Spatial organization within animal populations is often thought to reflect the outcome of strategies implemented by each individual to enhance its reproductive success and survival (Clutton-Brock Reference CLUTTON-BROCK1989). Thus, while females usually focus on the acquisition of food and breeding sites, male dispersion is more often determined by the distribution and availability of females (Clutton-Brock Reference CLUTTON-BROCK1989). Due to these factors, intraspecific competition for space could lead to the adoption of a territorial strategy, whenever the benefits of territorial defence are higher than the costs (Brown & Orians Reference BROWN and ORIANS1970). Among small mammals, two main hypotheses have been proposed to explain the occurrence of territoriality in females. Ostfeld (Reference OSTFELD1990) proposed that females should defend food resources, so the distribution and availability of food items should determine the cost–benefit relationship of adopting a territorial strategy. However, Wolff (Reference WOLFF1993) developed a hypothesis, based on small rodents, that females should defend nest sites in order to avoid infanticide, the so-called pup-defence hypothesis.
Territoriality has been studied in many species of mammal; however, little is known about its occurrence in didelphid opossums (Didelphimorphia: Didelphidae). In the present study, our main objective was to evaluate patterns of home-range overlap between and within sexes in Marmosops paulensis (Tate 1931), a semelparous didelphid, with a diet composed mainly of fruits and invertebrates (Leiner & Silva Reference LEINER and SILVA2007b) and a breeding season from September to February (Leiner et al. Reference LEINER, SETZ and SILVA2008). As we expected females to be territorial (Croft & Eisenberg Reference CROFT, EISENBERG, Armati, Dickman and Hume2006), the hypotheses of Ostfeld (Reference OSTFELD1990) and Wolff (Reference WOLFF1993) were tested by evaluating if territoriality is widespread throughout the year, in a way that it would be related to resource, and not to pup defence. Although there is no evidence of infanticide among opossums, the pup-defence hypothesis could also be applied to taxa in which young suffer a high predation risk and/or nest sites are limited (Wolff Reference WOLFF1993).
The study was carried out in Parque Estadual Intervales (24°16′S, 48°25′W), an Atlantic Forest area situated in São Paulo state, municipality of Ribeirão Grande, south-eastern Brazil. Home ranges were assessed using capture–mark–recapture techniques on a single large trapping grid covering 2.8 ha (Figure 1), located in mature secondary-growth vegetation. Marmosops paulensis was captured during monthly trapping sessions of five consecutive nights from August 2002 to July 2004 using 80 Sherman live traps (Model XLF15, 10.5 × 12 × 37.6 cm). Traps were baited with a mixture of banana, oatmeal, peanut butter and bacon, and checked daily. All captured individuals were marked with numbered ear-tags and the following data recorded: sex, body mass and reproductive condition. Females were considered reproductive when they had swollen nipples or carried young in the pouch, while male reproductive condition was not assessed.
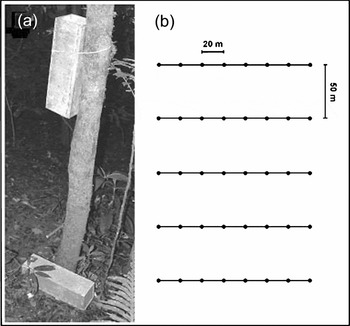
Figure 1. Configuration of the trapping grid, and position of traps used to capture Marmosops paulensis individuals at the study site. Trapping point, containing one Sherman trap in the ground, and the other affixed to a tree, at approximately 1.5–2.0 m (a). Trapping grid containing five parallel lines, separated by 50 m in order to cover a larger area of secondary-growth forest. Each line had eight trapping points (represented by dots) separated by 20 m (b). This trapping design and trap position (always set in the vertical position, with opening upward) have already proved to be efficient to capture terrestrial and scansorial small mammals (Freitas & Fernandez Reference FREITAS and FERNANDEZ1998).
Home-range areas were estimated through the Minimum Convex Polygon (MCP) method, using the program CalHome (Kie et al. Reference KIE, BALDWIN and EVANS1996). To increase reliability, ranges were calculated only for individuals that were captured at least five times. This criterion, adopted in earlier small-mammal studies (Pires et al. Reference PIRES, FERNANDEZ and FREITAS1999), was followed because the MCP method is highly dependent on the number of captures per individual. To evaluate patterns of overlap in home range between and within sexes, we used only individuals that met the five-capture criterion. Overlap was calculated using the program Image Tools 3.
Home range and percentage of overlap were calculated for three males and six females (Table 1) during three periods of equal trapping effort (2400 trap-nights each): two reproductive seasons, from September 2002 to February 2003 and from September 2003 to February 2004, and one non-reproductive season from March 2004 to August 2004. During both reproductive seasons combined, there was no difference (U = 1.59, P = 0.11) in the home-range sizes of males (median = 0.37 ha, range = 0.31–1.5 ha, n = 3) and females (median = 0.30 ha, range = 0.25–0.35 ha, n = 4). We combined reproductive seasons because (1) among didelphids, males usually present larger home ranges than females during the reproductive season, due to sexual dimorphism and to the fact that male dispersion in this period is related to female spatial organization (Cáceres & Monteiro-Filho Reference CÁCERES and MONTEIRO-FILHO2001), and (2) mean female movements during the reproductive season are quite similar between years (Leiner, unpubl. data), thus allowing pooling periods. Although we failed to find a difference between sexes, male ranges were much more variable than those of females (Levene's F test = 19.6, P = 0.006). Outside the breeding season, home ranges could be estimated for two females; both were 0.14 ha.
Table 1. Home-range data for six females and three males Marmosops paulensis (all with at least five captures), and summary of sex, body mass, study period (period in which the individual was captured) and number of captures (including, in parentheses, the number of captures at different trapping points) of these individuals. F (preceding the identification number): female, M: male. M–F overlap: left number represents the percentage of overlap between the studied individual and males, and right number represents the percentage of overlap between the studied individual and females.

There was no overlap among females during the three study periods (Figure 2). In order to illustrate better the relative positions of individuals, those animals that were captured three or four times were included in Figure 2. This allowed us to observe that when a female ceased to be captured inside the trapping grid (i.e. F1, F2 and F5), a new female would occupy its home range (Figure 2a). The disappearance of females from the study grid could be due to emigration, or it is also possible that these females were present but not captured at that particular time interval. The fact that these females were not captured afterwards agrees with the emigration possibility. In this way, the observation that when F1, F2 and F5 left the trapping grid and emigrated, F3, F4 and F6 occupied the whole ranges or part of the areas used by those previous females, gives extra support to the lack of overlap among females (Figure 2a). In contrast, home ranges of two males overlapped one another and the home ranges of six other females (Figure 2a, b). In this period, mean male–male home-range overlap was 30% (range = 12–49%), while mean male–female overlap was 72% (range = 30–100%). During the non-reproductive season, we observed male–female overlap, but could not estimate the percentage (Figure 2c).
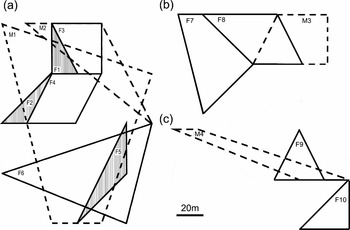
Figure 2. Patterns of home range overlap in a population of Marmosops paulensis. Black lines represent female home ranges, broken lines represent male home ranges, and hatched polygons represent home ranges of those females that ceased to be captured inside the trapping grid, and had their areas encompassed by new females. Individuals captured during the first reproductive season, from September 2002 to February 2003 (a); individuals captured during the second reproductive season, from September to December 2003 (b); individuals captured during the non-reproductive period, from March to July 2004 (c).
Due to the small number of recaptures, estimates of home-range size for M. paulensis (overall mean ± SD = 0.40 ± 0.42 ha, n = 9) in this study should be viewed with caution. Based on the effects of body mass on home-range size of sexually dimorphic species (Harestad & Bunnell Reference HARESTAD and BUNNELL1979), such as M. paulensis (Mustrangi & Patton Reference MUSTRANGI and PATTON1997; Table 1), and the assumption that home ranges of males and females are determined by different factors in small mammals (Ostfeld Reference OSTFELD1990), we expected M. paulensis males to present larger home ranges than females. The lack of difference between sexes agreed with a previous study in this species using the spool-and-line technique, which also failed to find any difference between the sexes (Leiner & Silva Reference LEINER and SILVA2007a), although our results could also be due to small sample sizes and low recapture rate.
The total absence of overlap among females during the reproductive season suggests territoriality. Due to the high costs of lactation in marsupials (Harder et al. Reference HARDER, HSU and GARTON1996), M. paulensis should defend food resources to maximize the chances of rearing well-fed young, especially since this species is semelparous (Leiner et al. Reference LEINER, SETZ and SILVA2008). During the reproductive season, M. paulensis consumes mostly fruits from Piper plants, which produce a few ripe fruits per night, occur in patches and exhibit high spatial and temporal predictability (Leiner & Silva Reference LEINER and SILVA2007b), favouring the occurrence of territorial behaviour in females. The limited number of females captured during the non-reproductive season (n = 2) raises difficulties to the evaluation of female spacing patterns in this period. However, the fact that the two females were captured at least seven times gives more reliability to the observed lack of overlap between them. This result allows speculating about the inapplicability of the pup defence hypothesis, although increasing sample sizes are necessary to point out the occurrence of female territoriality during the non-reproductive season, and the processes behind the adoption of this strategy in this period.
Male ranges overlapped both with other males and with females during the breeding season. In contrast to females, male reproductive success is determined by the availability of females (Ostfeld Reference OSTFELD1990), so that male spatial organization should be a function of female spatial distribution. Hence, when females have large, exclusive territories, it becomes impractical for males to defend access to each female (Soderquist Reference SODERQUIST1995). This seems to be the case in M. paulensis. Males, instead of guarding a single female, probably move between female territories, trying to obtain more matings. In the common shrew (Sorex araneus), this tactic is highly successful, and enables males to produce more offspring (Stockley et al. Reference STOCKLEY, SEARLE, MACDONALD and JONES1996). In this system, males compete for females and dominance is based on body size (Clutton-Brock Reference CLUTTON-BROCK1989, Oakwood Reference OAKWOOD2002). Two species of marsupial, Didelphis virginiana and Antechinus stuartii, are known to mate promiscuously, with larger males gaining access to more receptive females and fathering most young (Holleley et al. Reference HOLLELEY, DICKMAN, CROWTHER and OLDROYD2006, Ryser Reference RYSER1992). In M. paulensis, evidence of wounding in males during the reproductive season could be related to male dominance hierarchy in this species, although future studies should test this hypothesis, by evaluating the role of body size on male reproductive success.
ACKNOWLEDGEMENTS
We are grateful to Chris Dickman, Marco Mello, Marcelo Gonzaga and two anonymous reviewers for the valuable suggestions on the first version of the manuscript. We are indebted to Parque Estadual Intervales for their logistical support and to CAPES and FMB for their financial support.





