INTRODUCTION
In forested ecosystems, vegetation structure is an important selective pressure that shapes the ecology and behaviour of arboreal animals (Emmons & Gentry Reference EMMONS and GENTRY1983, García et al. Reference GARCÍA, ZAMORA and AMICO2011, MacArthur & MacArthur Reference MACARTHUR and MACARTHUR1961). In particular, stem diameter and surface rugosity influence the activities of cursorial arboreal animals (Clay et al. Reference CLAY, BAUER, SOLIS and YANOVIAK2010, Morse et al. Reference MORSE, LAWTON, DODSON and WILLIAMSON1985). Such characteristics interact with body size to influence community parameters, for example, by acting as a template for species interactions and habitat partitioning (Kaspari & Weiser Reference KASPARI and WEISER1999, Sinervo & Losos Reference SINERVO and LOSOS1991, Yanoviak & Kaspari Reference YANOVIAK and KASPARI2000). Here we examine such patterns among ants that use climbing plants as foraging pathways in a lowland tropical forest.
Ants are a particularly good focal taxon for investigating the importance of habitat structure on ecology and behaviour. As cursorial, central-place foragers, ants tend to minimize travel costs by selecting the most efficient pathways to a resource patch (Clay et al. Reference CLAY, BAUER, SOLIS and YANOVIAK2010, Dussutour et al. Reference DUSSUTOUR, NICOLIS, DENEUBOURG and FOURCASSIÉ2006, Farji-Brener et al. Reference FARJI-BRENER, BARRANTES, LAVERDE, FIERRO-CALDERÓN, BASCOPÉ and LÓPEZ2007, Fewell Reference FEWELL1988, Ydenberg et al. Reference YDENBERG, WELHAM, SCHMID-HEMPEL, SCHMID-HEMPEL and BEAUCHAMP1994). Given the prevalence of competition in ant communities (Davidson Reference DAVIDSON1998, Hölldobler & Wilson Reference HÖLLDOBLER and WILSON1990), such patterns likely influence species coexistence. Most studies addressing such questions have focused on epigeic or leaf-litter ants, which can access resource patches from multiple directions (Adler et al. Reference ADLER, LEBRUN and FEENER2007, Davidson Reference DAVIDSON1998). In contrast, the relatively linear and reticulate structure of arboreal habitats constrains foragers to narrow access routes. Thus, patterns of arboreal ant worker distribution within forests should partly reflect their direct interactions with structural characteristics of the vegetation.
Many tropical arboreal ants appear to preferentially use the stems of climbing plants (i.e. lianas) when foraging (S.Y., pers. obs.). This pattern likely arises from two factors. First, climbing plants provide persistent physical connections between trees (Gentry Reference GENTRY, Putz and Mooney1991, Schnitzer & Bongers Reference SCHNITZER and BONGERS2002), expanding the accessible resource base for cursorial arboreal animals by bridging natural gaps in the vegetation (i.e. crown shyness; Emmons & Gentry Reference EMMONS and GENTRY1983, Ng Reference NG1977, Putz Reference PUTZ1984). Second, the narrow pathways and smooth surfaces provided by stems of climbing plants may facilitate rapid discovery of patchy resources (Clay et al. Reference CLAY, BAUER, SOLIS and YANOVIAK2010). Despite these potential contributions to foraging efficiency, not all climbing plant stems in tropical forests are used by ants (S.Y., pers. obs.), and the factors influencing the distribution of ant workers among available stems remain unexplored.
Here we examine associations between the body size of foraging ants and basic characteristics of the stems of climbing plants they use when foraging. We predicted that the largest ants in the La Selva forest (e.g. Paraponera clavata (F.); c. 20 mm body length) would be excluded from the smallest stem diameters, whereas the smallest ants (e.g. Brachymyrmex spp.; c. 1 mm body length) would show no relationship with stem size (Figure 1). We also postulated that body size distributions of ants foraging on climbing plants would be bimodal on relatively rough stems, because very small ants can travel within furrows and large ants can step over them, as has been demonstrated with litter ants (Kaspari & Weiser Reference KASPARI and WEISER1999). In contrast, we expected that ants foraging on relatively smooth stems would exhibit a unimodal body size distribution (Yanoviak & Kaspari Reference YANOVIAK and KASPARI2000).

Figure 1. Predicted relationships between ant body size (log10 body length) and stem size (log10 diameter). The largest ants occurring on a stem are expected to be biomechanically constrained by stem size (relationship A). This pattern ends at the inflection point (*), which indicates the smallest stem diameter that is typically occupied by the largest ant foragers in the forest (in this case, Paraponera clavata). The minimum ant size on stems is expected to be unrelated to stem size (C; i.e. the smallest arboreal ants, Brachymyrmex spp., occur on all stem sizes).
METHODS
This study was conducted at the La Selva Biological Station, Costa Rica (10.43°N, 84.00°W), in June–August 2008 and in January 2010. La Selva is a mosaic of primary and secondary lowland tropical rain forest and receives c. 4000 mm y−1 of rainfall (McDade et al. Reference MCDADE, BAWA, HESPENHEIDE and HARTSHORN1994). All data were collected between 08h00 and 16h00 in fair weather.
We surveyed ants on the stems of climbing plants along 51 distinct trail segments in the forest. Focal trail segments ranged 50–100 m long and were distributed among 15 different trails (cumulative distance c. 5 km). We surveyed each trail segment for at least 1 h, during which climbing plants on one side of the trail were visually scanned for the presence of ants at heights between 1 and 2 m above the ground. Ants were collected into alcohol, and the diameter of the stem at the ant's location was measured with calipers. We also classified each stem as woody or herbaceous and ranked its surface rugosity as relatively smooth or furrowed.
We repeated the stem survey on the opposite side of each trail segment as described above. However, to reduce the probability of under-sampling small or cryptic ants, we placed baits (tuna mixed with honey) on the climber stems to attract foraging workers and to maximize the range of stem sizes examined in the project. Consequently, the size distribution of baited stems differed from that of unbaited stems (G = 59.0, df = 9, P < 0.001; Figure 2). As above, the diameter of each stem was measured at the bait location with calipers. We collected a representative sample of the ants present at a bait after 30 min. For both surveys (unbaited and baited) all sampled stems were within 5 m of the trail and each trail segment was surveyed only once.
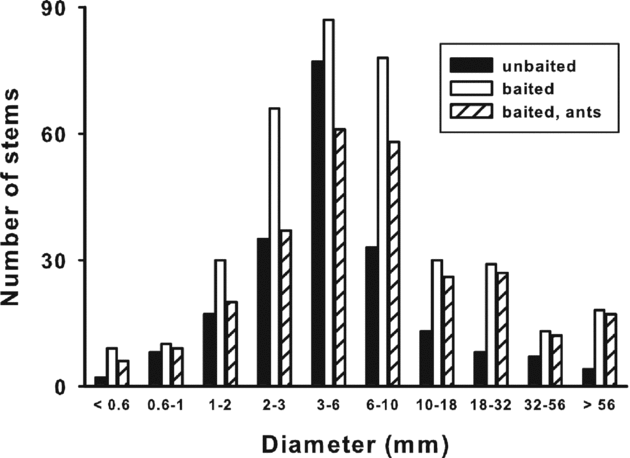
Figure 2. Frequency distributions of stem diameters of climbing plants along selected trails in the forest at La Selva Biological Station, Costa Rica. Data were obtained by measuring stem diameters of plants naturally used by foraging ants (filled bars) or that were baited to attract ants (open bars). Most (79%) baited stems attracted ants (hatched bars). The two baited distributions are statistically similar to each other, but differ from the unbaited distribution due to sampling bias, particularly for large stems.
We used the average body length (measured from the clypeus to the apex of the gaster) of up to five workers of each ant species as an estimate of body size. We only measured mid-size workers of polymorphic species. Worker body length was correlated with mass (r = 0.944, P < 0.001, n = 336). Ants were identified to species or morphospecies, and vouchers were deposited at the Instituto Nacional de la Biodiversidad (INBio) in Costa Rica.
Most analyses were applied to data from baited and unbaited stems separately, because the baits affected ant behaviour. We used linear regression to test the predictions illustrated in Figure 1 by analyzing diameter–body length relationships for the largest and smallest ants across the full range of stem size categories. We then divided the stem size distribution into 10 bins of 0.25 log10 units (ranging from <0.6 mm to >56 mm; Figure 2) and used stem diameters for the five largest and five smallest ants in each bin as raw data for the analysis (hereafter, large-ant and small-ant data sets).
We ran a series of regressions on the large-ant data set to determine the range of stem diameters that have the strongest effect on ant body size, and to establish the minimum stem diameter beyond which the length of the largest ants does not vary with stem size (i.e. the inflection point, Figure 1). We began with the complete large-ant data set and progressively removed ants grouped in the largest stem diameter categories (i.e. five ants at a time) for each subsequent regression. We determined the inflection point based on the regression model that provided the largest R2 value and significant slope with power > 0.80, while still meeting assumptions of normality and constant variance. Bonferroni–adjusted α was used to determine statistical significance.
To examine possible bait effects on ant distribution, we used G-tests (Sokal & Rohlf Reference SOKAL and ROHLF1995) to determine if the size distribution of baited stems with ants present differed from the size distribution of all baited stems, and that of unbaited stems. We similarly compared the body-size distribution of ant species between rough and smooth stem classes for the entire data set. We used a nested ANOVA to assess differences in average ant body size between stem type (woody vs. herbaceous) and rugosity (rough vs. smooth) within stem types. Data were tested for normality before analysis (Shapiro–Wilk W) and log-transformed when necessary to correct variance heterogeneity (Sokal & Rohlf Reference SOKAL and ROHLF1995). All t-tests assumed equal variance (as determined by F tests). Least-square means are given for tests involving more than one factor, and all means are reported ± 1 SE.
RESULTS
We collected 58 ant species from 671 climber stems in the study (ant species list is available from the authors). The predicted relationship between stem diameter and body length of the largest ants was supported for workers on unbaited stems (Figure 3, regressions A and B). Subsequent regressions showed that the strongest stem size–ant size relationship occurred over the range of stem diameters from < 0.6 mm to 3.2 mm (F1,13 = 22.1, R2 = 0.63, P < 0.001; Figure 3, regression A), which revealed an inflection point of c. 3.2 mm for this ant community. The largest ants (Paraponera clavata and Pachycondyla spp.) were not found on unbaited stems < 2.7 mm and < 1.5 mm in diameter, respectively. At stem diameters > 3.2 mm, the body length of the largest ants did not vary with stem size (F1,18 = 3.89, R2 = 0.18, P = 0.064, α = 0.01; Figure 3, regression B). Likewise, our prediction that small ants would be broadly distributed among stem sizes (Figure 1) generally was supported; Brachymyrmex spp., Pheidole spp. and other ants < 2 mm in body length were found on the full range of stem diameters (Figure 3).

Figure 3. Observed relationship between ant body length and the stem diameter of climbing plants in the forest at La Selva Biological Station, Costa Rica. Data from baited stems are indicated by crosses and unbaited stems by circles. Filled circles represent data used in regressions (i.e. small-ant and large-ant data sets from unbaited stems). Some data are obscured by overlap. Regressions A and B illustrate the positive relationship between large ant size and stem size for stem diameters ≤ 3.2 mm (body length = 5.17(stem diameter)1.28), and the lack of relationship for stem diameters > 3.2 mm, respectively (Figure 1). Dashed lines indicate 95% confidence limits. Line C illustrates the lack of significant relationship between stem diameter and the body length of the smallest ants on unbaited stems (Figure 1). All regression analyses used data from unbaited stems only.
Most (79%) of the 467 baited stems attracted ants (Figure 2). In contrast to the results for unbaited stems, there was no linear relationship between stem size and body size of the largest ants on baited stems (F < 5.15, R2 < 0.22, P > 0.033, α = 0.01). Large ants (P. clavata and Pachycondyla spp.) occasionally used very small stems (≤ 1 mm diameter) when attracted by baits (Figure 3).
The different results obtained for baited and unbaited stems was not an artifact of the range of stem sizes sampled. The size distribution of baited stems with ants present was similar to that of unbaited stems up to 3.2 mm (G = 4.33, df = 3, P = 0.23), and the size distribution of baited stems with ants present did not differ from that of all baited stems (G = 7.3, df = 9, P = 0.61; Figure 2). The average diameter of unbaited stems (8.4 ± 1.20 mm) was similar to the diameter of baited stems (10.4 ± 0.80 mm; df = 669, t = 1.29, P = 0.198), but average ant body length was smaller on baited stems (3.6 ± 0.22 mm) than on unbaited stems (5.8 ± 0.29 mm; df = 572, t = 7.44, P < 0.001).
The body-size distribution for ant species occurring on rough stems exhibited greater departures from unimodality than for ant species on relatively smooth stems (Figure 4), although neither distribution followed expectations derived from a normal curve (G > 14.0, df = 5, P < 0.012). Departures from normality were largely due to fewer Pheidole spp. (1.8–3.2 mm body length) and the absence of Pachycondyla spp., Odontomachus spp. and Pseudomyrmex gracilis (F.) (10–18 mm body length) on rough stems. Woody stems had larger ants (5.2 ± 0.32 mm) than herbaceous stems (3.4 ± 0.53 mm; F1,428 = 9.26, P = 0.003), but contrary to our expectations, there was no significant difference in average ant size between relatively smooth and rough stems within woody and herbaceous categories (F2,428 = 1.45, P = 0.24). Analyzing baited and unbaited stems separately did not change the outcome nor the direction of size differences.

Figure 4. Ant species frequency among different body length classes on relatively rough (open bars) and smooth stems (filled bars) of climbing plants in the forest at La Selva Biological Station, Costa Rica. Fifty-eight ant species were observed using one or more of the 671 stems observed in the study; 48 ant species occurred on smooth stems and 23 on rough stems.
DISCUSSION
Most information regarding resource discovery and dominance in ants is derived from observations of foragers on the ground or litter, which can potentially access food patches from all sides (Adler et al. Reference ADLER, LEBRUN and FEENER2007, Davidson Reference DAVIDSON1998, Hölldobler & Wilson Reference HÖLLDOBLER and WILSON1990). By contrast, the reticulate structure of the domain of arboreal ants (i.e. above-ground vegetation) physically limits foraging to relatively narrow linear pathways. Here we show that small-scale structural properties of such pathways affect the foraging behaviours of arboreal ants. Specifically, we show that ants do not use the full range of stems available in the forest, and that stem diameter influences the distribution of workers based on body size. In the La Selva forest, this effect is most evident in the distribution of large ants (> 5 mm body length) among stem diameters less than c. 3 mm. However, this ecological filter becomes quite porous when ants are attracted to an especially rich food source; large ants will (slowly) traverse relatively small stems to access baits. Similar patterns occur with respect to temporal activity in ants; diurnal species will forage at night if provided with high quality resources (Davidson et al. Reference DAVIDSON, COOK and SNELLING2004, Yanoviak et al. Reference YANOVIAK, MUNK and DUDLEY2012). Consequently, ant ecologists should reconsider the common practice of using artificially high quality resources (e.g. a rich mix of protein and carbohydrates) as bait, particularly for behavioural studies focused on foraging.
Despite the strong bait effects, the general relationships between stem characteristics and forager body size described here are likely to be widespread, assuming the distribution of ants among stems partly reflects biomechanical constraints and the costs of locomotion. Although we did not include kinematic or phylogenetic analysis in this study, similar body size–habitat structure relationships occur in leaf litter and epigeic ant assemblages (Farji-Brener et al. Reference FARJI-BRENER, BARRANTES and RUGGIERO2004, Kaspari & Weiser Reference KASPARI and WEISER1999, Sarty et al. Reference SARTY, ABBOTT and LESTER2006), and may explain differences in body size distributions between arboreal and litter ants (Yanoviak & Kaspari Reference YANOVIAK and KASPARI2000). We also did not quantify stem roughness, but our field observations and our results comparing relative stem roughness suggest that arboreal ants walk effectively on a broad range of substrate rugosities. This likely reflects the resilience and versatility of the alternating tripod gait of cursorial insects (Reinhardt et al. Reference REINHARDT, WEIHMANN and BLICKHAN2009, Sponberg & Full Reference SPONBERG and FULL2007), although such biomechanical patterns remain largely unresolved for ants.
On large spatial scales, the quantitative details of the body size–stem size relationship we observed should vary predictably among ant communities based on their body size composition. Both ants and climbing plants have broad biogeographic ranges (Gentry Reference GENTRY, Putz and Mooney1991, Hölldobler & Wilson Reference HÖLLDOBLER and WILSON1990), and ant worker size tends to vary with latitude (Cushman et al. Reference CUSHMAN, LAWTON and MANLY1993). Thus, replication of this project at various locations representing gradients in ant size distribution would appropriately test the general applicability of our results.
Physical habitat characteristics provide the foundation upon which biological communities develop in ecological and evolutionary time (MacArthur & MacArthur Reference MACARTHUR and MACARTHUR1961, Southwood Reference SOUTHWOOD1988, Tews et al. Reference TEWS, BROSE, GRIMM, TIELBÖRGER, WICHMANN, SCHWAGER and JELTSCH2004), and diversification in plant structure likely played a role in the evolutionary diversification of ants (Moreau et al. Reference MOREAU, BELL, VILA, ARCHIBALD and PIERCE2006). Although plants provide many different resources for ants (Rico-Gray & Oliveira Reference RICO-GRAY and OLIVEIRA2007), plant structure is particularly relevant to ant ecology – it is a defensible environmental feature that influences access to distant energy sources. Thus, vegetation structure should affect ant community structure on ecological time scales via effects on foraging efficiency and the frequency of aggressive interactions (Bentley Reference BENTLEY1981, Davidson et al. Reference DAVIDSON, SNELLING and LONGINO1989, Yanoviak & Kaspari Reference YANOVIAK and KASPARI2000). Such effects probably would be revealed by experimental manipulation of substrate characteristics and staged interactions among potential competitors.
This study was not designed to include analyses of species richness; however, the cumulative number of species found on stems was nearly double that found at similar heights on tree trunks in the same forest (Clay et al. Reference CLAY, BAUER, SOLIS and YANOVIAK2010). Here, as in the study by Clay et al. (Reference CLAY, BAUER, SOLIS and YANOVIAK2010), collecting ants 1–2 m above the ground under-sampled obligate canopy species (e.g. some Cephalotes spp.) while capturing ground-nesting species occasionally foraging on understorey vegetation (e.g. Ectatomma ruidum (Roger)). A replicate study conducted entirely in the canopy may give different results with respect to stem size distributions and the relationship between stem diameter and ant body length.
Among the conspicuous living components that constitute tropical forests, all available evidence indicates that climbing plants, especially lianas, are the canopy component most likely to show a rapid response to climate change, especially increased CO2 (Körner Reference KÖRNER2009, Phillips et al. Reference PHILLIPS, VÁSQUEZ MARTÍNEZ, ARROYO, BAKER, KILLEEN, LEWIS, MALHI, MENDOZA, NEILL, VARGAS, ALEXIADES, CERÓN, FIORE, ERWIN, JARDIM, PALACIOS, SALDIAS and VINCETI2002); indeed, liana abundance has been increasing at an accelerating rate over the past few decades (Phillips et al. Reference PHILLIPS, VÁSQUEZ MARTÍNEZ, ARROYO, BAKER, KILLEEN, LEWIS, MALHI, MENDOZA, NEILL, VARGAS, ALEXIADES, CERÓN, FIORE, ERWIN, JARDIM, PALACIOS, SALDIAS and VINCETI2002, Wright et al. Reference WRIGHT, CALDERÓN, HERNANDÉZ and PATON2004). Thus, understanding the relevance of lianas and other climbing plants to the behaviour and community structure of animals that use them is important to predicting future patterns of biodiversity.
ACKNOWLEDGEMENTS
We thank S. Letcher for useful discussions and B. Guenard for assistance with ant identifications. The Organization for Tropical Studies (OTS) and the staff of the La Selva Biological Station provided logistical support. The Costa Rican Ministerio del Ambiente y Energía (MINAE) provided permits. Participation of CS and CH was facilitated by the National Science Foundation La Selva REU program and OTS course 2010–1, respectively. We thank the members of both programs for their helpful insights. Comments from an anonymous reviewer improved the manuscript. The project was supported by grants from the National Geographic Society (7896–05) and NSF (IOS–0843120) to SY.






