understorey (Table 1). Nine out of 31 recorded species were separated by stratum, but only A. lituratus considered as having affinities with the canopy, and C. perspicillata and D. rotundus as typically understorey species had statistical support for the INDVAL test (Table 2).

Figure 2. Ordination of understorey (solid circle) and canopy (open circle) bat faunas in eight sampling sites in the Atlantic Forest of Rio Doce State Park, eastern state of Minas Gerais, Brazil. A non-metric multidimensional scaling ordination was performed based on 31 bat species recorded.
Table 2. Outputs from an Indicator Value test using the more representative species (≥ 10 individuals) in a study on the vertical stratification of bat communities of eight Atlantic Forest sampling sites at Rio Doce State Park, eastern state of Minas Gerais, Brazil. *Indicates statistically significant. Note that only three species had significantly distinct abundances between the strata.

Indeed, the highly cluttered space/gleaning frugivore (HCGF) A. lituratus was captured mostly at the canopy as indicated by the INDVAL test (P=0.04; Table 3) while highly cluttered space/gleaning omnivores (HSGO) and uncluttered space/aerial insectivores (USAI) were captured only in the canopy, although a single individual of M. aztecus (an aerial insectivorous bat) was netted in the understorey (Table 1). Highly cluttered space/gleaning nectarivores (HSGN) and highly cluttered space/gleaning sanguinivore (HSGS) were mostly captured in the understorey, and highly cluttered space/gleaning carnivore species were captured only in the understorey (Figure 3).
Table 3. Bat trophic guilds and their most associated vertical strata (C – canopy, U – understorey) based on data from eight Atlantic Forest sampling sites at Rio Doce State Park, eastern state of Minas Gerais, Brazil. Guild acronyms as in Table 1. *Indicates statistically significant. Note that HSGF was only guild distinct in abundance between strata.


Figure 3. Mean and standard deviation of captures of bats in the canopy (a) and understorey (b) in eight sampling sites in the Atlantic Forest of Rio Doce State Park, eastern state of Minas Gerais, Brazil. Bat species were grouped in trophic guilds (acronyms as in Table 1). *Indicates trophic guilds restricted to only one stratum.
DISCUSSION
Our data from the Atlantic Forest in south-eastern Brazil shows that local bat communities are not homogeneously distributed across vertical strata of the forest, and are structured in canopy and understorey assemblages. Canopy foragers include A. lituratus and C. villosum, and other species represented by few captures such as small frugivores (Vampyressa pusilla and Platyrrhinus recifinus), some small gleaning insectivores (Micronycteris minuta and Gardnerycteris crenulatum) and aerial insectivorous molossids (Molossus and Nyctinomops). Patterns similar to ours have been observed for other tropical forests in the Amazonia (Bernard Reference BERNARD2001, Henry et al. Reference HENRY, BERRIÈRE, GAUTIER-HION and COLYN2004, Hodgkison et al. Reference HODGKISON, BALDING, ZUBAID and KUNZ2004, Kalko & Handley Reference KALKO and HANDLEY2001, Marques et al. Reference MARQUES, PEREIRA and PALMEIRIM2015, Pereira et al. Reference PEREIRA, MARQUES and PALMEIRIM2010, Sampaio et al. Reference SAMPAIO, KALKO, BERNARD, RODRÍGUEZ-HERRERA and HANDLEY2003, Simmons & Voss Reference SIMMONS and VOSS1998). In studies in Belém, Brazilian Pará State, C. villosum was also considered a canopy species (Kalko & Handley Reference KALKO and HANDLEY2001), while in the Manaus area, in Brazilian Amazonas State, molossids of the genus Molossus and Nyctinomops have also been recorded usually flying above the trees and roosting in or foraging very close to the canopy (Bernard Reference BERNARD2001, Kalko & Handley Reference KALKO and HANDLEY2001, Sampaio et al. Reference SAMPAIO, KALKO, BERNARD, RODRÍGUEZ-HERRERA and HANDLEY2003). Moreover, in our sampled sites, C. perspicillata and D. rotundus have affinities with the understorey stratum (P≤5%; Table 2), a similar pattern to that observed in Amazonian sites (Bernard Reference BERNARD2001, Kalko & Handley Reference KALKO and HANDLEY2001, Pereira et al. Reference PEREIRA, MARQUES and PALMEIRIM2010, Sampaio et al. Reference SAMPAIO, KALKO, BERNARD, RODRÍGUEZ-HERRERA and HANDLEY2003).
Several other species have been strictly or mostly sampled in understorey, such as the nectarivore G. soricina, several gleaning insectivores and carnivores, and the recently described disk-winged bat Thyroptera wynneae (Velazco et al. Reference VELAZCO, GREGORIN, VOSS and SIMMONS2014). Thyroptera roosts mainly in unrolled leaves of Heliconia and Musa, dead leaves of Cecropia, and palm trees found in the understorey, edges and gaps of PERD. Indeed understorey roost selection may increase the chances of captures of several bat species in ground level mist-nets, including species of gleaner insectivore phylostomids, and aerial insectivore emballonurids and thyropterids that use ground hollows, termite hollows and fallen trunks (Dalponte et al. Reference DALPONTE, GREGORIN, ESTEVES-COSTA, ROCHA and MARCELINO2016, Simmons & Voss Reference SIMMONS and VOSS1998, Voss et al. Reference VOSS, FLECK, STRAUSS, VELAZCO and SIMMONS2016). Our second hypothesis, addressing the absence of stratification by trophic guild was just partially confirmed because most of the recorded guilds occurred in both strata though with distinct captures in each stratum (Table 1). For example, molossids – which are exclusive insectivores – were caught only in canopy nets (with one exception – Table 1), while frugivores were mostly in the understorey. On the other hand, gleaning frugivore (HSGF) guild presented differences between strata (Table 3).
Other approaches may help to refine the information on the use of the space by bats in tropical forests. Findings based on the study of stable isotopes (N and C) point to a more complex scenario for bats. For example, Rex et al. (Reference REX, MICHENER, KUNZ and VOIGT2011) revealed that some frugivores (including A. lituratus) were mostly netted in the understorey but, in fact, they had fed on plant species of canopy, as indicated by isotopic analyses. In our study, A. lituratus was netted in both strata, but mostly in the canopy.
Although our effort may not be sufficient to answer questions on vertical stratification for all species, especially those that are rarely captured or able to detect and avoid nets, we would suggest that the main limitation regarding our results is the need for long-term sampling study at PERD to be able to record uncommon species. On the other hand, this is the first attempt to test the vertical stratification of bat assemblages in the Atlantic Forest, and we argue that we have detected significant patterns that may be tested in the long-term studies. Based on the single study of Bergallo et al. (Reference BERGALLO, ESBÉRARD, MELLO, LINS, MONGOLIN, MELO and BAPTISTA2003) approximately 1000 captures may be necessary to estimate the richness of phyllostomid bat communities in Atlantic Forest sites and even an increased number of captures for Amazonian sites (Sampaio et al. Reference SAMPAIO, KALKO, BERNARD, RODRÍGUEZ-HERRERA and HANDLEY2003). In the same way, Meyer et al. (Reference MEYER, AGUIAR, AGUIRRE, BAUMGARTEN, CLARKE, COSSON, VILLEGAS, FAHR, FARIA, FUREY, HENRY, HODGKISON, JENKINS, JUNG, KINGSTON, KUNZ, GONZALEZ, MOYA, PONS, RACEY, REX, SAMPAIO, STONER, VOIGT, STADEN, WEISE and KALKO2010) proposed that long-term studies (˃10 y) and many plots may be necessary to access some patterns and tendencies in tropical bat communities based on abundance. Accessing and sampling the forest canopies have inherent methodological limitations making large sampling efforts frequently impractical (Lowman & Nadkarni Reference LOWMAN and NADKARNI1995, Lowman & Wittman Reference LOWMAN and WITTMAN1996, Nakamura et al. Reference NAKAMURA, ROGER, CAO, CREEDY, FAYLE, FREIBERG, HEWITT, ITIOKA, KOH, MA, MALHI, MITCHELL, NOVOTNY, OZANNE, SONG, WANG and ASHTON2017). We therefore recognized that the sample sizes, especially in the canopy, are limited, but our data are sufficient to show differences at the level of the assemblages, and for a few common species.
The importance of vertically extended sampling
Bat inventories focused exclusively on understorey sampling in the Atlantic Forest may be particularly limiting to access specific taxa. This is the case of molossids. Because molossids are high-flyers, and usually forage well above the canopy, they are hardly recorded with mist nets set in the forest interior or in the understorey (Kalko et al. Reference KALKO, HANDLEY, HANDLEY, Cody and Smallwood1996). Indeed, although PERD now has a diverse record of molossid bats including recently described new species (Eumops chimaera – Gregorin et al. Reference GREGORIN, MORAS, ACOSTA, VASCONCELLOS, POMA, SANTOS and PACA2016), most of those records were obtained from roost searching or sporadic captures with nets especially set intercepting flyways or foraging sites. Usually, little or no representation of molossids is observed in datasets from ground-level mist net samples in the Atlantic forests (Dias et al. Reference DIAS, PERACCHI and SILVA2002, Martins et al. Reference MARTINS, CARVALHO, DIAS, FRANÇA, OLIVEIRA and PERACCHI2015). In our study, with the exception of a single individual of M. aztecus, all the molossids recorded came from canopy nets in one of the sites, resulting in a high species richness (five species) and number of captures (41 bats).
Other families and species are also frequently misrepresented in inventories focused on the understorey level. Marques et al. (Reference MARQUES, PEREIRA and PALMEIRIM2015), working with bat detectors in the flooded forests of the Amanã Sustainable Development Reserve, in the Brazilian Amazonia, recorded much more activity in Emballonuridae, Molossidae and Vespertilionidae at the canopy level. Emballonurids are also usually recorded in canopy nets (e.g. Diclidurus spp., Saccopteryx spp. – Kalko & Handley Reference KALKO and HANDLEY2001). They were absent from our canopy nets, but have been previously recorded in PERD mostly due to roost searching, and often associated to water bodies (Tavares & Anciães Reference TAVARES and ANCIÃES1998, Tavares et al. Reference TAVARES, PERINI and LOMBARDI2007). Indeed the history of bat species inventories at PERD (Gregorin et al. Reference GREGORIN, VASCONCELLOS and GIL2015, Reference GREGORIN, MORAS, ACOSTA, VASCONCELLOS, POMA, SANTOS and PACA2016; Tavares Reference TAVARES2013, Tavares & Anciães Reference TAVARES and ANCIÃES1998, Tavares et al. Reference TAVARES, PERINI and LOMBARDI2007; Velazco et al. 2014) emphasize the need to adopt mixed methodologies (Marques et al. Reference MARQUES, PEREIRA and PALMEIRIM2015, Meyer et al. Reference MEYER, AGUIAR, AGUIRRE, BAUMGARTEN, CLARKE, COSSON, VILLEGAS, FAHR, FARIA, FUREY, HENRY, RODGKISON, JENKINS, JUNG, KINGSTON, KUNZ, GONZALEZ, MOYA, PATTERSON, PONS, RACEY, REX, SAMPAIO, SOLARI, STONER, VOIGT, STADEN, WEISE and KALKO2011) to uncover the diversity of the bat fauna at the reserve, including the use of bat detectors, roost searching and canopy sampling.
Conservation implications of changing the vertical structure of the forest
Neotropical forests are heterogeneous environments for bats in terms of vertical strata (Yang et al. Reference YANG, SCHAAF, STRAHLER, KUNZ, FULLER, BETKE, WU, WANG, THERIAULT, CULVENOR, JUPP and NEWNHAM2013) and the canopy shelters high richness and dynamic activity (Bernard Reference BERNARD2001, Kalko & Handley Reference KALKO and HANDLEY2001, Marques et al. Reference MARQUES, PEREIRA and PALMEIRIM2015, Rex et al. Reference REX, MICHENER, KUNZ and VOIGT2011). Reduction or loss of tree canopy is the first and one of the most harmful effects of deforestation in tropical forests, affecting several bat species (Fenton et al. Reference FENTON, CUMMING, RAUTENBACH, CUMMING, CUMMING, FORD, TAYLOR, DUNLOP, HOVORKA, JOHNSTON, PORTFORS, KALCOUNIS and MAHLANGA1998, Russo et al. Reference RUSSO, CISTRONE and JONES2007). The Atlantic Forest suffered a severe process of habitat loss, and has been reduced to ~8% of its original size (Ribeiro et al. Reference RIBEIRO, METZGER, MARTENSEN, PONZONI and HIROTA2009).
As demonstrated in our study and others elsewhere, the loss or lowering of mature forests and, consequently, of the forest canopy may affect whole bat assemblages associated to these upper strata. Besides direct effects on bats per se, this will also affect other bat-mediated ecological processes such as pollination and seed dispersal of many plant species (Tabarelli et al. Reference TABARELLI, AGUIAR, GIRÃO, PERES and LOPES2010). Such changes in the canopy structure can potentially disrupt seed-delivery services provided by some frugivores, as well as favour the dominance of bat species that feed mostly on small-seeded understorey plant species, or, more dramatically, promote a biotic plant community homogenization in response to a regional-scale proliferation of some pioneer species (Melo et al. Reference MELO, DIRZO and TABARELLI2006, Santo-Silva et al. Reference SANTO-SILVA, ALMEIDA, MELO and TABARELLI2013).
On the other hand, the increase in relative abundance of bat-associated species in the understorey may produce positive bias, accelerating the colonization and regeneration of disrupted habitats (Tabarelli et al. Reference TABARELLI, AGUIAR, GIRÃO, PERES and LOPES2010). In all cases, in a scenario where human-modified landscapes prevail – such as in south-eastern Brazil – conserving the integrity of the canopy of mature forests may directly contribute not just to the persistence of bats, but of a large portion of the local biodiversity of inland Atlantic Forest sites.
ACKNOWLEDGEMENTS
We thank the PERD team for all logistical assistance and efforts to make this work possible. We also are in debt with all the students who have participated in field trips and to Instituto Estadual de Florestas (IEF-MG) for permission to study in the PERD. We thank Brock Fenton and two anonymous reviewers for helpful criticism and improvement on an earlier version of the manuscript. This study was partially supported by FAPEMIG Process APQ-01451-11 (R. Gregorin), V. C. Tavares is supported by a PNPD/CAPES fellowship programme and E. Bernard is supported by a fellow's grant from CNPq.








