INTRODUCTION
Species-specific differences in survival and growth responses to light during seedling establishment may contribute to the coexistence of these species (Bloor & Grubb Reference BLOOR and GRUBB2003, Dalling & Hubbell Reference DALLING and HUBBELL2002, Garwood Reference GARWOOD, Estrada and Fleming1986). One proposed mechanism by which tree species coexist is through niche partitioning along gradients of light availability. Many studies, including shade house (Agyeman et al. Reference AGYEMAN, SWAINE and THOMPSON1999, Ashton Reference ASHTON1995, Bloor & Grubb Reference BLOOR and GRUBB2003, Hall et al. Reference HALL, MEDJIBE, BERLYN and ASHTON2003) and gap (Ashton et al. Reference ASHTON, GUNATILLEKE and GUNATILLEKE1995, Dalling et al. Reference DALLING, WINTER and HUBBELL2004, Pearson et al. Reference PEARSON, BURSLEM, GOERIZ and DALLING2003), have been carried out to test this mechanism within forests of mixed species assemblages, but evidence remains equivocal (Dalling et al. Reference DALLING, WINTER and HUBBELL2004, Sack & Grubb Reference SACK and GRUBB2001). Seed mass is a key life-history trait, which is closely related to seedling relative growth rates (RGR) and survival for forest tree species (reviewed by Leishman et al. Reference LEISHMAN, WRIGHT, MOLES, WESTOBY and Fenner2000). Morphological and functional characteristics of species change with environmental conditions may also determine growth rates (Bazzaz Reference BAZZAZ1979, Reich et al. Reference REICH, TJOELKER, WALTERS, VANDERKLEIN and BUSCHENA1998a, Reference REICH, WALTERS, TJOELKER, VANDERKLEIN and BUSCHENA1998b; Veneklaas & Poorter Reference VENEKLAAS, POORTER, Lambers, Poorter and Van Vuuren1998). For example, relative growth rates have been shown to be correlated with photosynthesis and respiration rates (Reich et al. Reference REICH, WALTERS, TJOELKER, VANDERKLEIN and BUSCHENA1998b), specific leaf area (Poorter Reference POORTER1999), leaf area ratio (Poorter & Remkes Reference POORTER and REMKES1990, Veneklaas & Poorter Reference VENEKLAAS, POORTER, Lambers, Poorter and Van Vuuren1998), and resource allocation to defence and/or storage (Coley et al. Reference COLEY, BRYANT and CHAPIN1985, Kobe Reference KOBE1997, Lambers & Poorter Reference LAMBERS and POORTER1992). In heterogeneous light environments, the coexistence of forest species with different seed sizes may through trade-off between high-light growth rates and low-light survival.
Tree species can be classified functionally as pioneer or non-pioneer based on the light requirements for seed germination and seedling establishment (Swaine & Whitmore Reference SWAINE and WHITMORE1988). In contrast to pioneer and shade-tolerant species, plants within the non-pioneer light-demanding guild can germinate in the forest understorey, need shade for early growth but then require release (Hawthorne Reference HAWTHORNE1995), and can adapt to survive and grow in a wide range of light conditions (Montgomery Reference MONTGOMERY2004). Tests for species-specific differences in survival and growth responses among tree species have included both shade-tolerant and pioneer species in tropical forests (Dalling & Hubbell Reference DALLING and HUBBELL2002, Kitajima Reference KITAJIMA1994, Kobe Reference KOBE1999, Sánchez-Gómez et al. Reference SÁNCHEZ-GÓMEZ, VALLADARES and ZAVALA2006, Walters & Reich Reference WALTERS and REICH2000), and produced varying results. However, there has been little study of performance trade-offs between survival and growth in non-pioneer light-demanding species. The mechanism of non-pioneer light-demanding tree species co-existence is not very clear.
This paper describes a study where nine co-occurring non-pioneer light-demanding tree species were grown under three simulated light environments (low light, 2% daylight; moderate light, 13% daylight; high light, 46% daylight) in Hainan in China. We examined the survival and growth of plants and tested hypotheses concerning the coexistence of tree species along light resource gradients by examining the trade-offs between seedling survival and RGR (both in mass and in height) at a range of light levels. Specifically, the hypotheses were tested as following: (1) seed mass is positively correlated with low-light survival, and negatively correlated with RGR in either low or high light; (2) there are important morphological or physiological trade-offs, particularly trade-offs between seedling low-light survival and high-light RGR across species; (3) there are crossovers in rank order performance of species between low and high light
METHODS
Study site and species
Our study was carried out in a tropical tree garden at Jianfengling Experimental Station (Research Institute of Tropical Forestry, Chinese Academy of Forestry) in Hainan Province, China (18°23′–18°52′N, 108°36′–109°05′E). The mean annual temperature of this region is 24.5 °C, and the mean temperatures of the coldest and hottest months are 14.3 °C and 27.3 °C, respectively. Snow and frost do not occur in this region. Rainy and dry seasons are clearly demarcated, with the dry season occurring from December to April and the wet season from May to October. Annual rainfall is approximately 2200 mm, 80–90% of which falls during the rainy season. Ascending from the lowest foothill to the mountain peak (1412.5 m), air temperature declines from 25 °C to 17–19 °C, and annual precipitation increases from 1300 to 3500 mm (Jiang & Lu Reference JIANG and LU1991, Li Reference LI, Li, Chen and Zhou2002). Early-successional forests dominate most mountains of Hainan Island (Li Reference LI, Li, Chen and Zhou2002). Forest gaps of 20–400 m2 constitute a large proportion of the forested area, and the light intensity in the understorey is 4–14% of full sunlight (Jiang et al. Reference JIANG, WANG and ZANG2002).
The nine tree species studied are common in seasonal tropical rain forests of Hainan and southern China and differ in seed mass and leaf phenology. Six epigeal and three hypogeal species were studied, with the seed reserve dry mass varying from about 1000 mg for Castanopsis fissa to about 1 mg for Homalium hainanense (Table 1). Seeds were collected from various rain-forest locations ranging from about 300–800 m altitude on Jianfengling Mountain between July 2007 and December 2008.
Table 1. Characteristics of the nine non-pioneer, light-demanding tree species examined in this study. Initial mass and height values were obtained by destructive harvesting of 10 individuals per species at the beginning of the 12-mo growth period.

Shade treatments
We established different light environments in three shade houses using neutral-density shade cloth. Photosynthetically active radiation (PAR) in each shade house was 46% ± 0.3%, 13% ± 0.2% and 2% ± 0.3% full daylight, based on comparisons of treatment vs. outdoor instantaneous readings made at midday under clear skies using LI-190SA Quantum sensors (Li-Cor, Lincoln, NE, USA). These light levels are comparable with those found in gaps and in the forest understorey in the study area (Jiang et al. Reference JIANG, WANG and ZANG2002).
Growth experiment
Seeds were germinated in shallow trays filled with forest topsoil. Once the first pair of leaves or photosynthetic cotyledons was fully expanded, seedlings were carefully dug up and planted individually into plastic pots (25 cm high, 23 cm diameter) containing a mixture of 3:1:1 forest soil, pond sludge and washed river sand, and then randomly allocated to a light treatment. Twenty plants of each species were assigned to each treatment. Dates of seedling transplant and treatment assignment varied based on seed collection and germination times. Once the plants were set up in the shade houses, initial harvests were taken for a subsample of each species (10 plants per species). We recorded leaf area, stem height and seedling dry mass (seedlings were oven-dried at 70 °C for 48 h) for these initial harvests.
Plants received natural rainfall, supplemented by hand-watering during dry periods (>1 d without rain). Any seedlings dying in the first 2 wk immediately following transplant were replaced. Following seedling transfer into the shade houses, survival censuses were initiated and carried out monthly throughout the experiment. We labelled as dead those individuals that had lost all their aerial structures, did not have any photosynthetically active leaves (i.e. green and flexible leaves) and exhibited loss of stem flexibility in the upper third portion of the plant. Plants were relocated within the shade houses regularly during the experimental period to reduce possible positioning effects. Seedlings were sprayed with a fungicide solution (50% Carbendazim, Pesticide Technology Development Co., Ltd., Wuhan Scarlett, China) twice during the experiment in order to control fungal infections. None of the mortality events showed signs of a fungal-infection-mediated death. The growing period between initial and final harvest was 12 mo for each species. No leaf abscission was observed in the high- or low-light treatments during the study period.
Photosynthetic measurements
In the wet season of 2008, we selected five fully expanded, young to medium-aged and apparently healthy leaves of each species (one leaf per individual) in each shade treatment for photosynthesis measurements. Photosynthetic measurements were taken from 09h00 to 11h30 under clear skies (July–November 2008). The measurement order of treatments and seedlings was randomized each day. Maximum photosynthetic capacity (Amax) was measured using a portable leaf chamber and open-system infrared gas analyser (IRGA) (LI-6400; Li-Cor Inc., Lincoln, NE, USA). Amax was measured at ambient CO2 concentrations (approximately 370 mmol mol−1) and 1500 mmol m−2 s−1 photosynthetic photon flux density (PFD) provided by a red-blue light source (6400–02B; Li-Cor Inc., Lincoln, NE, USA). Ambient temperature ranged from 24 °C to 28 °C, and leaf chamber temperature was about 25 °C. Amax was induced in a stepwise fashion at PFD levels of 500, 700 and 1000 mmol m−2 s−1 before it was measured at 1500 mmol m−2 s−1. The dark respiration rate (Rd) was measured in dark-adapted leaf blades.
Seedling measurements and derived parameters
All seedlings were grown for 12 mo, commencing September–December 2007. At the end of the study, 10 seedlings of each species in each treatment were destructively harvested, and morphological characteristics were measured. Seedlings were separated into roots, stems and leaves, and dried at 72 °C in a forced air oven for 48 h before quantifying dry mass. Roots were washed carefully prior to drying. Leaf area of all leaves of each seedling was measured using a LI-COR LI-3000 leaf area meter (Li-Cor Inc., Lincoln, NE, USA). Plant dry mass values did not include cotyledon remains as in many cases these had dropped off. The following growth parameters were derived from basic measurements: Relative growth rate in mass (RGRm, g g−1 mo−1) = (lnW2−lnW1)/(T2−T1), Relative growth rate in height (RGRh, cm cm−1 mo−1) = (lnH2−lnH1)/(T2−T1), W2 and W1 are the final and initial (i.e. mean of the plants from the initial harvest) total dry weights per plant, H2 and H1 are the final and initial total heights per plant, and T2−T1 is the growth time interval (12 mo). Specific leaf area (SLA, cm2 g−1) = leaf area/leaf mass. Leaf mass ratio (LMR, g g−1) = leaf mass/total plant mass. Stem mass ratio (SMR, g g−1) = (stem + petiole mass)/total plant mass. Root mass ratio (RMR, g g−1) = root mass/total plant mass. Leaf area ratio (LAR, cm2 g−1) = total leaf area/total plant mass. Net assimilation rate (NAR, g cm−1 mo−1) = [(W2−W1) (lnA2−lnA1)]/[(A2−A1) (T2−T1)], W2 and W1 are the final and initial (i.e. mean of the plants from the initial harvest) total dry weights per plant, A2 and A1 are the final and initial total leaf area per plant, and T2−T1 is the growth time interval (12 mo). Cotyledon masses were excluded. Absolute growth was also calculated in terms of biomass and height.
Data analysis
Patterns of variation among species and simulated light environment in seedling allocation pattern and physiology, and their contribution to growth, were explored using ANOVA models. Analyses were performed on the means of the seedlings of each species grown on each shade house (n = 10 per species per treatment). Differences in final seedling mass (and height), and RGRm (and RGRh) among light treatments were examined separately using one-way ANOVAs; if significant treatment differences were detected (P < 0.05) then Tukey's multiple range test was used for mean separation. Data were transformed where necessary to meet the assumptions of normality and homogeneity of variance. The relationships between seed mass and RGRm (or RGRh) in 13% (or 46%) sunlight, and seedling survival in 2% sunlight were determined using simple linear regression. Pearson's correlation coefficients were calculated between seedling survival in 2% sunlight and both RGRm and RGRh in the 13% and 46% treatments.
RESULTS
All seedling attributes measured responded to variation in light treatment (Table 2). The most responsive variables were RGRm, followed by Amax and SLA. Species also varied significantly for all attributes measured, and in many cases, including RGR, inherent differences among species accounted for less variation than did light responses. Similarly, interactions between species and light treatment were significant with the exception of Rd (Table 2).
Table 2. F values and significance values from ANOVA for analysis of variance of total biomass, total mass relative growth rate (RGRm), relative height growth rate (RGRh), root mass ratio (RMR), stem mass ratio (SMR), leaf mass ratio (LMR), specific leaf area (SLA), leaf area ratio (LAR), net assimilation rate (NAR), maximum photosynthetic capacity in area (Amax) and dark respiration rate (Rd). Three light levels and nine species are considered as fixed factors.

Seedling survivorship
We found interspecific differences in seedling survival in all light treatments (Figure 1). In low-light treatment, Eriobotrya deflexa, Artocarpus styracifolius and Sterculia alata experienced the highest survival, indicating higher shade tolerance than other species included in the study. Homalium hainanense experienced the lowest survival, indicating that this was the least shade-tolerant of the nine species examined.

Figure 1. Relationships between survival in deep shade (2% sunlight) and relative mass growth rate (RGRm) (a), and relative height growth rate (RGRh) (b) for seedlings of nine tree species. Relative growth rates (both RGRm and RGRh) are for seedlings of nine tree species grown at two different levels of irradiance in experimental shade houses: 46% sunlight (solid line, solid squares) and 13% sunlight (dashed line, hollow squares). Each point represents a species mean.
Seedling survival in deep shade showed a significant negative relationship with both moderate-light RGRm (r = −0.788, P = 0.012) and high-light RGRm (r = −0.694, P = 0.038). The correlation was stronger with RGRm in moderate light than in high light (Figure 1a). Seedling survival in deep shade also showed a significant negative relationship with moderate-light RGRh (r = −0.835, P = 0.005), but contrary to expectations, survival was not correlated with high-light RGRh (r = −0.549, P = 0.126) (Figure 1b). Seedling survival in low-light treatment was significantly correlated with the dry mass of seed reserves (r = 0.945, P < 0.001) (Figure 2).

Figure 2. Relationship between survival in deep shade (2% sunlight) and seed mass for nine tree species. Species codes are given in Table 1.
Relative growth rates in moderate and high light
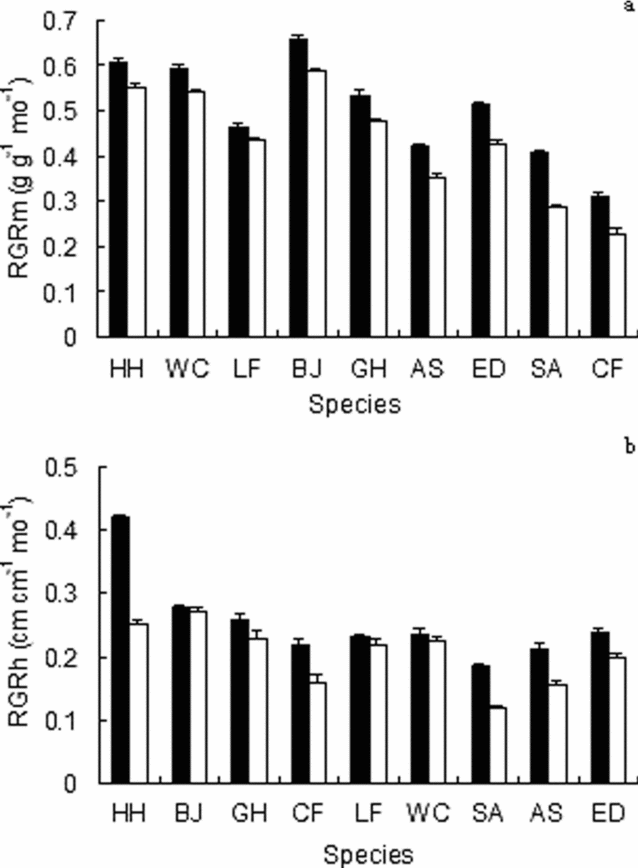
Relative mass growth rates (RGRm) showed a wide spread of values among species (Figure 3a). In both high and moderate light, RGRm values did not correspond linearly with seed mass (Figure 3a). Relative growth rates in height (RGRh) also showed comparable variation in the two treatments (Figure 3b). Species differed in initial seedling height, but differences in RGRh led to a number of crossovers in the rank order of species according to height (Figure 3b).
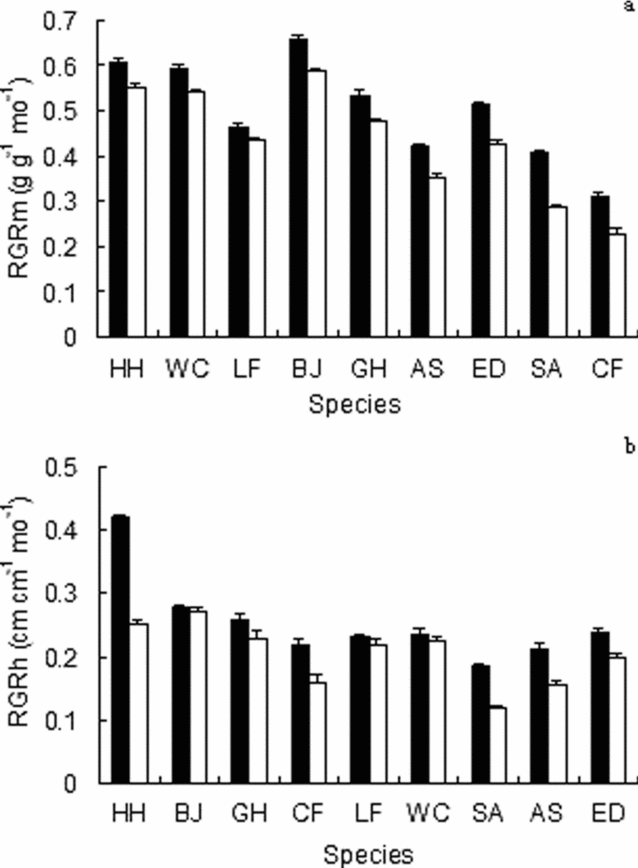
Figure 3. Relative mass growth rate (RGRm) (a), and relative height growth rate (RGRh) (b), for seedlings of nine tree species grown in both 46% (solid columns) and 13% (hollow columns) of full daylight. Mean (± SE) is shown; n = 10 seedlings per species per treatment. Species for RGRm are arranged according to seed mass. Species for RGRh are arranged according to initial seedling height. Species codes are given in Table 1.
No significant correlation existed between low-light and high-light RGRm (r = −0.301, P = 0.431) or between low-light and moderate-light RGRm (r = −0.349, P = 0.357) across species (Figure 4a). RGRh in high (and moderate) light both decreased with increasing low-light RGRh (r = −0.628, P = 0.070 and r = −0.455, P = 0.218, respectively) (Figure 4b). High-light RGRh was not significantly correlated with high-light RGRm across species (r = 0.612, P = 0.079), but moderate-light RGRh showed a strong positive relationship with moderate-light RGRm across species (r = 0.916, P < 0.001).

Figure 4. Relationships between RGRm (a), and RGRh (b) in low light (2% sunlight) vs. moderate light (13% sunlight) and high light (46% sunlight) treatments for seedlings of nine tree species. Each point is a species mean.
Relative growth rates and seed reserves
RGRm and RGRh were negatively correlated with seed reserve dry mass in both high- and moderate-light treatments (Figure 5). The correlation was stronger in moderate light than in high light for both RGRm (Figure 5a) and RGRh (Figure 5b).

Figure 5. Relationships between dry mass seed reserve and RGRm (a), and RGRh (b) for seedlings of nine tree species grown at two different levels of irradiance in experimental shade houses: 46% full daylight (solid line, solid squares) and 13% full daylight (dashed line, hollow squares). Each point is a species mean.
Final seedling height was not correlated with initial seedling height either in moderate light (r = −0.314, P = 0.411) or in high light (r = −0.068, P = 0.862), nor was it correlated with seed reserve mass in either moderate light (r = −0.198, P = 0.609) or high light (r = −0.290, P = 0.450). Initial differences in height were overcome by differences in RGRh. Final seedling mass was negatively correlated with seed mass in both moderate light (r = −0.812, P = 0.007) and high light (r = −0.681, P = 0.043). Initial differences in mass were overcome by differences in RGRm. Cross-overs in rank growth rate between gap sizes were therefore clear.
Relative growth rates and seedling traits
Interspecific variation in RGRm was associated with different plant traits depending on light level (Table 3). In high-light conditions, RGRm was significantly correlated with net assimilation rate (NAR) across species but was not correlated with leaf area ratio (LAR) or specific leaf area (SLA) (Table 3). Neither RGRm nor RGRh was significantly correlated with stem mass ratio (SMR), root mass ratio (RMR), or leaf mass ratio (LMR). RGRh was also not significantly correlated with NAR, LAR or SLA; although RGRh tended to increase somewhat with increasing NAR, this trend was not statistically significant. LMR was negatively correlated with SMR (r = −0.769, P < 0.05) but not with root mass ratio (RMR) (r = −0.310, P > 0.05).
Table 3. Correlation matrix for seedling traits of nine species grown in 13% and 46% sunlight. Values are Pearson's correlation coefficients (n = 10), with significant correlations indicated by asterisks: *P < 0.05, **P < 0.01. Numbers below the diagonal refer to plants grown in 13% daylight, whereas values above the diagonal refer to plants grown in 46% sunlight.

In moderate light, RGRm and RGRh were both significantly correlated with NAR but not with LAR or SLA. RGRm was significantly positively correlated with SMR but was not correlated with either RMR or LMR. RGRh was not correlated with SMR, RMR or LMR. LMR was significantly negatively correlated with RMR (r = −0.58, P < 0.05) and SMR (r = −0.60, P < 0.05).
DISCUSSION
Interspecific variation in survival and growth
Our results showed obvious species-specific differences in survival and growth among the light treatments, consistent with previous studies focusing on tropical species (Bloor Reference BLOOR2003, Sánchez-Gómez et al. Reference SÁNCHEZ-GÓMEZ, VALLADARES and ZAVALA2006, Seiwa Reference SEIWA2007). Contrary to our expectation, seedlings of all species subjected to the low-light treatment exhibited a high survival, suggesting that the non-pioneer light-demanding species of Hainan generally exhibit a degree of shade tolerance during the early establishment stages. However, the higher-than-expected survival may have resulted from the short duration (i.e. 12 mo) of our study. Baraloto et al. (Reference BARALOTO, FORGET and GOLDBERG2005a) found that larger seedlings did survive better within the first year, but they were more likely to die in the subsequent 4-y period.
Our results exhibited clear trade-off between low-light survival and high-light growth of seedlings, which was consistent with previous studies that included both shade-tolerant and pioneer species (Kitajima Reference KITAJIMA1994, Kobe Reference KOBE1999, Walters & Reich Reference WALTERS and REICH2000; but see Bloor & Grubb Reference BLOOR and GRUBB2003). This trade-off was found with respect to mass growth but not with respect to height growth, which is probably due to the complex effects of shade on height growth (Sánchez-Gómez et al. Reference SÁNCHEZ-GÓMEZ, VALLADARES and ZAVALA2006). Height growth in response to shade is a shade avoidance strategy; with poor light interception efficiency in shaded environments, shade avoiders have adapted to grow rapidly toward light (Pearcy et al. Reference PEARCY, MURAOKA and VALLADARES2005). Our results also suggested a potential negative relationship (not statistically significant, however) between low-light and high-light RGR (both in mass and in height), which was similar to results of studies of tropical tree seedlings (Agyeman et al. Reference AGYEMAN, SWAINE and THOMPSON1999), but different from some studies that found a trade-off between RGR in low vs. high light conditions, or a positive correlation between RGR in low vs. high light conditions (Bazzaz Reference BAZZAZ1979, Bloor & Grubb Reference BLOOR and GRUBB2003, Kitajima Reference KITAJIMA1994, Osunkoya et al. Reference OSUNKOYA, ASH, HOPKINS and GRAHAM1994, Poorter Reference POORTER1999). Thus, growth rates at different light levels may not be the simple result of differences between shade-tolerant and light-demanding species, but may be determined by intrinsic plant constraints unrelated to shade tolerance, as suggested by Bloor & Grubb (Reference BLOOR and GRUBB2003). Results in this study revealed that variation in RGRm among the study species was much greater in the moderate light than in the high light (Figures 1, 3), resulting in a more obvious performance trade-off in the moderate light. The results may link to the differences in light response and plant flexibility among non-pioneer light-demanding tree species.
Life history as a determinant of growth response
In agreement with general trend, we found a significant relationship between survival and seed size across species. Our results indicated that seed size was negatively correlated with relative mass growth and absolute growth in both moderate-light and high-light conditions, which was similar to relationships that have been observed in woody plants of widely varying shade tolerance (Cornelissen et al. Reference CORNELISSEN, CASTRO-Diez and HUNT1996, Dalling et al. Reference DALLING, WINTER and HUBBELL2004, Grubb & Metcalfe Reference GRUBB and METCALFE1996, Kitajima Reference KITAJIMA1994, Osunkoya et al. Reference OSUNKOYA, ASH, HOPKINS and GRAHAM1994, Paz & Martinez-Ramos Reference PAZ and MARTINEZ-RAMOS2003, Reich et al. Reference REICH, TJOELKER, WALTERS, VANDERKLEIN and BUSCHENA1998a, Reference REICH, WALTERS, TJOELKER, VANDERKLEIN and BUSCHENA1998b; Walters & Reich Reference WALTERS and REICH2000). Small-seeded tree species in our study had higher RGRh under moderate-light levels; this strategy may be obligatory for seedlings to allow them to escape potentially high mortality rates (Seiwa Reference SEIWA2007). To escape shading by neighbours, particularly in competitive, early-successional habitats, early rapid height growth is advantageous for seedling establishment (Ross & Harper Reference ROSS and HARPER1972, Seiwa Reference SEIWA2000, Reference SEIWA2007) because increasing seedling height can dramatically improve the light conditions experienced by the seedlings (Givnish Reference GIVNISH1982). Seed size had a critical effect on species-specific differences in seedling performance along the irradiance gradient in our study. The correlations and constraints we observed in the early stages of the experiment weakened during subsequent growth, which led to species cross-over in RGR, similar to observations from other studies (Baraloto et al. Reference BARALOTO, GOLDBERG and BONAL2005b, Bloor & Grubb Reference BLOOR and GRUBB2003).
Physiological and allocational responses to variation in light availability
The wide variation in RGR among species has been explained mainly by plant morphological variables (Atkin et al. Reference ATKIN, BOTMAN and LAMBERS1996, Cornelissen et al. Reference CORNELISSEN, CASTRO-Diez and HUNT1996, Marañón & Grubb Reference MARAÑÓN and GRUBB1993, Poorter & Remkes Reference POORTER and REMKES1990). RGR was only found to be positively correlated with NAR in our study, similar to results that were found in 12 neotropical pioneer tree seedlings grown in simulated forest gaps in Barro Colorado Nature Monument (Dalling et al. Reference DALLING, WINTER and HUBBELL2004). SLA and LAR have been assumed to be good indicators of interspecific variation in RGR among species and across environmental gradients (Cornelissen et al. Reference CORNELISSEN, CASTRO-Diez and HUNT1996, Poorter & Remkes Reference POORTER and REMKES1990, Westoby Reference WESTOBY1998). However, we found no direct correlation between SLA or LAR and RGR either in high-light or in moderate-light treatments, which was similar to the findings of several previous studies (Bloor & Grubb Reference BLOOR and GRUBB2003, Dalling et al. Reference DALLING, WINTER and HUBBELL2004, Lusk & Pozo Reference LUSK and POZO2002, Poorter Reference POORTER1999). Significant correlations between SLA and RGR may largely reflect the difference between slow-growing, shade-tolerant species with low and largely invariant SLA and fast-growing pioneers with relatively high SLA (Dalling et al. Reference DALLING, WINTER and HUBBELL2004). Shipley (Reference SHIPLEY2006), in a meta-analysis of growth, found that NAR but not LAR was the best predictor of RGR, as found in this study (see also Antúnez et al. Reference ANTÚNEZ, RETAMOSA and VILLAR2001, Ruiz-Robleto & Villar Reference RUIZ-ROBLETO and VILLAR2005). The relative importance of LAR in determining RGR may depend on the irradiance (Shipley Reference SHIPLEY2002). No correlation was also found between Amax and RGR across treatments, similar to results found in neotropical pioneer species (Dalling et al. Reference DALLING, WINTER and HUBBELL2004) and Quercus species (Quero et al. Reference QUERO, VILLAR, MARAÑÓN, ZAMORA, VEGA and SACK2008). Quero et al. (Reference QUERO, VILLAR, MARAÑÓN, ZAMORA, VEGA and SACK2008) proposed that a correlation between Amax and RGR would be strongest only for plants of a range of species grown under high irradiance and water supply; the correlation would be weakened due to interspecific variation in the plasticity of LAR and leaf physiology across a gradient of resource supply. This study fails to support several proposed relationships between growth performance and morphological and functional characteristics of species. Thus, these variables contribute little to variation in growth performance within the non-pioneer light-demanding functional group.
Performance trade-off and species coexistence
Results in this study provide some evidence supporting the hypothesis of niche-partitioning gradients of light availability (i.e. species co-existence) in spatially heterogeneous light environments in forests undergoing secondary succession. This evident trade-off between low-light survival and high-light RGR also supports the idea of light gradient partitioning in heterogeneous light environments (Baraloto et al. Reference BARALOTO, GOLDBERG and BONAL2005b, Dalling & Hubbell Reference DALLING and HUBBELL2002, Kitajima Reference KITAJIMA1994, Kobe Reference KOBE1999, Sánchez-Gómez et al. Reference SÁNCHEZ-GÓMEZ, VALLADARES and ZAVALA2006, Walters & Reich Reference WALTERS and REICH2000). Differences in RGR led to a number of crossovers in the rank order of species according to height and dry mass over time in the current study. A shifting competitive hierarchy among species in a given light regime over time may interact with species-specific responses to environmental variables, thereby promoting species co-existence.
ACKNOWLEDGEMENTS
We are grateful to Prof. Yide Li for assistance during the study, to Prof. Shiman Huang for his assistance in identifying tree species and to Mr. Chaoyong Wang for assistance in seed collection. We also gratefully acknowledge the staff of the Jianfengling Experimental Station (Research Institute of Tropical Forestry, Chinese Academy of Forestry) for their field assistance. This research was funded by National Natural Science Foundation of China (30570298 and 30430570) and Special Research Program for Public-Welfare Forestry (‘Responses of forests to climate change and adaptive strategy of forestry in China’, Grant No. 200804001).