INTRODUCTION
In tropical forests, light is spatially and temporally heterogeneous and considered to be the most limiting resource for plant growth, especially in the understorey (Chazdon et al. Reference CHAZDON, WILLIAMS and FIELD1988, Poorter & Werger Reference POORTER and WERGER1999). Many studies have shown that light strongly influences growth responses in terms of size, leaf dynamics, morphology, architecture, carbon gain and allocation (Bloor & Grubb Reference BLOOR and GRUBB2004, Canham Reference CANHAM1989, Denslow et al. Reference DENSLOW, SCHULTZ, VITOUSEK and STRAIN1990, Poorter Reference POORTER1999, Schmid & Bazzaz Reference SCHMID and BAZZAZ1994, Sterck et al. Reference STERCK, MARTÍNEZ-RAMOS, DYER-LEAL, RODRÍGUEZ-VELÁZQUEZ and POORTER2003, Whitmore & Brown Reference WHITMORE and BROWN1996). The effect of light availability rather than soil resources, i.e. nutrients and water, is expected to determine the performance of plants in forest conditions (Baltzer et al. Reference BALTZER, THOMAS, NILUS and BURSLEM2005, Baraloto et al. Reference BARALOTO, BONAL and GOLDBERG2006, Denslow et al. Reference DENSLOW, SCHULTZ, VITOUSEK and STRAIN1990, Engelbrecht et al. Reference ENGELBRECHT, COMITA, CONDIT, KURSAR, TYREE, TURNER and HUBBELL2007, Poorter Reference POORTER1999, Turner et al. Reference TURNER, BROWN and NEWTON1993, but see Baker et al. Reference BAKER, BURSLEM and SWAINE2003a, Reference BAKER, SWAINE and BURSLEMb). Leaf traits are known to vary with productivity of a site, but for tropical forests these relationships have mainly been shown in relation to light conditions (Sterck et al. Reference STERCK, POORTER and SCHIEVING2006). In general, however, it can be predicted that with increasing site productivity (high light, or increasing soil fertility) leaf longevity reduces while photosynthetic capacity and specific leaf area (leaf area:leaf mass ratio) typically increase (Ackerly & Bazzaz Reference ACKERLY and BAZZAZ1995). Plants growing in poor soils have slower leaf dynamics than plants from rich soils, and these plants may invest more in defences and suffer less damage by herbivores (Boege & Dirzo Reference BOEGE and DIRZO2004, Coley Reference COLEY1988, Coley et al. Reference COLEY, BRYANT and CHAPIN1985). With respect to growth, it has been suggested that soil nutrients and water availability might not be a limiting factor and that light is the dominant factor in tropical forests (Denslow et al. Reference DENSLOW, SCHULTZ, VITOUSEK and STRAIN1990, Turner et al. Reference TURNER, BROWN and NEWTON1993, Whitmore & Brown Reference WHITMORE and BROWN1996). For example, in fertilization experiments in Costa Rican and Malaysian forest, the addition of nutrients did not significantly affect growth rate in any light environment (Denslow et al. Reference DENSLOW, SCHULTZ, VITOUSEK and STRAIN1990, Turner et al. Reference TURNER, BROWN and NEWTON1993). Other authors show, however, that soil nutrients and water availability influence growth and plant development significantly (Baker et al. Reference BAKER, BURSLEM and SWAINE2003a, Reference BAKER, SWAINE and BURSLEMb; Baltzer et al. Reference BALTZER, THOMAS, NILUS and BURSLEM2005, Baraloto et al. Reference BARALOTO, BONAL and GOLDBERG2006, Boege & Dirzo Reference BOEGE and DIRZO2004, Burslem et al. Reference BURSLEM, GRUBB and TURNER1996). Most of the studies were however carried out under strong artificial fertilization contrast in shadehouses (Baraloto et al. Reference BARALOTO, BONAL and GOLDBERG2006, Burslem et al. Reference BURSLEM, GRUBB and TURNER1996, Gunatilleke et al. Reference GUNATILLEKE, GUNATILLEKE, PERERA, BURSLEM, ASHTON and ASHTON1997), while only few studies report on natural field conditions (Baker et al. Reference BAKER, SWAINE and BURSLEM2003b, Boege & Dirzo Reference BOEGE and DIRZO2004). The interaction effect for the availability of light and soil resources remains particularly poorly studied under field conditions.
We explored how tree saplings respond to light level and availability of soil resources in terms of leaf traits and whole plant growth. For this purpose, we studied the shade-tolerant tree species Brosimum alicastrum in a tropical forest in the south-east of Mexico. We compared crown growth, leaf traits and herbivory patterns between saplings from closed-forest sites and open gaps on rich soils and on poor soils (in nutrient and water availability) and predicted that saplings released from resource scarcity (i.e. saplings at high light exposure on rich soils) will (1) produce cheap leaves (high SLA) at high rates, which are sensitive to herbivore damage, and (2) grow more rapidly in size. We expect effects from either light or soil conditions, and particularly their interaction might limit strongly sapling performance.
METHODS
Study site
This study was conducted near the Chajul Biological Field Station (l6°07′12″N–90°55′31″ W), in the Montes Azules Biosphere Reserve (MABR), within the Lacandona region in south-east Mexico. The climate is warm and humid, with an average annual precipitation of 3850 mm and a dry season between February and April with less than 100 mm of rain per month. The mean annual temperature is 25°C.
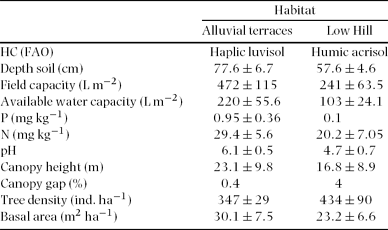
In the region a mosaic of vegetation types is present including lowland evergreen rain forest, semi-deciduous forests on karst outcrops, and savanna-like vegetation. MABR covers an area of 331 230 ha and around 3400 species of vascular plant, of which 573 are trees, have been recorded (Martínez et al. Reference MARTÍNEZ, RAMOS and CHIANG1994). The structure and composition of the lowland rain forest varies with soil and topography (Celedón Reference CELEDÓN2006, Ibarra-Manriquez & Martínez-Ramos Reference IBARRA-MANRIQUEZ and MARTÍNEZ-RAMOS2002, Siebe et al. Reference SIEBE, MARTÍNEZ-RAMOS, SEGURA-WARNHOLTZ, RODRÍGUEZ-VELÁZQUEZ, SÁNCHEZ-BELTRÁN and Sigmarangkir1996). This study included two contrasting geomorphologic units (henceforth ‘habitats’) contrasting in nutrient content and water availability: (1) alluvial terraces along the rivers; and (2) low hills (altitude: 115–300 m asl). Previous studies found that the first are characterized by flat and fertile soils with good availability of N, P and water while the latter are poor sandy or limestone soils low in nutrients and water availability (Table 1). At the low-hill sites gaps are more often created, as suggested from the high gap cover (4%) on low-hill sites compared to alluvial sites (0.4%, Table 1). The average canopy height is higher at alluvial (23 ± 2.6 m) than across low-hill sites (16 ± 0.6 m) (M. van Breugel, unpubl. data). Dialium guianense (Aubl.) Sandwith (Fabaceae); Guarea glabra Vahl (Meliaceae); Ampelocera hottlei (Standl.) Standl. (Ulmaceae); Spondias radlkoferi Donn. Sm. (Anacardiaceae); Licania platypus (Hemsl.) Pittier (Chrysobalanaceae); Cupania dentata DC. (Sapindaceae) and Brosimum alicastrum Sw. (Moraceae) are among the dominant canopy species in both habitats. More details on soil properties and characteristics of the tree communities are presented in Table 1 (Celedón Reference CELEDÓN2006, Ibarra-Manriquez & Martínez-Ramos Reference IBARRA-MANRIQUEZ and MARTÍNEZ-RAMOS2002, Martínez-Ramos & Rodriguez-Velazquez unpubl. data).
Table 1. Soil and tree community characteristics of the habitats studied at Chajul Biological Field Station, south-east Mexico.
Study species
Brosimum alicastrum Sw. (Moraceae) is found from Mexico to the northern part of South America. Its distribution range includes humid, sub-humid and sub-dry tropical forests. It has been found to be locally abundant over its whole range, but its maximum height decreases with decreasing water availability (Pennington & Sarukhán Reference PENNINGTON and SARUKHÁN1998). In our study region B. alicastrum is more abundant on the low hills (20 ind. ha−1 ≥ 10 cm dbh), where it can reach 25 m height and 80 cm dbh and 1.45 m2 ha−1 of basal area. In contrast on alluvial terraces it presents lower densities (6 ind. ha−1 ≥ 10 cm dbh), but it can reach up to 150 cm dbh and 35 m height, and double basal area (2.9 m2 ha−1) (Martínez-Ramos & Rodriguez-Velazquez, unpubl. data). Brosimum aliscastrum is a shade-tolerant tree species with abundant regeneration in the understorey (Pennington & Sarukhán Reference PENNINGTON and SARUKHÁN1998, Rodriguez-Velazquez et al. Reference RODRIGUEZ-VELAZQUEZ, MARTÍNEZ-RAMOS, DYER-LEAL, González, Dirzo and Voght1997).
Data collection
In March 2000, saplings of Brosimum alicastrum were selected along a trail near the Chajul station that crosses alluvial terraces and low hills. For both habitats, we selected saplings in high (gap) and low (understorey) canopy openness, using a crown illumination index (Clark & Clark Reference CLARK and CLARK1992). For an accurate estimation of the effects on the response variables and to be able to compare individuals from gaps and understorey only recent (≤ 12 mo) and large (≥ 200 m2) gaps were used. Additionally we controlled variation selecting only non-reproductive saplings of comparable height (between 1 m and 1.50 m) with little or no herbivore damage. Individuals from gaps were exposed to overhead light or to crown completely exposed to vertical and lateral light. In contrast understorey saplings had no direct light or low to medium lateral light (Clark & Clark Reference CLARK and CLARK1992). Due to the specific conditions we required and to avoid pseudoreplication only one individual per gap was selected and saplings were separated by 100–1000 m from each other. From the combination of soil and light factors four different treatments resulted with differences in soil and canopy openness and a total of 67 individuals recorded, although for analyses those saplings broken during the study period were discarded (number of individuals per treatment: 13–17).
For every sapling we measured height and crown diameter in two perpendicular directions. Apical meristems of leaf-bearing shoots and leaves were counted and permanently marked. Leaf length was measured for all leaves and used to estimate leaf area. Crown area was calculated for each sapling, as an ellipse from the two perpendicular crown diameters and then used to calculate leaf area index (LAI), as total leaf area divided by crown area. After 1 y, height crown diameter, LAI, leaf area, apical meristems and leaves were again measured and counted and growth or change for every attribute was calculated. In this way we were able to detect growth of meristems and shed and newly produced leaves for the whole period of study. Selected saplings had little or no herbivory at either site and for the second census we estimated herbivory levels for all newly produced leaves, using visual damage classes of 20% (0%, ≤ 20%, ≤ 40%, ≤ 60%, ≤ 80%, ≤ 100%).
For each treatment we selected two leaves from five different saplings at each site (40 leaves) and determined length, leaf area and dry weight for each one. All saplings were growing close to the saplings from the growth study. Leaf area was measured with a leaf area meter (DeltaT Devices, Cambridge, England). Leaves were then dried for 72 h at a temperature of 70 °C, whereupon dry weight was measured. Average specific leaf area (SLA) was calculated per treatment as leaf area divided by dry weight.
Soil and vegetation data
The alluvial terraces and low-hill sites selected for our study differ significantly in soil properties (Celedón Reference CELEDÓN2006, Siebe et al. Reference SIEBE, MARTÍNEZ-RAMOS, SEGURA-WARNHOLTZ, RODRÍGUEZ-VELÁZQUEZ, SÁNCHEZ-BELTRÁN and Sigmarangkir1996). The alluvial terraces are characterized by deeper soils with a higher field capacity, a higher water capacity, a higher pH, higher levels for the major nutrients, and they supported higher stands than the low hills (Table 1). We considered field capacity and and available water capacity as proxies of water availability. Average available P and N are provided as indicators of soil nutrient availability. Tree community attributes are based on censuses of trees with a dbh ≥ 10 cm in three to five 0.5-ha plots in each habitat (M. Martínez-Ramos, unpubl. data). Canopy height and canopy gap area was measured above a 5 × 5-m grid in the 0.5-ha plots, means (± SD) are based on data lumped per habitat (M. van Breugel, unpubl. data). We thus consider the alluvial-terrace sites as a more productive soil than the low-hill sites.
Analyses
All 40 harvested leaves were pooled and used to determine the relationship between leaf length and leaf area. The best fit was provided by a power function and was highly significant (leaf area = 0.138 (leaf length)2.1, R2 = 0.93, P < 0.001). This regression was used to calculate the area of all individual leaves and the total leaf area of every sapling from the growth study.
Mean leaf life span (LLS) in months was calculated using the widely used formula from King (Reference KING1994):
where na is the mean number of leaves alive in a year, and np and ns are the mean number of leaves produced and leaves shed per year, respectively.
We tested for differences between the four treatments with regard to new meristem production (a branching rate), leaf area production, height growth, leaf area index, leaf life span, specific leaf area, percentage of leaves damaged by herbivores, and leaf area loss per damaged leaf. Data were analysed using generalized linear models (Crawley Reference CRAWLEY1993) testing two factors and their interaction: soil (rich versus poor) and canopy openness (high and low). Initial height and leaf area did not differ between treatments and were therefore not included as covariates (two-way analysis of variance Initial height: F 1,56 = 1.43, P = 0.235, Initial leaf area: F 1,56 = 1.61, P = 0.332). A two-way ANOVA with normal error distribution and identity link function were applied in the case of the continuous variables height growth, leaf area, leaf area index, leaf life span and specific leaf area (Crawley Reference CRAWLEY1993). Prior to analysis, we tested for normality and homogeneity of variance with the Kolmogorov–Smirnov test and the Bartlett's test respectively and ln-transformations were applied when required. Meristem production was quantified using counts, therefore an analysis of deviance using a Poisson error distribution and a log-link function was used. For leaves with herbivory and percentage of leaf area eaten, due to the binary nature of these data, an analysis of deviance with a binomial error distribution and logit link function was applied. In models with binomial and Poisson errors, the deviance explained by each factor approximates chi-square values (Crawley Reference CRAWLEY1993), which were then used to test factor effects. To correct for over-dispersion problems, rescaling was performed when needed. When a factor in the analysis of deviance or ANOVA models was significant, post hoc tests were conducted to determine significance of differences among treatments using Bonferroni pair-wise contrasts (Crawley Reference CRAWLEY1993). The analyses of deviance and variance were conducted using Glim 3.7 (Royal Statistical Society, London, UK) and the K-S normality and Bartlett's homogeneity of variances test were performed using the Minitab ver. 14 statistical package.
RESULTS
We did not find differences among treatments in any measured structural trait (height, leaf area index, number of apices) at the first census (data not shown). However, almost all traits referring to growth, leaf dynamics and herbivory differed significantly between different sites. Saplings on rich soils had a higher SLA (∼17 m2 kg−1) than saplings on poor soil (∼15 m2 kg−1) and unexpectedly SLA was not significantly affected by canopy openness (Figure 1a; Table 2). Leaf life span (LLS) was, however, unaffected by soil (Table 2). Gap saplings had shorter LLS (∼20 mo) than those from the low canopy openness (∼29 mo; Figure 1b).
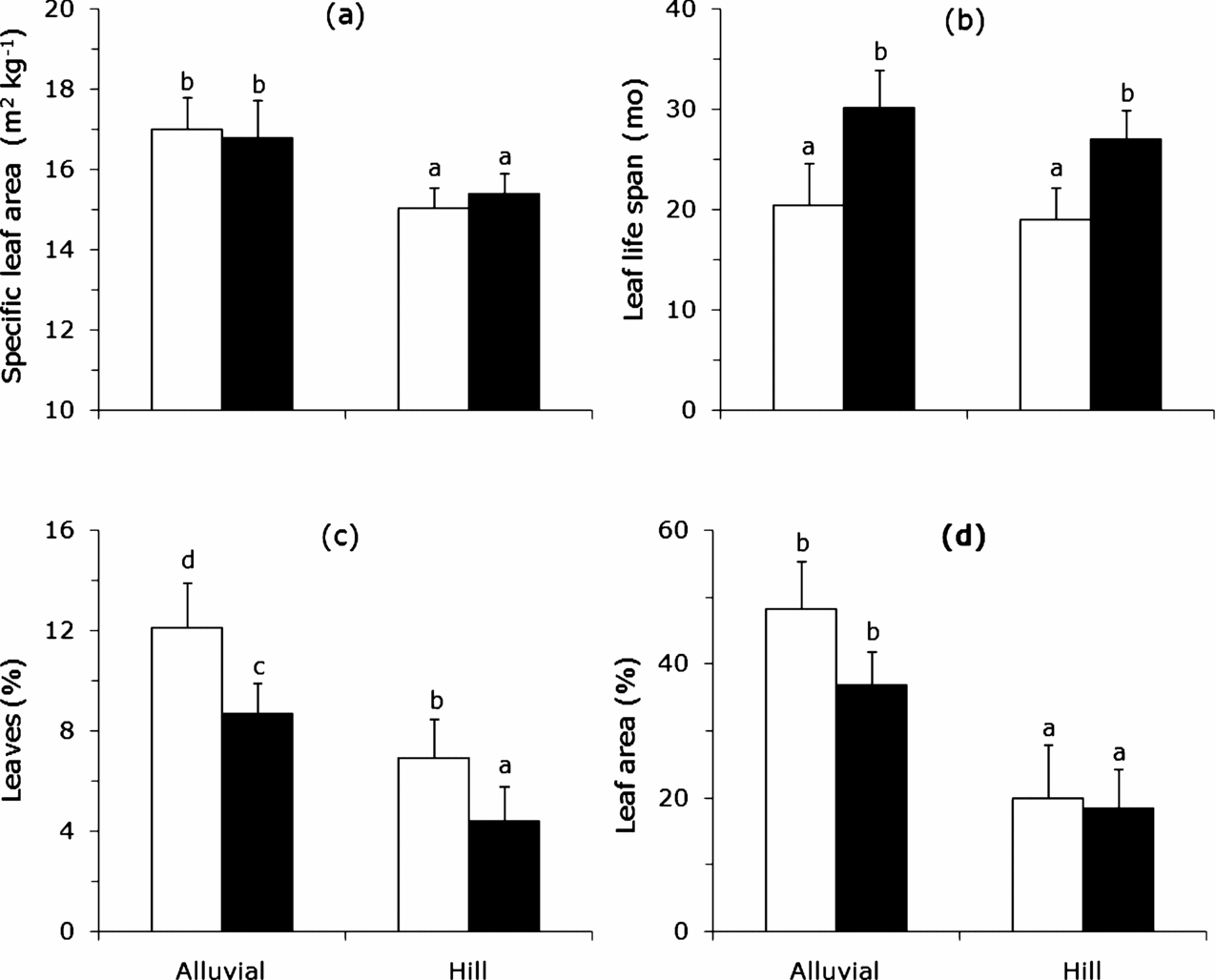
Figure 1. Leaf traits of saplings of Brosimum alicastrum in a tropical forest in south-east Mexico growing in high (alluvial) and low (hill) soil resources with different light levels, open bars represent gaps (high canopy openness) and filled bars understorey (low canopy openness). Specific leaf area (a); Leaf life span calculated from King (Reference KING1994) (b); Percentage of leaves damaged (c); Percentage of leaf area damaged (d). Values presented are untransformed. Bars with the same superscript letter are not significantly different, but differ from those with different letter according to statistical analyses (at least P < 0.05).
Table 2. Statistics for leaf traits and crown growth in saplings of Brosimum alicastrum in four different treatments with contrasting soil and canopy openness at the Chajul Biological Field Station, south-east Mexico. SLA = Specific leaf area, LLS = Leaf life span, HL = Percentage of herbivory on leaves, HLA = Percentage of herbivory on leaf area, LAI = Leaf area index, F/χ 2: F refers to analysis of variance for SLA, height, leaf area and LAI and χ2 to analysis of deviance for LLS, HL, HLA and meristems. P values are provided and * indicates statistical significance.
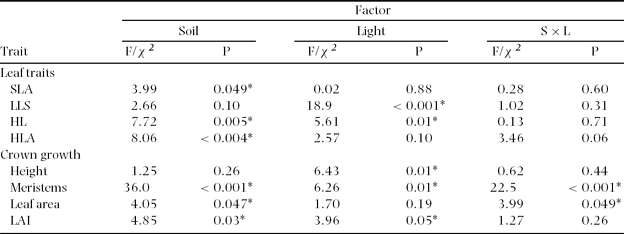
Trees in conditions with rich resource levels had more damaged leaves and were significantly more heavily damaged (Figure 1c, d). At one extreme, gap saplings on rich soils had 12% of their leaves damaged, while understorey saplings on poor soils only 4%. Moreover the former had 45% of their leaf area damaged and the latter only 19% and this damage was only affected by soil richness (Figure 1d; Table 2).
The soil-canopy openness interaction influenced the production of meristems and leaf area, but not height growth (Figure 2). Gap saplings on rich soils produced up to five times more meristems than the other saplings. Soil and light had minor independent effects, indicating that both may to some extent limit meristem production separately (Figure 2b; Table 2). The interaction effect on meristem production was accompanied by an interaction effect on leaf area production (Table 2). Gap saplings on the richer soils produced almost double the amount of leaf area compared to other saplings (Figure 2c). For height growth only a canopy-openness effect was observed: gap saplings grew 23 cm in height on average while understorey saplings grew only 12 cm. The height growth differences between poor and rich soils were not significant (Figure 2a). Leaf area index (LAI) decreased on average in all four treatments suggesting a general ontogenetic trend. The decline in LAI was, however, stronger under resource limitation, either from the light or soil (Figure 2d; Table 2). In general the interaction effect on leaf area and meristem production suggests that saplings grow at much higher rates when neither light nor soil resources were scarce.
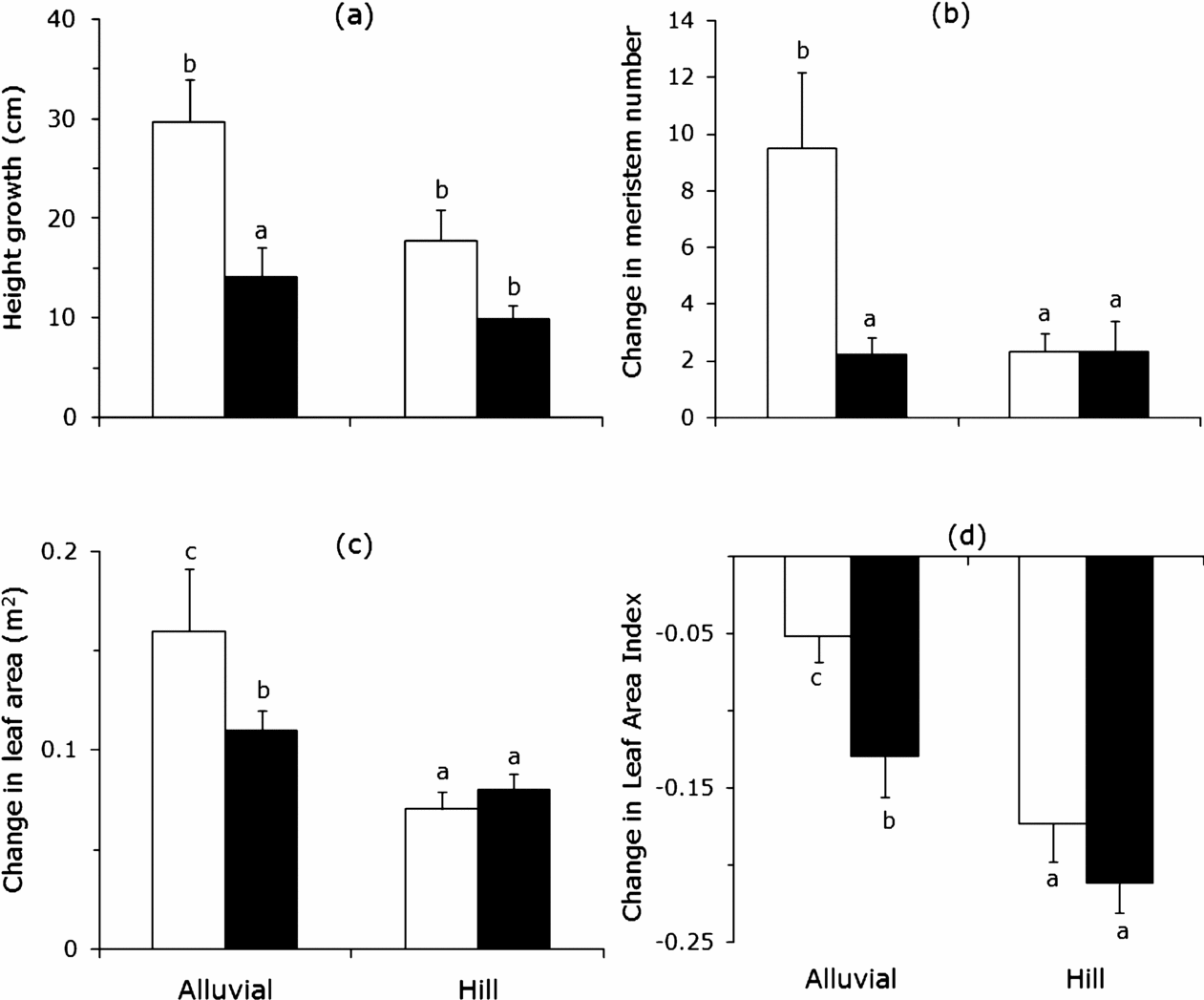
Figure 2. Annual response in plant traits of saplings of Brosimum alicastrum in a tropical forest in south-east Mexico growing in high (alluvial) and low (hill) soil resources sites with different light levels, open bars represent gaps (high canopy openness) and filled bars understorey (low canopy openness). Height growth (a); Change in the number of meristems (b); Change in leaf area (c); Decline in Leaf area index (LAI) (d). Values represented are untransformed. Bars with the same superscript letters are significantly similar, but differ from those with different letter according to statistical analyses (at least P < 0.05).
DISCUSSION
As indicated by soil analysis provided in Table 1, alluvial terraces provide better nutrient and water conditions to plants than low hills, and such differences might affect the performance of saplings of Brosimum alicastrum (Celedón Reference CELEDÓN2006, Ibarra-Manriquez & Martínez-Ramos Reference IBARRA-MANRIQUEZ and MARTÍNEZ-RAMOS2002, Siebe et al. Reference SIEBE, MARTÍNEZ-RAMOS, SEGURA-WARNHOLTZ, RODRÍGUEZ-VELÁZQUEZ, SÁNCHEZ-BELTRÁN and Sigmarangkir1996). In gaps, saplings changed from low to high light levels shortly (<1 y) before our study and were expected to respond to the abrupt light increase during the subsequent year, while understorey individuals grew at low light before and during our study were expected to continue their development typical to the heavily shaded understorey. Although it can be thought that the effects of soil may be present before the study was completed, we did not detect any differences in leaf area or other attributes at the initial stage. This might indicate that soil is an important factor when it interacts with light.
We predicted that saplings growing in rich resources (high light availability and rich soils) produce leaf area at a high rate and at low cost (high SLA), which might result in leaves that are sensitive to herbivore damage. These predictions were only partially confirmed. Saplings on rich soils had higher SLA than on poor soils but, unexpectedly, their SLA was not affected by light conditions. In other studies, saplings often respond in SLA to light availability (Montgomery & Chazdon Reference MONTGOMERY and CHAZDON2002, Poorter Reference POORTER1999, Sterck Reference STERCK1999, Vincent Reference VINCENT2006), both under field conditions (Sterck Reference STERCK1999) and shade-house experiments (Bloor & Grubb Reference BLOOR and GRUBB2004, Huante et al. Reference HUANTE, RINCON and CHAPIN1998, Knops & Reinhart Reference KNOPS and REINHART2000, Veenendaal et al. Reference VEENENDAAL, SWAINE, LECHA, WALSH, ABEBRESE and OWUSU-AFRIYIE1996, Vincent Reference VINCENT2006). In most other studies, saplings also had higher SLA on richer soils (Knops & Reinhart Reference KNOPS and REINHART2000, Paoli Reference PAOLI2006, Veenendaal et al. Reference VEENENDAAL, SWAINE, LECHA, WALSH, ABEBRESE and OWUSU-AFRIYIE1996). Thus, while the high SLA on rich soils agrees with the literature, the lack of any light effect on SLA does not. In other studies, it was also suggested that below-ground competition affects directly leaf attributes like SLA (Coomes & Grubb Reference COOMES and GRUBB1998a).
Saplings under high canopy openness had shorter leaf life spans than saplings under closed understorey conditions, but their leaf life span was not affected by soil richness. The light effect agrees with reports for saplings in natural light conditions (Rijkers et al. Reference RIJKERS, PONS and BONGERS2000, Sterck Reference STERCK1999), as well as under artificial light contrasts (Vincent Reference VINCENT2006). The short leaf life span coincides with high leaf production rates and, probably, with a high demand for translocation of nutrients from older leaves, thus reducing the leaf life span (Aerts Reference AERTS1996). The lack of soil effects is however not consistent with the literature. In most studies, saplings on poor soils produced long-lived leaves with a low SLA, low nutrient concentration and a low photosynthetic capacity (Baraloto et al. Reference BARALOTO, BONAL and GOLDBERG2006, Palmiotto et al. Reference PALMIOTTO, DAVIES, VOGT, ASHTON, VOGT and ASHTON2004, Poorter & Bongers Reference POORTER and BONGERS2006). We found indeed a lower SLA on poor soils, but not the expected longer leaf life span. Thus, while a lower SLA might indicate that saplings on poor soil better protect their leaves against herbivores; this protection did not pay off in the expected longer leaf life span (Coley Reference COLEY1988, Coley et al. Reference COLEY, BRYANT and CHAPIN1985, Poorter & Bongers Reference POORTER and BONGERS2006).
For leaf damage, we found strong interaction effects of soil and light resources. Saplings under high light on rich soils had the largest number of damaged leaves, and also had the highest herbivory level per damaged leaf. While SLA, which may be considered a proxy for protection against herbivores, might only account for the soil effect, low level of defences/high nutrient levels in exposed plants may account for the high damage at high light. Thus higher preferences by herbivores might be found because of low concentrations of secondary compounds/high nutritional value of leaves (Alonso & Herrera Reference ALONSO and HERRERA2003, Boege & Dirzo Reference BOEGE and DIRZO2004, Coley Reference COLEY1988). The strong interaction effects suggest that leaves are particularly preferred by herbivores when both conditions (high SLA and low defences/high nutrient levels) are met. This result is consistent with other empirical studies on other saplings (Boege & Dirzo Reference BOEGE and DIRZO2004, Coley Reference COLEY1988, Coley et al. Reference COLEY, BRYANT and CHAPIN1985). These results indicate that saplings, due to high investment of resources in growth, might save fewer resources for defence. Our results are also consistent with the fact that anti-herbivory defences (deduced from low herbivory) are negatively correlated with leaf life span (Eichhorn et al. Reference EICHHORN, COMPTON and HARTLEY2006). However, we cannot exclude the possibility that differences in the abundance of herbivores contribute to the differences in damage between different sites.
We predicted that saplings from high light availability and rich soils would grow more rapidly in size, and thus expect strong interaction effects from light and soil conditions on sapling performance. This prediction was confirmed for two of the three growth parameters, i.e. the new meristem production and leaf area production. For height growth, we only found a positive effect of increasing light availability. The positive effect of light on height growth agrees with earlier studies on this species (Ramos & Grace Reference RAMOS and GRACE1990), and many other species in other tropical forest communities (Poorter Reference POORTER1999, Sterck Reference STERCK1999). Although no soil effect was found for height growth, soil resource availability strongly contributed to the number of meristems (a measure of branching rate) and leaf area production. Possibly these latter crown parameters are better proxies for biomass growth than height growth alone, which might only depend on the response in the top leader shoot (Sterck Reference STERCK1999). It can be concluded that in natural conditions, both the soil resources (nutrients and water) and light conditions limited growth of Brosimum saplings.
Leaf area index, estimated over 1 y, reduced in all saplings probably owing to the overall size increase. This decline in LAI was particular strong at low resource levels. Normally gap plants compared to shade plants have higher leaf area index and variation in LAI is determined more by leaf area than by crown area (Poorter & Werger Reference POORTER and WERGER1999, Sterck Reference STERCK1999). Bearing in mind this reasoning, the strong decrease in leaf area index on poor soil at low light levels might result from the slow leaf area production.
The present study suggests how the availability of soil resources and light influence the plastic responses in plant traits that underlie plant performance. As has previously been demonstrated, light availability and soil resource availability might influence physiological, ontogenetic and allometric attributes in different ways (Coomes & Grubb Reference COOMES and GRUBB1998b, Engelbrecht et al. Reference ENGELBRECHT, COMITA, CONDIT, KURSAR, TYREE, TURNER and HUBBELL2007). This study suggests that saplings of Brosimum alicastrum growing at high resource levels invested more in expansion, while saplings from low resource levels invested more in defences and durable leaves (low SLA), and that is reflected by low herbivore damage. This study emphasizes the importance of plastic responses to both soil and light resources, even in tropical rain forests that are particularly well known for their growth limitation by light.
ACKNOWLEDGEMENTS
We thank David Burslem, Emily Swaine and Simon Queenborough for comments on this paper. Ian Turner, editor of the Journal of Tropical Ecology, provided crucial feedback for the final version of this paper. We acknowledge the Chajul Biological Field Station and Centro de Investigaciones en Ecosistemas, UNAM, Mexico for supporting this research. Jorge Rodriguez and Miguel Martínez-Ramos are also acknowledged for supporting field work and for providing some unpublished data. LL was granted with a scholarship from CONACYT-Mexico (No. 163218) and Becas Complementarias-SEP-Mexico. MvB was financially supported by WOTRO W85-326 and FS by WOTRO grant W01-53.






