INTRODUCTION
The orchid family is renowned for its enormous diversity of pollination mechanisms and unusually high occurrence of non-rewarding flowers compared to other plant families (Dressler Reference DRESSLER1981, van der Cingel Reference VAN DER CINGEL2001, van der Pijl & Dodson Reference VAN DER PIJL and DODSON1966). Although non-rewarding flowers have evolved in at least 32 angiosperm families (Renner Reference RENNER, Waser and Ollerton2006), it is most prevalent within the orchid family: approximately 6500 out of the 7500 angiosperm species that are thought to be pollinated through deception are orchids (Jersáková et al. Reference JERSÁKOVÁ, JOHNSON and KINDLMANN2006, Renner Reference RENNER, Waser and Ollerton2006). The mechanisms by which non-rewarding orchids attract pollinators vary from generalized food deception, through specific mimicry of other flowers to sexual deceit (leading in many cases to pseudocopulation) (Jersáková et al. Reference JERSÁKOVÁ, JOHNSON and KINDLMANN2006).
A special case of deception involves sapromyiophily. Sapromyiophilous plants deceive their pollinators by producing odours of decay and mimicking the decaying flesh in which these flies normally lay their eggs (van der Cingel Reference VAN DER CINGEL1995). These plants are generally pollinated by carrion or dung flies. The flies are not interested in the flowers as such, but go to the flower expecting to find rotting protein. The set of features that describe the sapromyiophily pollination syndrome includes radial flowers (often with great depth) with dark colours (most often brown, purple and greenish), and specific floral blends characterized by sulphur compounds, giving the flowers a putrescent odour (Feinstein et al. Reference FEINSTEIN, PURZYCKI, MORI, HEQUET and BERKOV2008, van der Pijl & Dodson Reference VAN DER PIJL and DODSON1966). The occurrence of sapromyiophily is not restricted to the Orchidaceae alone and has been found in other plant families as well, including Aristolochiaceae, Asclepiadaceae and Araceae, illustrating a case of convergent evolution (Jersáková et al. Reference JERSÁKOVÁ, JOHNSON and KINDLMANN2006). Within the orchid family, this deception mechanism is mainly confined to tropical and subtropical areas (Jersáková et al. Reference JERSÁKOVÁ, JOHNSON and KINDLMANN2006).
The pantropical genus Bulbophyllum (subfamily Epidendroideae) is the largest genus of the orchid family, encompassing approximately 2400 epiphytic species (Tan & Nishida Reference TAN and NISHIDA2007). Due to the occurrence of a well-balanced see-saw lip (labellum), which is attached to the base of the floral column by a small springy hinge, species of Bulbophyllum are assumed to be pollinated by flies (Bartareau Reference BARTAREAU1994, Borba & Semir Reference BORBA and SEMIR1998, Nishida et al. Reference NISHIDA, TAN, WEE, HEE and TOONG2004, Tan et al. Reference TAN, NISHIDA and TOONG2002, Teixeira et al. Reference TEIXEIRA, BORBA and SEMIR2004). Sapromyiophily has also been reported in the genus (Dressler Reference DRESSLER1981, van der Pijl & Dodson Reference VAN DER PIJL and DODSON1966), but surprisingly this pollination mechanism has never been described in much detail (van der Cingel Reference VAN DER CINGEL2001).
The Mascarene Archipelago harbours approximately 15 species of Bulbophyllum. Fly-pollination has been suggested for these species (Jacquemyn et al. Reference JACQUEMYN, MICHENEAU, ROBERTS and PAILLER2005), but their pollination mechanisms are largely unknown, and to date no pollinators have been identified. In this study, we focused on the pollination biology of Bulbophyllum variegatum Thouars. This species displays large reddish flowers that emit a very unpleasant urine-like scent, suggesting a probable case of sapromyiophilous pollination. To verify this hypothesis, field work and floral measurements were conducted in Réunion in order to determine the pollination system of B. variegatum, and to quantify its reproductive success across its natural range.
METHODS
Study species
Bulbophyllum variegatum Thouars is endemic to Madagascar and Mascarene Islands (Fischer et al. Reference FISCHER, GRAVENDEEL, SIEDER, ANDRIANTIANA, HEISELMAYER, CRIBB, SMIDT, SAMUEL and KIEHN2007). This species is a robust epiphytic orchid and shows an unusually high degree of host preference for the tree Agarista salicifolia (Ericaceae) (Lancaster Reference LANCASTER2004). Roberts (Reference ROBERTS2001) considers this orchid to be a common species in lowland rain forests of the east and the south-east of Réunion (occurring from sea level to c. 800 m asl, Figure 1) and in coastal forests in Madagascar, but it is rare in Mauritius. The species is characterized by a single pair of dark green, strap-like, acuminate leaves emerging from a flattened, round pseudobulb. Flowers are large and reddish and emit a very unpleasant urine-like scent during the day. The flowering period lasts for about 2 mo from the beginning of December to the end of January.
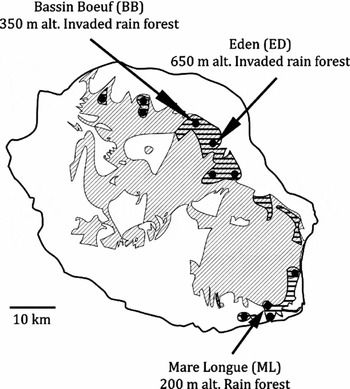
Figure 1. Distribution of Bulbophyllum variegatum on La Réunion. Diagonal shading indicates current natural habitats that are remaining on Réunion, after Strasberg et al. (Reference STRASBERG, ROUGET, RICHARDSON, BARET, DUPONT and COWLING2005); black points indicate precise localities where the species has been encountered among 121 recorded sites, see Jacquemyn et al. (Reference JACQUEMYN, MICHENEAU, ROBERTS and PAILLER2005); horizontal shading indicates potential geographical range for the species; black arrows indicate study sites.
Study sites
This study was conducted on Réunion. Together with Mauritius and Rodriguez, Réunion (c. 2500 km2) belongs to the Mascarene Archipelago, situated c. 800 km east of Madagascar (55°39ʹE, 21°00ʹS) in the western Indian Ocean. It is a young volcanic island (c. < 3 My), dominated by two massifs, Piton de la Fournaise (2619 m) (one of the most active volcanoes in the world) and Piton des Neiges (3070 m) (the highest point in the Indian Ocean). On Réunion, in contrast to Mauritius where less than 1% of the original vegetation still remains (Strahm Reference STRAHM1993), approximately 30% of original habitats have been well preserved (Strasberg et al. Reference STRASBERG, ROUGET, RICHARDSON, BARET, DUPONT and COWLING2005). The Mascarene Archipelago together with Madagascar and other nearby Indian Ocean islands belongs to one of the 34 biodiversity hotspots (Mittermeier et al. Reference MITTERMEIER, GIL, HOFFMAN, PILGRIM, BROOKS, MITTERMEIER, LAMOREUX and DA FONSECA2005, Myers et al. Reference MYERS, MITTERMEIER, MITTERMEIER, DA FONSECA and KENT2000).
Our study was carried out during two consecutive flowering seasons (2006 and 2007) at three different localities: (1) Mare Longue (hereafter ML), considered to be the best-preserved lowland wet forests of Réunion, (2) Bassin Boeuf (hereafter BB) and (3) Eden (hereafter ED), which are situated in more disturbed and fragmented areas that have been severely altered by the invasion of alien plants (Figure 1).
Variation in floral traits
Floral traits were measured in 2007 on eight plants in ML, and seven plants in ED. Three fully opened flowers per individual were randomly collected and preserved in 70% ethanol prior to floral measurements. A total of 20 morphological characters were measured to the nearest 0.01 mm using digital callipers. Differences in floral traits between populations were investigated using Wilcoxon rank sum tests.
Scent analysis
Floral volatiles were analysed using the solid-phase micro-extraction (SPME) technique (Zang & Pawliszyn Reference ZANG and PAWLISZYN1993) and GC/MS analyses described in Micheneau et al. (Reference MICHENEAU, FOURNEL, GAUVIN-BIALECKI and PAILLER2008). Two wild specimens from each study site were collected in bud, cultivated at the laboratory during the flowering period and returned to the field after experiments. The flower headspace was captured for 6 h during the optimum fragrance emission, from 12h30 to 18h30. To ensure reproducibility, each experiment was repeated twice by using the same flower. The retention index of the constituents was determined by Kovats method using n-alkanes (C8–C22) as standards (Kovats Reference KOVATS, Giddings and Keller1965). Compounds were identified by comparison of their retention indices and their mass spectral fragmentation with those reported in Adams (Reference ADAMS2001) and Arctander (Reference ARCTANDER1994), and stored on the MS ‘Nist 2002’, ‘Wiley 7’ or home-made libraries (i.e. built up from pure substances).
Breeding system and compatibility
In order to investigate breeding and compatibility systems of B. variegatum, hand-pollination experiments were conducted on randomly chosen plants in ML (11 plants in 2006 and 10 in 2007), and in ED (11 plants in 2007). Prior to flower anthesis, plants were enclosed in fine-mesh cotton bags to exclude both pollinators and predators. Three pollination treatments were performed between 9h00 and 15h00 on clear days. Treatments (colour-coded with a drop of acrylic paint on flower pedicels) were assigned randomly on different flowers from the same inflorescence. Two to four flowers per inflorescence were used for each treatment. These three treatments were: (1) no manipulation, to detect the ability to set fruit in the absence of pollinator (i.e. auto-pollination sensu Catling Reference CATLING and Arditti1990); (2) self-pollination to quantify self-compatibility; and (3) cross pollination. Fruit set was recorded 2 mo after hand pollination.
After ripening, all fruits were collected, and the percentage of seeds with normal embryos was quantified on five fruits for both self- and cross-pollination treatments. For each fruit, 100 seeds (three replicates) were observed under a microscope (× 40), and the length and width of five embryos per fruit were measured. Student's t-tests were used to compare fruit set, the proportion and size of mature embryos between flowers that were self- and cross-pollinated.
Natural fruit set
During 2006 and 2007, additional individuals were used at the three study sites to determine (1) the number of flowering plants, (2) the number of flowers per inflorescence, and (3) the fruit production (reproductive success under natural conditions). Fruit sets were recorded 2 mo after the flowering period, and were calculated as the ratio of mature fruits to open flowers per individual. Kruskal–Wallis and Wilcoxon tests were used for comparisons between populations.
Pollinator observations
Pollinator observations were performed from 10h00 to 16h00 (depending on the rain), either by eye or by using a digital video camera (Sony DCR-TRV16E) fixed on a tripod. The camera was hidden in the forest, 3 m from a patch of several individuals. Before and after each videotape or visual session, each flower of target individuals was examined for pollen removal and/or deposition.
RESULTS
Variation in floral traits
Plants usually produced pendulous inflorescences of 10–15 purplish flowers, emitting a foul, urine-like, odour during the day. The lip (averaging 9.50 mm long and 8.80 mm wide) is purple and yellow and immobile. Flowers are about 18 mm deep, and the flower opening (i.e. the distance between the base of the lip and the pollinarium) averages 8 mm. The column is 4 mm long. The pollinarium consists of two hard, yellow pollinia, which are attached to a single viscidium. No significant differences between the two populations (ML and ED) were found for each the 20 floral characters measured (data not shown).
Scent analysis
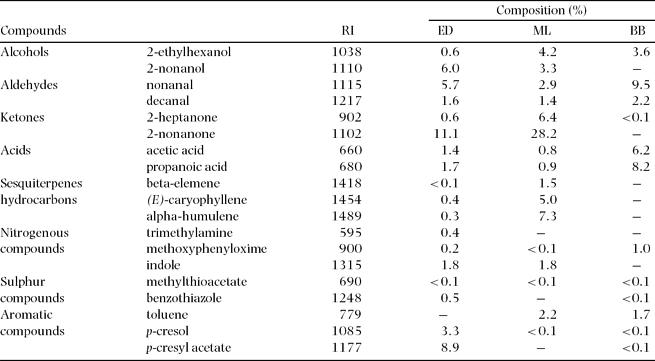
The composition of the identified headspace of the three Bulbophyllum variegatum populations revealed 51 compounds, accounting for approximately 66.8% (sample 1, ED), 85.3% (sample 2, ML) and 47.3% (sample 3, BB) of the total composition. Only major compounds (19 in total) are shown in Table 1, where they are presented according to their chemical classes, and their elution order on SPB-5 column.
Table 1. Major headspace volatile compounds of flowers in three populations of Bulbophyllum variegatum (ED: Eden; ML: Mare Longue; BB: Bassin Boeuf). RI = Retention index relative to C5-C22n-alkanes on SPB-5 non-polar capillary column.
Breeding system and compatibility
Bulbophyllum variegatum was not able to set fruit without pollinators in the two studied populations (pollinator exclusion experiments). We failed to detect any difference in fruit set between self- and cross-pollination treatments whatever the population: fruits arising from self-pollinations averaged 44% (data from ML 2006, 47 flowers; ML 2007, 40 flowers; and ED 2007, 31 flowers), and 53% from cross-pollinations (data from ML 2006, 40 flowers; ML 2007, 19 flowers; and ED 2007, 28 flowers). This result was confirmed by the proportion of mature embryos in the two treatments. No significant difference in the number or size of mature embryos was observed between the two treatments (percentage of mature embryos from self- and cross-pollinations: 71.3% and 70.3% respectively; mean embryo size: 870 μm2 and 823 μm2 respectively).
Natural fruit set
Few plants produced an inflorescence, particularly at ML where only five plants flowered in 2006 among the 103 target individuals (data not shown) and 127 plants flowered in 2007 among the 721 target individuals (Figure 2). However, flowering plants were significantly more frequent in the two other populations: 15 out of 60 plants flowered in BB in 2007, and 178 out of 604 flowered in ED in 2007 (Figure 2).

Figure 2. Natural reproductive success of Bulbophyllum variegatum in three different populations on Réunion. Percentage of flowering plants in 2007 (a). Number of flowers per plant (mean of 2006 and 2007) (b). Percentage of fruit set (mean of 2006 and 2007) (c). ML: Mare Longue; BB: Bassin Boeuf; ED: Eden. Bars represent SD. Population means from the same characters sharing a similar letter (a–c) were not significantly different at P < 0.05 using the Wilcoxon test.
In each of the three populations, levels of natural fruit set were significantly higher in the preserved forest of ML (19.9% in 2006, N = 45 flowering plants; and 24.3% in 2007, N = 125 flowering plants) compared with the disturbed forests of BB (8.1% in 2006, N = 63 flowering plants; and 5.4% in 2007, N = 15 flowering plants) and ED (0.5% in 2006, N = 63 f flowering plants; and 1.2% in 2007, N = 178 flowering plants), even if the number of flowers per plant was significantly lower at ML (Figure 2).
Pollinator observations
Although visitors were mostly small flies and ants, these were ineffective pollinators probably because they were too small to remove the pollinarium. The only efficient pollinator that we observed was a hitherto undescribed, medium-sized fly belonging to the Platystomatidae (M. Martinez, pers. comm.) (Figure 3). Only one individual has been caught, and sex determination was not possible. Adult insects are approximately 60–90 mm long. They have a dark robust body with a metallic lustre and brown-spotted wings (Figure 3). The head is wider than long. The flies seem to be attracted by the odour of the orchid that is emitted during the day. Flies were never found in flowers before daylight. In a typical pollinator visit, the fly lands on the inflorescence or directly on the flower, most often on the sepals. The insect then progresses toward the column by walking on the lip, which is channelled at its base, just below the viscidium. When the insect goes deep into the flower, its thorax comes into contact with the viscidia (without any movements of the lip). When the fly retreats from flowers, both pollinia become fixed on the back of its thorax (Figure 3). No feeding or laying behaviour has been observed during the pollination process.

Figure 3. Pollinator of Bulbophyllum variegatum on La Réunion. Fly observed visiting flowers (a). Flies with pollinarium fixed to the thorax and captured having to visit the flower at the ML population (b, c). Red and yellow paint marks on the flower (a) were used to determine natural fruit set and number of visited flowers.
Pollinating insects were only observed in ML in 2006 (29 visits during 2845 min of observations, with tree pollinia removal events on two inflorescences), and were never observed either in ML in 2007 (510 min of observations), or in ED in 2007 (300 min of observations).
DISCUSSION
Sapromyiophily: a new orchid pollination system recorded on Réunion
The only effective pollinator that we observed on B. variegatum flowers was a species of fly belonging to the Platystomatidae. This family frequently contains pollinators of Asclepiadaceae (Lumer & Yost Reference LUMER and YOST1995) or Araceae (Gibernau Reference GIBERNAU2003). In the Araceae, the inflorescence deceives the pollinators by mimicking the laying site of the pollinator. The insects visit the inflorescence in order to complete their reproductive cycle. Through this deceptive attraction, the insects achieve pollination but without actually receiving any reward (Gibernau Reference GIBERNAU2003). Given that no other pollinators were observed, this study therefore supports the first proven case of sapromyiophily in the genus Bulbophyllum in the Mascarene Archipelago.
These flies are certainly attracted by the heavy unpleasant scent emitted by the orchid during the day. Although it was not possible from our results to identify active compounds that were responsible for pollinator attraction, potential roles of each volatile compounds as key chemical signals in flower–pollinator interactions can be highlighted using Flavor-Base vers. 2007 software (Leffingwell & Associates, Canton, Georgia, USA). As shown in Table 1, chemical compounds that were identified in B. variegatum floral blends cover a large variety of chemical classes and some of them may be in accordance with the unpleasant olfactory impression produced by the orchid, as for example acetic acid, propanoic acid, methylthioacetate and p-cresol, that were all present in the headspaces of our sampling. More precisely, the unpleasant urine-like character of B. variegatum floral scent could be attributed to indole, p-cresol and 2-heptanone (Arctander Reference ARCTANDER1994). All these three volatiles have been recorded as being attractive to pollinating flies, with p-cresol showing the greatest activity (Dobson Reference DOBSON, Dudareva and Pichersky2006). This first investigation of B. variegatum floral scent composition remains preliminary, and further studies are needed to determine more clearly which of the identified component(s) are responsible for the attraction of the fly pollinator. However, the few floral chemical components that have been identified so far within the genus Bulbophyllum as playing a key role in plant–pollinator interaction mainly encompass methyl eugenol (aromatic compound) and ketones. These compounds have been mostly identified in fruit-fly pollinated species, and were not found in the scent of B. variegatum. For example, the floral fragrance of Bulbophyllum cheiri acting as a synomone contained methyl eugenol as a major component. This chemical attracts and serves as sex pheromone precursor for males of methyl eugenol-sensitive Bactrocera species during pollination (Nishida et al. Reference NISHIDA, TAN, WEE, HEE and TOONG2004, Tan et al. Reference TAN, NISHIDA and TOONG2002). The floral fragrance of Bulbophyllum vinaceum also contains methyl eugenol as the largest component that attracts Bactrocera dorsalis and Bactrocera unimacula to aid in pollination in highland forests of Sabah, Malaysia (Tan et al. Reference TAN, TAN and NISHIDA2006). Additionally, several raspberry-ketone-sensitive species, such as Bactrocera albistragata, B. caudatus, B. cucurbitae (the melon fly) and B. tau, are attracted to Bulbophyllum apertum subsp. verrucosum (Nabawan population found in Sabah) via raspberry ketone, which is the major chemical component in the floral fragrance. The floral raspberry ketone is consumed and sequestered into the male fruit fly unchanged in B. cucurbitae (Tan & Ritsuo Reference TAN and RITSUO2005). Furthermore, both methyl eugenol- and raspberry ketone-sensitive Bactrocera species are attracted to flowers of Bulbophyllum patens that release zingerone, which is the largest volatile component in the floral fragrance (Tan & Nishida Reference TAN and NISHIDA2000). Zingerone is reported to be responsible for the attraction of fruit flies Bactrocera by Bulbophyllum baileyi (Tan & Nishida Reference TAN and NISHIDA2007).
Despite several qualitative and quantitative differences between the populations, the headspace compositions of the three Bulbophyllum variegatum samples were all similar to flower scent composition of fly-pollinated orchids, and are characterized by n-alkyl-ketones, n-alkyl-aldehydes, n-alkyl-alcohols, aromatic compounds and few terpenes (as opposed to the orchids pollinated by bees for which the chemical composition usually reveals an abundance of terpenes, i.e. mono- and sesquiterpenes) (da Silva et al. Reference DA SILVA, BORBA, SEMIR and MARSAIOLI1999, Knudsen et al. Reference KNUDSEN, ERIKSSON, GERSHENZON and STAHL2006). Our analyses also revealed the presence of nitrogenous and sulphur compounds (Table 1). It is worth mentioning that sulphur compounds, which are known for their very powerful odours, even at low concentrations, are rarely encountered in the composition of orchid fragrances (Knudsen et al. Reference KNUDSEN, ERIKSSON, GERSHENZON and STAHL2006).
Self-compatible but pollinator-dependent orchid
The genus Bulbophyllum is well known to display flowers that are mainly adapted to fly-pollination (Nishida et al. Reference NISHIDA, TAN, WEE, HEE and TOONG2004, van der Cingel Reference VAN DER CINGEL2001, van der Pijl & Dodson Reference VAN DER PIJL and DODSON1966). We have shown that no fruits were formed when flowers were bagged, indicating that spontaneous auto-pollinations do not occur, and that B. variegatum requires a pollinator to successfully develop fruits. Our results further support the hypothesis that the genus Bulbophyllum may be predominantly self-compatible (Catling Reference CATLING and Arditti1990), since in this study a high efficiency of fruit set was recorded from induced self-pollinations. This pattern is similar to what is observed at the family level: self-compatibility, as a result of the capacity to set fruits from self-pollen is largely dominant within Orchidaceae, resulting in no difference in the number of fruits between selfed and crossed treatments (Dressler Reference DRESSLER1993, Tremblay et al. Reference TREMBLAY, ACKERMAN, ZIMMERMAN and CALVO2005). However, it is not rare that self-pollinations affect seed production via a significant decrease in embryo formation in mature capsules (Tremblay et al. Reference TREMBLAY, ACKERMAN, ZIMMERMAN and CALVO2005), but this was not observed in B. variegatum. When visiting a flower, fly behaviour typically favours self-pollination (i.e. geitonogamy), as they make long-lasting visits, and visit many flowers on the same individual (Borba & Semir Reference BORBA and SEMIR1998). This seems to be supported in B. variegatum.
Reproductive success
Fruit production showed strong differences depending on the populations, averaging 22.1% at the preserved forest of ML (range = 19.9–24.3%), and 3.8% in disturbed and fragmented areas, highly altered by the invasion of alien plants (range = 0.5–8.1%). Flower production was extremely low at the ML site, but fruit set was surprisingly high in this preserved habitat, especially if compared with general means reported in the literature either for rewardless tropical orchids (11.5%: Neiland & Wilcock Reference NEILAND and WILCOCK1998), or for fly-pollinated orchids (11.9%: Tremblay et al. Reference TREMBLAY, ACKERMAN, ZIMMERMAN and CALVO2005). Although a few fly families rely on flowers for their food (e.g. Syrphidae, Bombyliidae and some Tachinidae), flies are not primarily involved in plant pollination, and are not particularly viewed as efficient pollinators (van der Pijl & Dodson Reference VAN DER PIJL and DODSON1966). This is particularly true within Orchidaceae where among all pollinator groups the lowest fruit set is suggested for species pollinated by flies (Tremblay et al. Reference TREMBLAY, ACKERMAN, ZIMMERMAN and CALVO2005). However, in contrast to myiophilous pollination where flies tend to visit unspecialized flowers when looking for nectar (van der Pijl & Dodson Reference VAN DER PIJL and DODSON1966), sapromyiophily constitutes a rather specialized pollination system, in which flies may become effective pollinators (van der Pijl & Dodson Reference VAN DER PIJL and DODSON1966). Our results suggest that sapromyiophilous pollination is locally efficient in Réunion. Significant differences in fruit set that have been found between preserved and disturbed habitats suggest that fly-pollinators may be less abundant in the disturbed areas and/or in the submontane habitats compared to the native lowland rain forest. Although we spent approximately 56 h observing B. variegatum flowers, pollinator visits were only observed in 2006. Observations of orchid pollinators are difficult, and pollination biology of epiphytic species is particularly neglected, presumably because of the difficulty and cost of accessing the plants (Damon & Valle-Mora Reference DAMON and VALLE-MORA2008). Visits by pollinators are unpredictable, ephemeral, rare or simply absent. Johnson et al. (Reference JOHNSON, PETER, NILSSON and AGREN2003) and Tremblay & Ackerman (Reference TREMBLAY and ACKERMAN2007) mention hundreds of hours of observation of flowering Lepanthes rupestris, over a period of 15 y, without observing a single pollinator, although seed capsules were clearly being produced.
Implications for conservation management
Isolated oceanic tropical islands are remarkable for the high proportion of endemic taxa among their floras. These floras are, however, subject to serious threats from various origins (Myers et al. Reference MYERS, MITTERMEIER, MITTERMEIER, DA FONSECA and KENT2000). Understanding a plant's breeding system and pollinator diversity can help to set conservation and management strategies because plant populations may be greatly impacted by a reduction in pollinator visitations (Havens Reference HAVENS1999, Kearns et al. Reference KEARNS, INOUYE and WASER1998). Pollination systems are often linked to plant rarity given the dependence on insect species for cross pollination as this relationship is required for seed production and fruit set to take place. A comprehensive investigation of the pollination system is a prerequisite to developing a conservation plan for any endemic species. The destruction of the natural vegetation of the Mascarene Islands by human activities has been described in detail by Cheke (Reference CHEKE and Diamond1987). Lowland forests now exist on Réunion only in a few inaccessible sites (Strasberg et al. Reference STRASBERG, ROUGET, RICHARDSON, BARET, DUPONT and COWLING2005). The size distribution of all populations of B. variegatum should be investigated to determine if sufficient recruitment is occurring and determine if the host tree populations are stable. Restriction to a single host species may place B. variegatum at a higher risk of extinction than other Bulbophyllum or orchid species. A recent study (Lancaster Reference LANCASTER2004) reports that the conservation of B. variegatum cannot be considered independently of Agarista salicifolia. Strong host specificity, the rarity of pollinators, disturbed habitat and probably mycorrhizal specificity, all can have a major impact on the long-term survival of B. variegatum.
ACKNOWLEDGEMENTS
We are grateful to Florence Ferreto, Nelly Folgoat, Erwan Nicolas and Gypsy Pineau for their help in the field and the Office National des Forêts for support. We thank Gunter Fischer for his helpful advice and an anonymous reviewer and the editor in chief for constructive comments on an earlier version of the manuscript. This research was supported by the Université de La Réunion (BQR2006–2009) and Conseil Régional de La Réunion.






