INTRODUCTION
Most dry forests are subject to distinct changes in water availability during the annual cycle (Bullock et al. Reference BULLOCK, MOONEY and MEDINA1995). These limitations may affect species composition and relative abundance within local assemblages of animals and plants, and also the life histories of individual species. Frugivorous and nectarivorous animals depend directly on these annual rhythms, as flowering and fruiting are tightly synchronized with seasonal water availability (Borchert et al. Reference BORCHERT, MEYER, FELGER and PORTER-BOLLAND2004, Foster Reference FOSTER, Leigh, Rand and Windsor1996). As their high mobility allows them to respond rapidly to changes in resource abundance, bats are especially well adapted to living in seasonally variable habitats (Fleming Reference FLEMING, Hunter, Ohgushi and Price1992). Nectar-feeding and frugivorous bats therefore provide excellent examples for studying seasonal responses of animals to changing resource situations.
The Neotropical nectar-feeding bats (Phyllostomidae: Glossophaginae) visit and pollinate the flowers of a wide array of plant species (Dobat & Peikert-Holle Reference DOBAT and PEIKERT-HOLLE1985, Fleming et al. Reference FLEMING, GEISELMAN and KRESS2009, Vogel Reference VOGEL1958). Special adaptations to their foraging style include hovering flight, reduced dentition and notably a long rostrum that may be seen as an indicator for the degree of specialization to nectar consumption, and correlates with operative tongue length (Freeman Reference FREEMAN1995, Muchhala Reference MUCHHALA2006, von Helversen Reference VON HELVERSEN, Barthlott, Naumann, Schmidt-Loske and Schuchmann1993, Winter & von Helversen Reference WINTER and VON HELVERSEN2003). Plants adapted to the pollination by glossophagine bats have large flowers that provide high nectar volumes, and frequently emit a strong odour (Tschapka & Dressler Reference TSCHAPKA and DRESSLER2002, von Helversen Reference VON HELVERSEN, Barthlott, Naumann, Schmidt-Loske and Schuchmann1993). Glossophagines may form local guilds of two to seven species co-occurring in the same area (Fleming Reference FLEMING2005) and occur in rain forests (Murray et al. Reference MURRAY, KINSMAN, BRONSTEIN, Nadkarni and Wheelwright2000, Tschapka Reference TSCHAPKA2004) as well as arid habitats like dry forests and deserts (Tschapka et al. Reference TSCHAPKA, SPERR, CABALLERO-MARTINEZ and MEDELLIN2008, Zortea Reference ZORTEA2003).
We studied a guild of nectar-feeding bats in the semi-arid Pacific West of Mexico and assessed seasonal changes during dry season and wet season. Our main hypothesis was that the seasonal increase of flowering bat-pollinated plant species in the west Mexican dry season (Stoner et al. Reference STONER, O.-SALAZAR, R.-FERNÁNDEZ and QUESADA2003) should provide an increase of available resources that may affect nectar-feeding bat guild composition, resource use and timing of reproductive events of the species. Specifically we tested the following predictions: we expected during the flower-rich dry season an increase in the number of nectar-feeding bat species foraging in the study area, such as found at other sites (Tschapka Reference TSCHAPKA2004). We also expected specific ecomorphological differences that can facilitate the respective seasonal foraging strategy observed for the species. We finally predicted the species to reproduce primarily during the dry season when floral resources are most abundant.
METHODS
Study area
The study took place near Callejones, Colima state, (18°49′N, 103°38′W) on the central Pacific coast of Mexico. Fieldwork was conducted during both wet (25 July–11 September 2002) and dry seasons (10 March–11 April 2003; 6 January–4 March 2004). The mountains surrounding the study area are covered with tropical deciduous forest up to 1000 m asl (Rzedowski Reference RZEDOWSKI1983). The wet season with more than 90% of the annual precipitation lasts from mid-June to October, followed by an extended dry season from November to May (Secretaria de Programación y Presupuesto 1981). We worked mainly in combined banana and coconut plantations covering large parts of the lower Rio Coahuayana valley (20–100 m asl) that were owned by local families and were less rigorously managed (e.g. less pesticide and fungicide use, less removal of male flowers, relatively low yield per area) than industrial plantations and were in close vicinity to the natural tropical deciduous forest on the surrounding hills.
Bat data
Confirming previous reports (Giannini & Brenes Reference GIANNINI and BRENES2001, Schaldach & McLaughlin Reference SCHALDACH and MCLAUGHLIN1960, Stephens & Tyson Reference STEPHENS and TYSON1975), our initial visual observations of bats frequently visiting banana inflorescences and subsequent exploratory mist-netting sessions showed that the banana plantations attracted, through their year-round availability of abundant floral nectar (Vandermeer Reference VANDERMEER and Janzen1983), a much higher density of nectar-feeding bats than the surrounding area, so mist nets were mostly set up in front or in close proximity to banana flowers. Mist-netting started 1 h after sunset to ensure capturing bats that had already foraged, and lasted 5 h. We mist-netted on 25 nights during the wet season and 57 nights during the dry season, for a total of 82 nights. Species were identified based on the current taxonomy of Wilson & Reeder (Reference WILSON and REEDER2005) and using the field key from Medellín et al. (Reference MEDELLÍN, ARITA and SÁNCHEZ1997). Bats not belonging to our target subfamily Glossophaginae were released immediately. The most abundant glossophagine species Glossophaga soricina and Leptonycteris yerbabuenae were only randomly sampled, taking data from approximately every fourth (G. soricina) or second (L. yerbabuenae) bat, in order to assure sufficient time for careful acquisition of pollen samples without cross-contamination from one animal to the next. Sex, age (adults, subadults, juveniles, according to the degree of ossification of the metacarpal–phalange joints), and reproductive condition (non-reproductive, pregnant, lactating, post-lactating) were recorded (Racey Reference RACEY and Kunz1988). We measured body mass with a spring balance (Pesola, Switzerland; precision: 0.1 g) and length of forearm by using calipers (precision: 0.1 mm). Only adult animals were used for morphological measurements; for the calculation of median body mass we additionally excluded pregnant females. Wing shape of bat species is related to flight style and consequently to specific foraging behaviour (Altringham Reference ALTRINGHAM1996, Norberg & Rayner Reference NORBERG and RAYNER1987). We assessed wing-shape by measuring the length of third and fifth finger with a ruler (precision 0.5 mm). Wing proportions were quantified using the Aspect Ratio Index (ARI) and the Wing Tip Index (WTI) (Findley et al. Reference FINDLEY, STUDIER and WILSON1972). High values of WTI occur together with high aspect ratio index among migrants and fast aerial hawkers (Norberg & Rayner Reference NORBERG and RAYNER1987). Low ARI is found in species with high manoeuvrability and pronounced hovering ability. Rostrum length of glossophagine species increases with the degree of specialization on a diet based on nectar and pollen (Heithaus Reference HEITHAUS and Kunz1982, Solmsen Reference SOLMSEN1998). Increased relative rostrum length decreases the bite force and poses limits on the consumption of harder food items (Aguirre et al. Reference AGUIRRE, HERREL, VAN DAMME and MATTHYSEN2002). Rostrum length was measured with a ruler (precision: 0.5 mm) as the distance between the centre of the eye and the tip of the lower lip (Tschapka Reference TSCHAPKA2004).
Diet
In order to obtain data on diet, pollen samples were collected from the bats' bodies using fuchsine-stained gelatine (Beattie Reference BEATTIE1971) and mounted on glass slides. Pollen was identified using a light microscope (magnification 100–400 ×), pollen key (Roubik & Moreno Reference ROUBIK and MORENO1991) and a reference collection assembled from locally found plants fitting the bat-pollination syndrome (Vogel Reference VOGEL1958, von Helversen Reference VON HELVERSEN, Barthlott, Naumann, Schmidt-Loske and Schuchmann1993). Our reference collection was supplemented by pollen samples taken from herbarium specimens (Herbario Nacional MEXU, UNAM) of bat-visited species known to occur in the study area (Alvarez & Sanchez-Casas Reference ALVAREZ and SANCHEZ-CASAS1997, Chávez Reference CHÁVEZ1975, Stoner et al. Reference STONER, O.-SALAZAR, R.-FERNÁNDEZ and QUESADA2003). Pollen grains within a sample that morphologically belonged to the same type were defined as a pollen encounter. Pollen grains were identified to the lowest possible taxonomic level. Pollen was scored on a presence/absence scale for each bat. Although no quantitative data on resource-use of individuals can be gained in this way (Thomas Reference THOMAS and Kunz1988), pollen presence/absence data obtained from many individuals may provide an estimate of the relative use of a particular plant species within a bat population (Arkins et al. Reference ARKINS, WINNINGTON, ANDERSON and CLOUT1999, Lobo et al. Reference LOBO, QUESEDA, STONER, FUCHS, HERRERIAS-DIEGO, ROJAS and SABORIO2003, Tschapka Reference TSCHAPKA2004). Plant species represented in a sample by fewer than five pollen grains were considered contamination and were not counted. The pollen-rich Cactaceae, the genera Ceiba, Bombax and Pseudobombax as well as the species Crescentia alata and Cleome spinosa had to exceed 10 pollen grains per sample in order to be scored. We chose this second threshold value because bats visiting these plants are usually covered with large amounts of pollen and therefore there is an increased risk for contamination through gloves or mistnets. We included in the analysis samples from five additional captures of the rare Musonycteris harrisoni obtained at the study site during preliminary visits in March 1996 and July 2001. After pollen collection, the bats were put into clean cloth bags for 1–2 h in order to obtain faecal material. Samples were stored in 70% alcohol and later scanned for the presence or absence of pollen, seeds and remains of plant tissues, soft-bodied arthropods, hard-bodied arthropods and small, externally undigested arthropods (mainly ants), using a dissecting scope.
Data analysis
Data were analysed using parametric and non-parametric tests using Sigma Stat 2.03 (SPSS Inc., Chicago, USA). Significance level α was set at 0.05. Niche overlap of glossophagine species was calculated using Morisita's index, utilizing algorithms from Krebs (Reference KREBS1989). Multivariate analysis of niche overlap of the resident bat species was performed with the software CANOCO for Windows 4.5 and visualized with CanoDraw 3.1 (ter Braak & Šmilauer Reference TER BRAAK and ŠMILAUER2002).
RESULTS
Bat captures
During 57 evenings in the dry season and 25 in the wet season we sampled 564 nectar-feeding bats (Phyllostomidae: Glossophaginae) belonging to four species: Glossophaga soricina (200 individuals in dry season, 83 in wet season), Leptonycteris yerbabuenae (136, 69), and Musonycteris harrisoni (30, 28) were found in both seasons in similar numbers. The fourth species Anoura geoffroyi (4, 14) was mostly captured during the wet season (Figure 1).
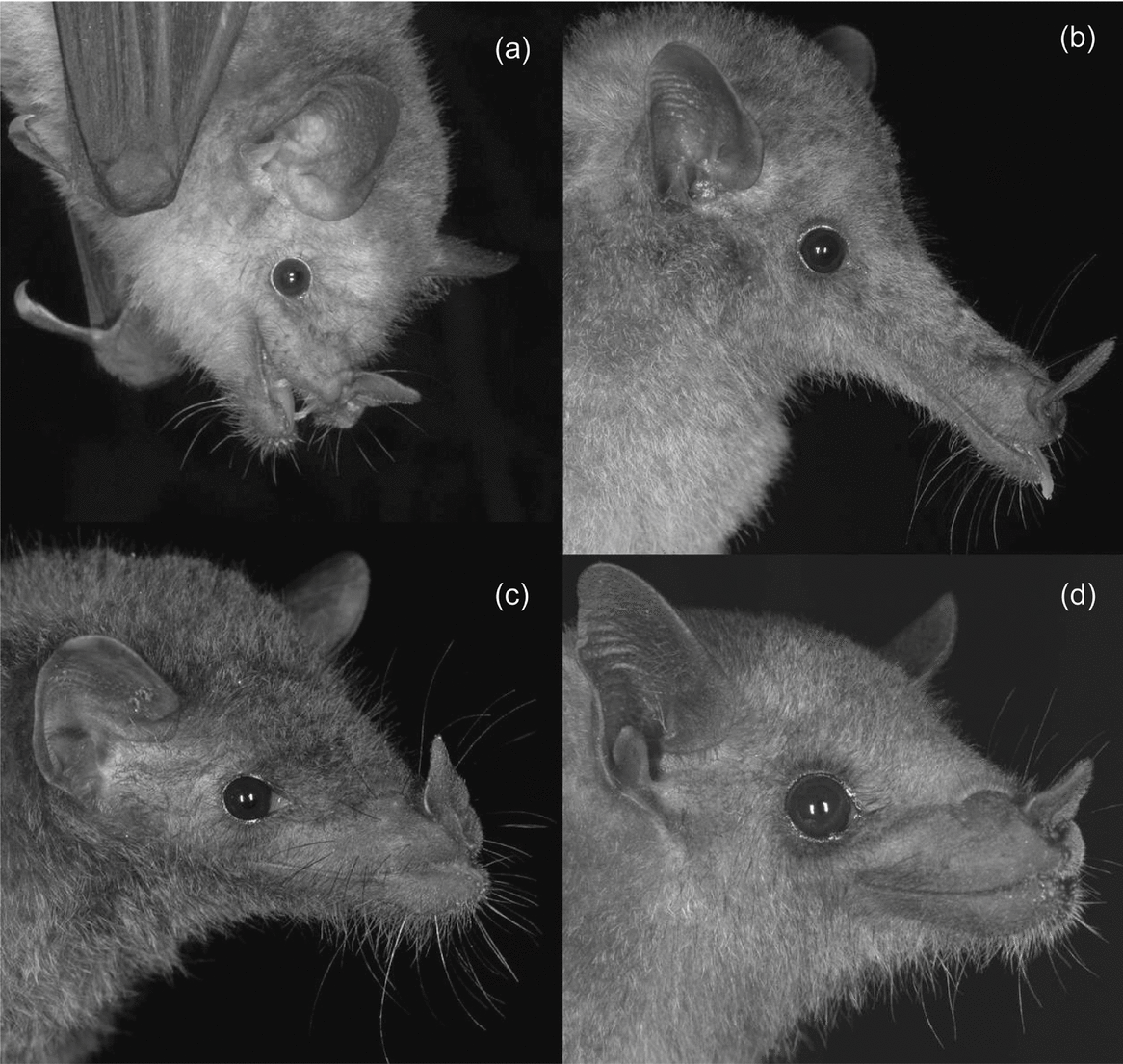
Figure 1. Glossophagine species found at Callejones, Colima: Glossophaga soricina (a), Musonycteris harrisoni (b), Anoura geoffroyi (c), Leptonycteris yerbabuenae (d).
We always caught more males than females of L. yerbabuenae, however, the proportion of females more than doubled during the dry season (sex ratio 1♀:16♂ wet season, χ2 = 54.9, df = 1, P < 0.001; 1♀:7♂ dry season, χ2 = 73.6, df = 1, P < 0.001). During the wet season we found also for G. soricina a sex ratio that was significantly skewed towards females (4♀:1♂, χ2 = 25.8, df = 1, P < 0.001). Sex ratios in the other two species were not significantly different from a 1:1 ratio.
Capture time of bats may reflect differences in species-specific activity patterns and foraging strategies. Individuals of Anoura geoffroyi were captured significantly later at night than all other species (ANOVA on ranks with Dunn's post hoc test; H = 9.83; df = 3, P < 0.05). While median capture time for Anoura geoffroyi was 22h50, all other species appeared earlier in the study area, around 22h10.
Ecomorphological characteristics of species
The guild consisted of one small species (G. soricina, mean (± SD) forearm length 36.6 ± 0.9 mm; body mass 9.7 ± 0.9 g), two middle-sized species (M. harrisoni, 43.1 ± 0.7 mm; 11.6 ± 1.0 g; A. geoffroyi, 43.2 ± 1.0 mm; 14.3 ± 1.2 g) and one large species (L. yerbabuenae, 53.9 ± 1.8 mm; 23.3 ± 2.3 g).
Differences in wing proportions may indicate species-specific foraging strategies. Anoura geoffroyi differed clearly from the other three species by having the narrowest and longest wings (highest values in both ARI and WTI), followed by L. yerbabuenae, M. harrisoni and G. soricina. Wing tip index (WTI) was highest in A. geoffroyi and decreased from G. soricina and M. harrisoni to L. yerbabuenae (Figure 2). All species differed significantly from each other in both wing indices (one-way ANOVA with Tukey test, P < 0.001).

Figure 2. Wing proportions of the four Glossophagine species. Plotted are aspect ratio index (ARI) versus wing tip index (WTI). Both ARI and WTI are presented as mean ± 1 SD, respectively. Number of individuals: Glossophaga soricina 184; Leptonycteris yerbabuenae 181; Anoura geoffroyi 14; Musonycteris harrisoni 43.
Within glossophagine bats rostrum length may be an indicator of the degree of specialization on a nectar diet. Musonycteris harrisoni had by far the longest rostrum with a median length of 19 mm (range = 17–21 mm; n = 50); Glossophaga soricina showed the shortest rostrum (median = 8.5 mm, range = 7.5–9.5 mm, n = 247) within the guild. Anoura geoffroyi (12 mm, 11.5–12.5 mm, n = 17) and L. yerbabuenae (12 mm, 10–13 mm, n = 204) were characterized by medium-sized rostra and were the only two species where rostrum size did not differ significantly from each other (Kruskal–Wallis ANOVA, H = 451, P < 0.001, post hoc test Dunn's Method at P < 0.05).
Food plants
We collected 370 samples in the dry season and 194 samples during the wet season. The 760 pollen encounters in these samples represented 28 different pollen types, 12 of which were identified to species level, nine to genus and six to family level. One very rare, but clearly distinguishable, pollen type could not be identified and was classified as ‘unknown’. The identified plants belong to 13 different families, encompassing herbs, shrubs and trees (Table 1). Pollen identification was possible only to family level in most Cactaceae and Malvaceae, and therefore these were classified as follows: Cactaceae comprised at least three different species of columnar cacti. While two flowered exclusively during the wet season (Cactaceae 1 and 2, probably Stenocereus sp. and Cephalocereus sp., based on distribution, flowering period and floral morphology), a third pollen type was one of the most important food plants during the dry season (Cactaceae 3) and probably corresponded to the most common columnar cactus species in the study area, Pachycereus pecten-aboriginum. In the family Malvaceae, pollen of Helicteres baruensis, Luehea sp. and Ceiba pentandra could clearly be distinguished from three other pollen types belonging to the species Pseudobombax ellipticum, Bombax palmeri and Ceiba aesculifolia. The last three species had very similar pollen morphology and overlapping sizes, and all of these three may be present in the samples. They all were formerly included in the family Bombacaceae, and we prefer using this name for the pollen type collectively representing them, as not all pollen could be assigned with certainty to one of these three species.
Table 1. Percentage of use (n pollen encounters/n bats sampled × 100) of 28 plant species in Western Mexico between 2002 and 2004 during dry and wet seasons as indicated by the analysis of pollen samples obtained from 564 nectar-feeding bats belonging to the four species Leptonycteris yerbabuenae (L), Musonycteris harrisoni (M), Glossophaga soricina (G) and Anoura geoffroyi (A). The second column gives the percentage of all bats carrying the particular pollen and approximates the overall importance of this plant for the bat guild. As bats may carry more than one pollen type, the sum exceeds 100%.

The total number of different pollen types found on a species varied between 10 (A. geoffroyi) and 19 (G. soricina) (Table 1). The species differed clearly in their use of nectar resources. While the pollen types most commonly found on M. harrisoni were Cactaceae 3, Bombacaceae and Crataeva palmeri, Leptonycteris yerbabuenae individuals visited Cleome spinosa and plants of the Bombacaceae complex most frequently, followed by Ceiba pentandra and Cactaceae 3. While no distinctly dominant plants were found in the diet of Anoura geoffroyi, Calliandra sp. 1 was found on almost a fifth of all individuals, followed by Crescentia alata and Luehea sp. Plant species most often visited by G. soricina were Cleome spinosa and, interestingly, also the insect-pollinated Cocos nucifera.
The abundance of the nectar-feeding bats within the plantations strongly indicates that they used banana flowers in both seasons as an additional nectar resource. As the cultivated bananas did not produce any pollen (Vandermeer Reference VANDERMEER and Janzen1983) this supplementary resource did not enter our niche analysis.
Seasonal differences in nectar resource use
Of the 28 pollen types, 11 (39.4%) were exclusively found during the dry season and 10 (35.7%) exclusively during wet season (July–August), the remaining seven plant species (25%) were available in both seasons. Only the cultivated coconut palm (Cocos nucifera) was used with similar high intensity in the two seasons and exclusively by G. soricina (Table 1).
There was a significantly higher occurrence of pollen on the glossophagine bats during the dry season; 91.9% of all bats carried pollen during the dry season but only 54.6% in the wet season (χ2 = 107, df = 1, P < 0.001). Within all three resident species we found a significant decrease in the number of pollen types present on individuals from the dry to the wet season (Mann–Whitney tests; all species P < 0.001), but not for the seasonal A. geoffroyi, probably because of the small number of animals captured during the dry season (Table 2).
Table 2. Number of pollen types found on individual bats in the two seasons (mean ± SD; number of sampled individuals; comparison by Mann–Whitney test).

Niche overlap
For the wet season, Morisita's Index shows highest niche overlap between the middle-sized A. geoffroyi and the large L. yerbabuenae (Table 3). The extremely high resource overlap of A. geoffroyi with all other species during the dry season is probably an artefact of the very low sample size (n = 4) for the species during this period. Not considering Anoura, highest overlap in the dry season occurs between M. harrisoni and L. yerbabuenae, that both fed heavily on cacti, Cleome spinosa and the Bombacaceae species. Lowest overlap occurred in both seasons between the residents G. soricina and M. harrisoni. Mean overlap between all species was 0.23 ± 0.2 in the wet season and 0.67 ± 0.23 for the dry season (0.58 ± 0.21 without A. geoffroyi).
Table 3. Seasonal niche overlap in nectar diet calculated using Morisita's Index based on the pollen from the fur of the bat species (Krebs Reference KREBS1989). Overlap index values top right are from the wet season; those bottom left are from the dry season.

A redundancy analysis (RDA) on pollen load provides a two-dimensional projection of a 28-dimensional resource space and illustrates the feeding niches of the coexisting bat species. While all species overlap widely during the dry season (Figure 3a), the degree of overlap changes drastically in the wet season and especially the resident species G. soricina and M. harrisoni differed distinctly in resource use, while both still overlapping with the large L. yerbabuenae (Figure 3b). However, the plot also shows large areas for both seasons that are occupied by single species, which points out that many bats fed on plants never or only rarely shared with other species.

Figure 3. Seasonal overlap in nectar use (RDA, CANOCO 4.5) of the Glossophagine species Glossophaga soricina (G), Leptonycteris yerbabuenae (L), Anoura geoffroyi (A) and Musonycteris harrisoni (M), as indicated by the pollen loads of the individuals during dry season (a) and wet season (b). Shown are the first (dry season 42.4% of variance, wet season 31.6%) and the second canonical axis (4.8%; 27.0%). Stars represent the centroid for each species; data points of individuals with identical pollen load have identical coordinates, so each symbol may represent several bats.
Seasonal use of supplementary resources
Faecal analysis revealed that some flower-visiting bat species varied seasonally in the utilization of fruits and insects in addition to pollen and nectar. While Leptonycteris yerbabuenae showed increased consumption of fruits and insects in the wet season, G. soricina used alternative food types over the entire year (Figure 4). We obtained few faecal samples from M. harrisoni and A. geoffroyi. Almost all samples of Musonycteris (5/6) showed pollen in their faeces. There was no indication of the use of fruits and only two individuals showed remains from soft-bodied arthropods. Faeces of A. geoffroyi showed over the entire year mainly hard-bodied arthropods while pollen was only found in two of eight faecal samples.

Figure 4. Feeding remains found in faecal pellets from the two common resident species Leptonycteris yerbabuenae (a) and Glossophaga soricina (b) during dry and wet season. Shown are the proportions of samples that contained the corresponding food types. As a faecal sample may contain more than one food type the sum for each of the two seasons can exceed 100%.
Timing of reproduction
Female G. soricina were found lactating during the dry as well as during the wet season, resulting in two birth peaks per year in the study area. Juveniles were commonly captured during both seasons.
Lactating and post-lactating females of the species M. harrisoni, Anoura geoffroyi and L. yerbabuenae were found exclusively during the dry season, suggesting a single dry-season reproductive peak. In M. harrisoni this is also supported by further evidence: (1) an adult non-reproductive female caught in the wet season in August 2002 was recaptured lactating in March 2003 during the dry season; (2) a single juvenile was caught in the dry season in March 2004. We never caught juveniles of A. geoffroyi or L. yerbabuenae. The low number of Anoura geoffroyi captured in the dry season prohibits a conclusive assessment of the annual reproductive patterns for this species.
DISCUSSION
Seasonal changes in local guild
The local nectar-feeding bat guild (an ensemble, sensu Fauth et al. Reference FAUTH, BERNARDO, CAMARA, RESETARITS, VAN BUSKIRK and MCCOLLUM1996) at our study site consisted of three resident species (G. soricina, M. harrisoni, L. yerbabuenae) that were present over the entire year, and one seasonal species (A. geoffroyi) that was captured mostly during the wet season. Over large parts of its distribution the large Leptonycteris yerbabuenae is only seasonally present at a given site (Cole & Wilson Reference COLE and WILSON2006), however, at our study site it was captured during wet and dry seasons. However, a doubling of the proportion of females in the dry season suggests a seasonal increase in numbers of females during the flower-rich dry season, corresponding to the pattern found by Stoner et al. (Reference STONER, O.-SALAZAR, R.-FERNÁNDEZ and QUESADA2003) in nearby Jalisco State. Seasonal changes in glossophagine guilds were found previously in the Costa Rican Atlantic rain forest, where two resident and two seasonal species (that took advantage of temporally high resource availability) co-occurred over the annual cycle (Tschapka Reference TSCHAPKA2004). Seasonal changes in phyllostomid species abundance have also been observed at other dry-forest sites, e.g. in the Costa Rican north-west, where the frugivores Carollia perspicillata and Artibeus jamaicensis differed significantly in abundance over the annual cycle (Stoner Reference STONER2001).
The number of nectar-feeding bat species found in Callejones corresponds to those reported from other dry habitats. Guilds containing smaller numbers of glossophagines were reported from Curaçao (two spp., Petit Reference PETIT1997), Venezuela (two spp., Sosa & Soriano Reference SOSA and SORIANO1993) and Colombia (two to three spp., Sánchez et al. Reference SÁNCHEZ, ALVAREZ, ARIZA and CADENA2007). Similar-sized or larger ones are known from Costa Rica (five spp., Stoner & Timm Reference STONER, TIMM, Frankie, Mata and Vinson2004) as well as from Jalisco, West Mexico (four spp., Stoner Reference STONER, Noguera, Quesada, Vega and Garcia-Aldrete2003).
Seasonal resource availability and bat mobility
Floral resources of dry forests are particularly abundant during the dry season (Perez et al. Reference PÉREZ, MARTÍNEZ-CORONEL and LÓPEZ2003) and the resident nectar-feeding bats at Callejones confirmed this by showing significantly higher pollen loads compared with the wet season. We initially hypothesized that there might be additional bat species during this period. However, the seasonal species Anoura geoffroyi appeared in the banana plantations of the study area almost exclusively in the flower-poor wet season. This apparent contradiction might not be the consequence of the resource situation at our study site, but rather reflect the food abundance within the unknown main habitat of A. geoffroyi. Probably A. geoffroyi were attracted to the banana flowers available year-round in our study area especially during the wet season, due to seasonal nectar shortages in other foraging areas nearer to its roosting site.
Anoura geoffroyi mainly roosts in larger colonies within caves, which may force the individuals to cover large distances between day roost and foraging sites (Ortega & Alarcon-D. Reference ORTEGA and ALARCON-D.2008, Ramirez-Pulido et al. Reference RAMIREZ-PULIDO, GALINDO-GALINDO, CASTRO-CAMPILLO, SALAME-MENDEZ and ARMELLA2001). The significantly late arrival of A. geoffroyi in the study area suggests that the animals did not actually roost in the study area, but that they commuted every night from a distant roosting place, such as the similar-sized Lonchophylla robusta in Costa Rica (Tschapka Reference TSCHAPKA2004). Individuals of Anoura geoffroyi showed two pollen types that were never found on any other species, indicating that these bats foraged not only within our study area but also along the way coming from a remote roost. The long and slender wings of Anoura geoffroyi, indicated by far the highest ARI, differed from all other species at the site. Such proportions are characteristic of bats that fly very fast (Altringham Reference ALTRINGHAM1996) and may allow A. geoffroyi to respond quickly to seasonal changes in local resource availability within a large area. In Central America and Mexico A. geoffroyi seems to be largely restricted to mountainous areas (Timm et al. Reference TIMM, WILSON, CLAUSON, LAVAL and VAUGHAN1989, Tschapka Reference TSCHAPKA2004, Villa-R. Reference VILLA-R.1967), while we caught the species almost at sea level. It would be very interesting to identify the entire annual home range for the Anoura individuals that temporarily foraged at Callejones. Probably, size of such home ranges of Anoura will cover multiple times those of strictly resident species, such as Glossophaga soricina or Musonycteris harrisoni.
Body size is another critical factor for mobility of bats that may distinctly affect foraging behaviour and local occurrence of bats. Large species are better adapted to flying over longer distances (von Helversen & Winter Reference VON HELVERSEN, WINTER, Kunz and Fenton2003). It is noteworthy that the two species showing evidence for seasonal movements, A. geoffroyi and especially L. yerbabuenae, are also the two largest species in the guild. Given that L. yerbabuenae routinely covers long distances (Fleming et al. Reference FLEMING, NUÑEZ, SILVEIRA and STERNBERG1993, Horner et al. Reference HORNER, FLEMING and SAHLEY1998, Rojas-Martinez et al. Reference ROJAS-MARTINEZ, VALIENTE-BANUET, DEL CORO ARIZMENDI, ALCANTARA-EGUREN and ARITA1999) we would expect similar wing shapes as in A. geoffroyi. However, its wing proportions clearly correspond more to the resident species M. harrisoni and G. soricina than to Anoura geoffroyi. Wing shape of Anoura might be additionally influenced by other factors, such as the main occurrence of the genus in highlands with lower air density (Tamsitt & Nagorsen Reference TAMSITT and NAGORSEN1982). Another reason might be connected to L. yerbabuenae being one of the largest glossophagine bats overall. At higher body masses the hovering flight necessary for feeding on most bat-flowers becomes distinctly more expensive than ordinary forward flight (Voigt & Winter Reference VOIGT and WINTER1999). Therefore, requirements of hovering flight could prevent the large Leptonycteris yerbabuenae from specializing entirely on fast movement.
The seasonal presence of Anoura geoffroyi and the increased proportion of female Leptonycteris during the dry season indicate that long distance (Cole & Wilson Reference COLE and WILSON2006) and regional movements may be common strategies for certain nectar-feeding bat species. Ongoing habitat conversion, fragmentation and destruction may jeopardize these movements. Conservation strategies need to provide suitable habitat connectivity, especially when also considering the possible consequences of global climate change that may even shift species boundaries (Peterson et al. Reference PETERSON, ORTEGA-HUERTA, BARTLEY, SÁNCHEZ-CORDERO, SOBERÓN, BUDDEMEIER and STOCKWELL2002).
Dietary adaptations and seasonal resource use
The conspicuously elongated rostrum of some glossophagine species is mostly correlated with tongue length and therefore with the flower depth bats may potentially reach into during foraging (Winter & von Helversen Reference WINTER and VON HELVERSEN2003, but see Muchhala Reference MUCHHALA2006). However, resource partitioning based on rostrum morphology seems not to be a major factor structuring the studied community. In spite of the distinct differences in rostrum length, e.g. between Musonycteris harrisoni and Leptonycteris yerbabuenae, many of the important food plants of Musonycteris have very short corollas. Probably, the disproportionately long rostrum and tongue of this species are mainly shaped by energetic factors and allow year-round access to the broadest range of nectar resources possible, while maintaining small body size and low food requirements (Tschapka et al. Reference TSCHAPKA, SPERR, CABALLERO-MARTINEZ and MEDELLIN2008).
Although we mist-netted mainly in the vicinity of banana plantations in order to maximize capture success, we found bats to utilize 28 plants from the nearby natural forest. Floral resource use varied distinctly between the four species and between the two seasons. Highest niche overlap among the species was found in the dry season, indicating that high availability of flowers such as Cleome spinosa and Cactaceae 3 (probably Pachycereus pecten-aboriginum) decreased competition for floral resources and allowed the species to converge in their diet on these abundant resources. During the wet season floral resources were less important in the bats' diet, overlap decreased and particularly the year-round co-occurring resident species G. soricina and M. harrisoni utilized distinct resources.
Glossophaga soricina supplemented its nectar diet in both seasons with fruits and insects, and had the shortest rostrum of all species, which probably provides enough bite force for regular consumption of moderately hard fruit material (Aguirre et al. Reference AGUIRRE, HERREL, VAN DAMME and MATTHYSEN2002, Freeman Reference FREEMAN1995). The use of fruits in G. soricina in both seasons contrasts with other studies, where Glossophaga spp. relied on fruits as a substitute food only in seasons of nectar scarcity (Bonaccorso Reference BONACCORSO1979, Tschapka Reference TSCHAPKA2004). This might be either an opportunistic reaction of the bats to the availability of nutritionally valuable fruits or to specific physiological demands that are not completely met by the nectar resources available at the study site. While we found no evidence of fruit consumption in the diet of the long-snouted M. harrisoni, the larger L. yerbabuenae fed also on fruits and insects during this flower-poor time.
Interestingly, in both seasons one of the most frequently found pollen types on the unspecialized Glossophaga soricina is not from a typical bat flower. Cocos nucifera, the cultivated coconut palm, flowers year-round and is mainly pollinated by small insects. The unmistakable pollen grains of this single Arecaceae in the area were present on almost 40% of all G. soricina. Our study presents the first record for the use of Cocos nucifera by neotropical flower-visiting bats. However, in Asia pollen of this palm species was found in the faeces of the flying fox Eonycteris spelaea. This species is known to visit the flowers of several plant species exclusively for pollen intake (Start Reference START1974), which might be also the reason for the use of C. nucifera by G. soricina. Similar opportunistic feeding habits were previously found for Glossophaga commissarisi, which regularly carried pollen from wind-pollinated Cecropia spp. (Tschapka Reference TSCHAPKA2004). At Cocos inflorescences the bats may also use the nectar that is copiously provided for pollinating bees. Similar to banana plants, both Cecropia and Cocos flower and fruit year round, so they may represent an aseasonal and reliable food resource for Glossophaga bats. There is growing evidence that unspecialized Glossophaga species opportunistically learn to use non-coevolved plants with accessible and large pollen or nectar supplies (Tschapka Reference TSCHAPKA2003).
As indicated by the high bat density in both dry and wet seasons, all species utilized also the cultivated bananas as a supplementary nectar resource. Low native flower availability in the wet season might even have caused an increase in banana nectar use, however, our methods could not resolve this. Banana plants may represent a valuable supplementary resource to nectar-feeding bats in areas where forest fragmentation and conversion to industrialized agriculture displace the natural plant species. However, we emphasize that the plantations in the study area are not industrialized high-performance plantations, but small family businesses with a less rigorous management system. While within industrialized plantations male banana inflorescences are routinely cut after initiation of fruit set, inflorescences at our study sites were available to the bats for a long time. Currently, the conversion into large commercial plantations that depend heavily on artificial irrigation and input of pesticides, fungicides and fertilizer is one of the major threats to the dry-forest ecosystems (Bullock et al. Reference BULLOCK, MOONEY and MEDINA1995). Nevertheless, supplementary planting of unmanaged bananas in cultivated landscapes, e.g. growing at the edge of larger monocultures, could mitigate habitat disruption effects by offering nectar resources especially also for migrating bats and might therefore be developed as a tool for conservation.
Reproductive patterns
Although our field work did not cover the entire year, our data permit an assessment of the reproductive strategies for at least the three resident species. Both Musonycteris harrisoni and Leptonycteris yerbabuenae were lactating only during the dry season. This suggests that reproduction of these species responded to floral resource availability in the deciduous dry forest that peaked in the dry season with the flowering of the abundant cactus Pachycereus pecten-aboriginum, various Bombacaceae species such as Ceiba pentandra, and several Capparaceae. In contrast, Glossophaga soricina reproduced in the study area both in the dry and the wet season, thus following the main pattern of the genus (Estrada & Estrada-Coates Reference ESTRADA and COATES-ESTRADA2001, Fleming et al. Reference FLEMING, HOOPER and WILSON1972, Ramirez-Pulido et al. Reference RAMIREZ-PULIDO, ARMELLA and CASTRO-CAMPILLO1993, Tschapka Reference TSCHAPKA2005, but see Petit Reference PETIT1997). While one birth peak coincided with high flower availability in the dry season, the increase of insect consumption during the wet season suggests that the second peak might profit from a higher insect abundance. In conclusion, all resident species took advantage of the dry-season flowering peak for their reproduction, thus supporting our initial hypothesis. Due to the small number of individuals of Anoura captured during the dry season, our data on reproduction on the species are limited, but as reproductively active individuals were captured only during the dry season they might also utilize the dry-season floral peak in their main habitat for reproduction.
Similar to L. yerbabuenae we found also a much smaller bias towards females during the wet season in G. soricina. As this species is not known to migrate, we assume that reproductive females either tried to minimize their foraging cost by limiting their flight time (Voigt Reference VOIGT2003) and mainly foraged in the nectar-rich banana plantations near our nets or that the maternity roosts were close to the plantations.
Conclusion
While our first prediction was not confirmed, we did find seasonal changes in species richness and sex ratio of some nectar-feeding bats. Morphological adaptations that facilitate tracking of seasonally variable resources over large areas (Fleming Reference FLEMING, Hunter, Ohgushi and Price1992) were found in wing shape (A. geoffroyi) and size (A. geoffroyi, L. yerbabuenae) of mobile species, thus confirming our second prediction. Finally, verifying our last prediction, most of the species reproduced in the dry season, corresponding to the highest availability of floral resources. Our study highlights the dynamics within communities that are exposed to drastic changes in resource availability. As two out of the four nectar-feeding bat species show demographic responses that suggest regional movements, connectivity of suitable habitats is a crucial factor for these species and may be of particular importance for the seasonally very variable dry forests.
ACKNOWLEDGEMENTS
We thank Don Xavier and Doña Lilia and the entire Moctezuma family for the hospitality and kind permission to work on their property at Callejones. Support for pollen identification came from E. Martínez-Hernandez (MEXU, Mexico) and H. Behling (University of Göttingen, Germany). Valuable assistance in the field came from E. Kalko, L. B. Vazquez, S. Gallo, R. Sanchez, Y. Rodriguez, V. Rosas-Guerrero, C. Rothenwöhrer, N. Becker and the Chapkitas. We thank Osiris Gaona for technical and cabinet support. Valuable comments on the manuscript came from L. F. Aguirre and two anonymous referees. Financial support for the study was provided by the German Academic Exchange Service (DAAD), National Geographic Society, the Programa para la Conservación de los Murciélagos de México (PCMM), CONACYT, and the Wildlife Trust Alliance. Scientific collecting permits were kindly provided by SEMARNAT (FAUT DGVS-0001).









