INTRODUCTION
Animal activity is body-mass dependent (Lindstedt et al. Reference LINDSTEDT, MILLER and BUSKIRK1986, Owen-Smith Reference OWEN-SMITH1988). Body mass is one of the most important axes of biological diversity and has major effects on activity patterns through metabolic and thermoregulatory processes, and predation and competition (Demetrius et al. Reference DEMETRIUS, LEGENDRE and HARREMÖES2009, Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012a). Relationships between body size and activity patterns have long intrigued ecologists (Van Schaik & Griffiths Reference VAN SCHAIK and GRIFFITHS1996) as such relationships influence home-range and daily movement distance of species (Garland Reference GARLAND1983, Goszczynski Reference GOSZCZYNSKI1986). Small-sized mammals often adjust their behaviour to minimize agonistic encounters with the superior or large-sized mammals.
Partitioning of the temporal niche reduces substantial competition in sympatric species that exploit common resources through decreased interspecific encounter rates (Bloch et al. Reference BLOCH, STEVENS and WILLIG2011, Guevara et al. Reference GUEVARA, GONZAGA, VASCONCELLOS-NETO and AVILÉS2011, Juliano & Lawton Reference JULIANO and LAWTON1990, Kronfeld-Schor & Dayan Reference KRONFELD-SCHOR and DAYAN2003, Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012a, Schoener Reference SCHOENER1974, Warren & Lawton Reference WARREN and LAWTON1987). Greater energy demands in large-sized animals necessitate longer foraging times of over 12 h each day due to higher energy requirements (Owen-Smith Reference OWEN-SMITH1988) than the smaller ones (Van Schaik & Griffiths Reference VAN SCHAIK and GRIFFITHS1996). For instance, large carnivores have larger home ranges and are dependent on large-sized prey which necessitates travelling over larger areas (McNab Reference MCNAB1963, Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012a) to tackle territoriality, reproduction and interference competition (Beltran & Delibes Reference BELTRAN and DELIBES1994, Garland Reference GARLAND1983, Goszczynski Reference GOSZCZYNSKI1986). Large herbivores normally spend more time in search of food than small herbivores due to their generalist nature and tolerance to low-quality food (Jarman Reference JARMAN1974, Weckerly Reference WECKERLY2013). Lower mass-specific metabolic needs enable them to manage a wider variety of food than small herbivores (Bell Reference BELL1971, Jarman Reference JARMAN1974).
Characterizing the factors underlying the activity patterns of sympatric species across varying body sizes from field investigations is a daunting task. In recent years ecologists have begun to recognize the importance of time as a mediator of ecological interactions (Harmsen et al. Reference HARMSEN, FOSTER, SILVER, OSTRO and DONCASTER2011, Lucherini et al. Reference LUCHERINI, REPPUCCI, WALKER, VILLALBA, WURSTTEN, GALLARDO, IRIARTE, VILLALOBOS and PEROVIC2009) however we still have scant knowledge of this phenomenon in mammal communities from the Oriental Region. Studies have tested the role of body size and feeding type of herbivores on activity time (du Toit & Yetnian Reference DU TOIT and YETNIAN2005, Mysterud Reference MYSTERUD1998, Owen-Smith Reference OWEN-SMITH1988, Weckerly Reference WECKERLY2013). Carnivore activity is related to prey activity (Harmsen et al. Reference HARMSEN, FOSTER, SILVER, OSTRO and DONCASTER2011, Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012a) and body size is strongly related to home range in mammals (Carbone et al. Reference CARBONE, TEACHER and ROWCLIFFE2007, Haskell et al. Reference HASKELL, RITCHIE and OLFF2002, Jetz et al. Reference JETZ, CARBONE, FULFORD and BROWN2004, Swihart et al. Reference SWIHART, SLADE and BERGSTROM1988). Traditionally, most of the studies documented mammal activity based on diurnal observations (Johnsingh Reference JOHNSINGH1981, Schaller Reference SCHALLER1967). However, quantification of body-size dependency on activity pattern over the 24-h cycle in free-ranging mammals using field data from camera-trap surveys has never been documented in India. The tropical reserves in the Western Ghats with high mammal diversity and many sympatric species provide us with the opportunity to test relationships between body size and overall activity pattern of mammals, which has never been studied before. Wide variation in body mass of herbivores (2.7–2088 kg) and carnivores (1.1–200 kg) with diverse ecological niches from our study region provided substantial opportunity to study this aspect of behavioural ecology. We predicted that body size and concentration of activity were negatively related, i.e. overall activity time increases with body size.
MATERIALS AND METHODS
Study area
The Mudumalai Tiger Reserve (11°32′–11°43′N, 76°22′–76°45′E) is a continuous stretch of pristine forest (Figure 1) positioned at the junction of Tamil Nadu, Karnataka and Kerala states, at an altitude ranging from 960 to 1266 m asl. This 321-km2 reserve is bounded with Wayanad Wildlife Sanctuary in the west, Bandipur Tiger Reserve in the north and Nilgiri North Forest Division in the south. The present study was carried out in an intensive study area of 187 km2. Vegetation types were classified into dry thorn, dry deciduous, moist deciduous, semi-evergreen, moist bamboo brakes and riparian fringe forests (Champion & Seth Reference CHAMPION and SETH1968). The park has a short dry season (January–April) and a long wet season (May–December). The south-west monsoon starts by May and ends in August while the north-east monsoon starts by September and ends in December. The rainfall has a marked east–west gradient, with the eastern areas getting the least amount of rain (1000–2000 mm y−1). Temperature ranges from 8°C in December to 35°C in April. The area supports a wide variety of large, medium and small-sized carnivores and herbivores.

Figure 1. Placement of camera trap stations for measuring animal activity in Mudumalai Tiger Reserve, Western Ghats.
Data collection and analysis
Camera trapping can collect valuable ecological information on multiple species with the advantage that it can sample larger numbers of individuals, which is a limitation in radio-collar studies (Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012a). Camera-trap data for our study were collected between November and April during 2008, 2009 and 2010 as a part of research on sympatric large carnivores, i.e. tiger, leopard and dhole within an intensive study area of 187 km2 covering deciduous (DD), semi-evergreen (SE) and dry thorn (DT) forests (Figure 1). Cameras were distributed uniformly across the sites every 1.8 km. Cameras were placed at a height of 20–30 cm as this spacing and height allowed the inclusion of individuals from all mammalian species in the study area. Camera stations were placed along roads, trails, stream-beds or near water holes to maximize photo-captures. Each station comprised a pair of passive infrared analogue camera traps DeerCam® DC300 (DeerCam, Park Falls, WI, USA) to maximize capture probability. According to the available extent of major forest types, we had 20 trap stations in deciduous forest, and 17 and 13 traps in semi-evergreen and dry thorn forests respectively. Camera trap stations were run for 24 h on an average of 2000 trap nights y−1. Cameras were loaded with 36-print, 200 American Standard Association (ASA) 35-mm film.
Photographs provided information on date and time of the picture taken which was used to study daily temporal activity patterns in mammals over 24 h. Sometimes individuals were photographed from only one of the two cameras operating at a single camera station. The event of capturing an individual animal, whether it was photographed by two camera traps or one at a station, was considered to be an independent record of that animal. On some occasions, individuals were captured more than once at a camera station during a short time period (<1min), thus to avoid pseudo-replications we considered the first capture of the animal as an independent record. Photographs without time were discarded from analyses.
We classified animals as either diurnal or nocturnal if the percentage of activity records >80% occurred during the day or night respectively. We considered cathemeral if animals were active throughout a 24-h period. In our analyses activity meant overall 24-h photographic observations/records of mammals without referring to any particular behaviour. We considered 06h01–18h00 as day and 18h01–06h00 as night. We assumed that there is not much variation between seasons. We also used the mean activity time of 24 h and angle of the circular activity (Kovach Reference KOVACH2011) to classify mammals as nocturnal/diurnal/cathemeral. The time of capture printed on photographs was used to create 24-h circular activity patterns for the study species using program Oriana 4.0 (Kovach Reference KOVACH2011). Due to the low sample size, all photo records of target species were pooled together across 3 y (2008–2010) to get overall activity patterns for each species. Mean body mass of mammals was taken from available literature (Karanth & Sunquist Reference KARANTH and SUNQUIST1992, Menon Reference MENON2003). We classified the study species into small, medium and large categories based on Karanth & Sunquist (Reference KARANTH and SUNQUIST1995).
The mean vector length (r) reflects the clustering of activity in time (Kovach Reference KOVACH2011). Using each independent time record, mean vector length in carnivores and herbivores over 24 h was generated to attain comparisons between species using the program Oriana 4.0 (Kovach Reference KOVACH2011). A group of observations (or individual vectors) have a mean vector that can be calculated by combining each of the individual vectors. The mean vector has two properties, its direction (the mean angle) and its length. The length ranges from 0 to 1; a larger value indicates that the observations are clustered more closely around the mean than a smaller one (Kovach Reference KOVACH2011). A longer mean vector means greater concentration of the data around the mean, and thus less likelihood of the data being uniformly distributed. Circular histograms of activity pattern for mammals were analysed from independent time records using the program Oriana 4.0 (Kovach Reference KOVACH2011). This allowed us to assess the 24-h activity of all mammal species. We calculated the slopes of mean vector length in relation to body mass using bivariate reduced major axis (RMA) models on log-transformed data. RMA regressions were performed between mean vector length and logarithmically transformed body mass of mammals using RMA software for reduced major axis regression available at http://www.bio.sdsu.edu/pub/andy/rma.html.
RESULTS
A total of 11267 independent photographs of 26 mammal species were obtained from 7200 camera trap days. The photographic records of five species were not included in the analyses due to low detections. Activity patterns of 21 mammalian species are summarized in Table 1. The tiger had a bimodal peak activity: from midnight to morning and just after sunset. Although primarily active at night and inactive during the hottest hours of the day, the tiger and the bear were cathemeral. The leopard also showed a cathemeral activity pattern. In contrast, small cats and the civet species were essentially nocturnal, dhole and mongoose species were diurnal exhibiting minimal activity during the hottest hours of the day (Figure 2).
Table 1. Activity pattern of mammals in Mudumalai Tiger Reserve, Western Ghats. The time of capture printed on camera trap photographs was used to create the circular mean activity time in 24 h to classify mammals as nocturnal/diurnal/cathemeral. Mean average body mass of mammals was taken from Prater (Reference PRATER1971), Menon (Reference MENON2003) and Karanth & Sunquist (Reference KARANTH and SUNQUIST1992). The mean vector length ranges from 0 to 1, a larger value indicates that the observations are clustered more closely around the mean than a smaller one (Kovach Reference KOVACH2011).

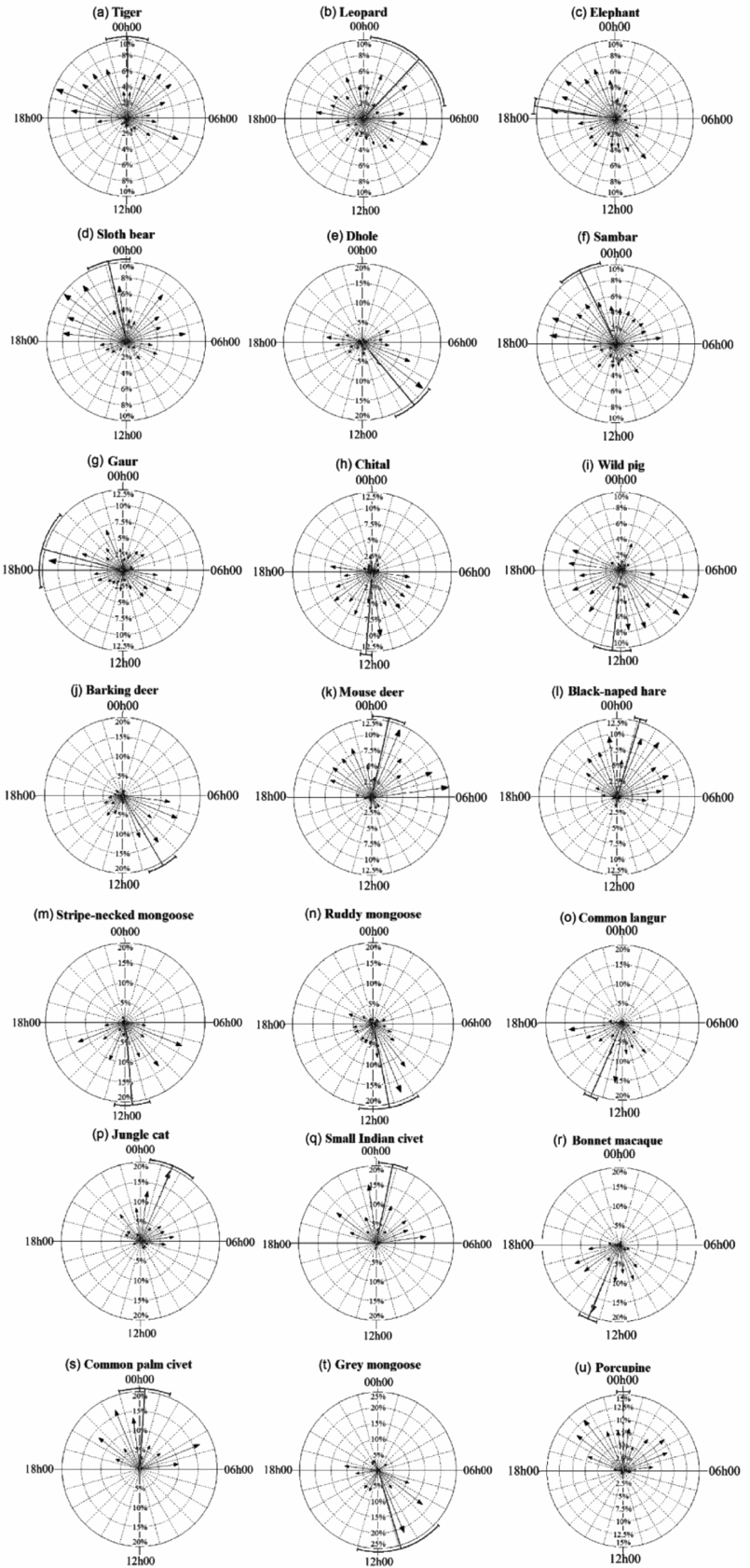
Figure 2. Temporal activity patterns of tiger (a), leopard (b), elephant (c), sloth bear (d), dhole (e), sambar (f), gaur (g), chital (h), wild pig (i), barking deer (j), mouse deer (k), black-naped hare (l), stripe-necked mongoose (m), ruddy mongoose (n), common langur (o), jungle cat (p), small Indian civet (q), bonnet macaque (r), common palm civet (s), grey mongoose (t), and porcupine (u) in Mudumalai Tiger Reserve, Western Ghats, India. Circular arrows of histogram plot for 24-h activity indicate: relative frequency of records in each hour and a longer arrow means greater clustering of the data around that hour, and thus less likelihood of the data being uniformly distributed.
Large herbivores (elephant, gaur and sambar) were cathemeral and increased their activities between 18h00 and 22h00. All medium-sized herbivores were active during the day, with barking deer and four-horned antelope being mostly active in the morning and evening, respectively. Other than primates small herbivores were exclusively nocturnal (Figure 2). Both primate species were active in the day with increased activity in the evening (Figure 2).
On the whole, large body-sized mammals showed a relatively even distribution of activity and low mean duration of activity concentration. There was a significant negative relationship between mean vector length and log-transformed body mass for mammal species (r2 = 0.60, N = 21, P = 0.001, Figure 3). The intercepts of the relationships between mean vector length and log-transformed body mass was as follows: (RMA intercept = 6.95 ± 1.17, RMA slope = −10.4 ± 2.45, 95%CI = −9.37 to −3.82, r2 = 0.56, P < 0.001) for carnivores, (RMA intercept = 7.72 ± 0.85, RMA slope = −9.73 ± 1.74, 95% CI = −13.7 to −5.80, r2 = 0.71, P < 0.001) for herbivores and (RMA intercept = 7.52 ± 0.74, RMA slope = −10.4 ± 1.51, 95% CI = −13.6 to −7.28, r2 = 0.60, P < 0.001) for all mammals. Increase in mean vector length was inversely related to increase in body size of mammals. That the mean vector length was higher in medium- to small-sized species (0.45–0.72) compared with large-sized mammals (0.4–0.09) proves the allometric relationship between overall activity time and body mass.

Figure 3. RMA regressions shows the relationship between body weight and mean vector length (Rayleigh Uniformity test score) of mammals in Mudumalai Tiger Reserve, Western Ghats, India. Increase in mean vector length was inversely related to increase in body size of mammals.
DISCUSSION
Our study shows that clustering of activity in time of large mammals is not equal to small mammals probably due to the energy expenditure for travel being more than for small mammals (Bonner Reference BONNER2006, Calder Reference CALDER1984, Glazier Reference GLAZIER2005, Peters Reference PETERS1983) as the former require a larger total quantity of food, but smaller animals require higher mass-specific quantities. The shorter activity (12 h) in most small mammals resulted in a higher mean vector. Large-sized mammals travel more widely to cover a variety of habitats resulting in dispersed activity in time. We observed that mean vector length in mammals was inversely related to body size as the proportion of time spent feeding to total activity in mammals declined with increasing body mass (Belovsky & Slade Reference BELOVSKY and SLADE1986). The total energy expenditure for large-bodied mammals is more than smaller mammals (Carbone et al. Reference CARBONE, TEACHER and ROWCLIFFE2007, Jetz et al. Reference JETZ, CARBONE, FULFORD and BROWN2004) thus explaining the clear demarcation in activity concentration across body sizes. Higher mass-specific metabolic rates and consumption of large quantities of food relative to body weight (Dial et al. Reference DIAL, GREENE and IRSCHICK2008, Weckerly Reference WECKERLY2013) in small-sized animals makes their activity more clustered at particular times of day.
The significant negative relationship between mean vector length and body mass of mammals in our study indicates that activity duration increased with body size in mammals. The larger species spend time feeding almost throughout the day while the smaller species achieve their daily food intake requirements by feeding at night and during twilight hours (Du Toit & Yetnian Reference DU TOIT and YETNIAN2005). The cathemeral activity of large herbivorous mammals suggests that they require longer foraging time (Van Schaik & Griffiths Reference VAN SCHAIK and GRIFFITHS1996).
In our region the small (1–10 kg) and medium-sized mammals (11–20 kg) were either nocturnal or diurnal, and the large-sized mammals (> 20 kg) were mostly cathemeral due to energy needs and associated feeding commitments. The selection for the optimal patterns of activity varies between species (Kronfeld-Schor & Dayan Reference KRONFELD-SCHOR and DAYAN2003). At the species level, activity of large carnivores such as tiger, leopard and dhole, is significantly correlated with their major prey activity (Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012a). The sloth bear was more active during late evening to midnight, and early mornings but less active during midday, likely to avoid intense heat conditions. All small-cat and civet species showed primarily nocturnal activity which is related to activity of nocturnal small-mammalian prey (rodents) and other potential prey species. Although body size is an important factor explaining activity patterns, interspecific competition between mammals and thermoregulation is considered to be a major constraint in small mammals as they lose more energy per unit body mass (Belovsky & Slade Reference BELOVSKY and SLADE1986, Carbone at al. Reference CARBONE, TEACHER and ROWCLIFFE2007), which explains why most of the small carnivores were active during the night and twilight hours. Mongoose species were mainly active during the day owing to their terrestrial feeding habits, better vision during the day and to avoid competition with similar-sized carnivores. Overall, activity duration of small carnivores is less than large carnivores as the former have comparatively low hunting costs than their larger cousins which includes hours of ambush, speedy chases, capture and killing (Carbone et al. Reference CARBONE, TEACHER and ROWCLIFFE2007, Gorman et al. Reference GORMAN, MILLS, RAATH and SPEAKMAN1998, Laurenson Reference LAURENSON1995).
The daily energy intake and expenditure by carnivores also corresponds with body size (Carbone et al. Reference CARBONE, TEACHER and ROWCLIFFE2007, Jetz et al. Reference JETZ, CARBONE, FULFORD and BROWN2004, McNab Reference MCNAB1963). At the community level, large carnivores have high metabolic energy expenditure mainly due to their wide-ranging habits, longer food-searching periods (Carbone et al. Reference CARBONE, TEACHER and ROWCLIFFE2007), territoriality, reproduction and interference competition (Beltran & Delibes Reference BELTRAN and DELIBES1994, Caro & Stoner Reference CARO and STONER2003, Durant Reference DURANT1998, Goszczynski Reference GOSZCZYNSKI1986). They generally consume prey species larger than their own mass which requires traversing large home ranges to maintain sufficient number of prey species that support their energy consumption. Small carnivores are mostly invertebrate feeders that can subsist on this diet due to their low absolute energy requirements (Carbone et al. Reference CARBONE, MACE, ROBERTS and MACDONALD1999, Kalle et al. Reference KALLE, RAMESH, SANKAR and QURESHI2012, Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012b). With the relatively higher metabolic rate of small mammals there is more clustering of activity in time possibly in order to forage more in relation to the amount of time spent for other activities compared with larger mammals (Illius & Gordon Reference ILLIUS and GORDON1992).
The ecological adaptations of mammalian herbivores allow smaller species to avoid competition with larger species (Owen-Smith Reference OWEN-SMITH2002). In the current study there was a clear relationship between herbivore activity and body-size categories where small-sized herbivores (except primates) were nocturnal, medium-sized herbivores were diurnal and large-sized herbivores were cathemeral. When compared with smaller, more-selective herbivores, large herbivores tend to forage more on abundant fibrous items of low nutritional content which leads to longer (Demment & Van Soest Reference DEMMENT and VAN SOEST1981, Illius & Gordon Reference ILLIUS and GORDON1992, Moen Reference MOEN1973) ruminating time compared with short feeding time in small herbivores (Maulfair et al. Reference MAULFAIR, ZANTON, FUSTINI and HEINRICHS2010, Mysterud Reference MYSTERUD1998, Robbins Reference ROBBINS1993, Weckerly Reference WECKERLY2013). In this context, different-sized herbivores can vary in aspects such as metabolic rates and digestive capacity (Demment & Van Soest Reference DEMMENT and VAN SOEST1985), foraging parameters or behaviour (Peters Reference PETERS1983). Larger species, with a lower per mass metabolic rate, need large amounts of food but can cope with relatively low food quality; whereas smaller species, with higher per mass metabolic rates, can cope with lower amounts of food but require a relatively high food quality (Olff et al. Reference OLFF, RITCHIE and PRINS2002). In some cases large grazers may not find short grass suitable enough but this could serve as useful forage for smaller species due to the variation associated with incisor breadth (Arsenault & Owen-Smith Reference ARSENAULT and OWEN-SMITH2008, Illius & Gordon Reference ILLIUS and GORDON1987) and this leads to the exclusion of larger species from preferred short-grass areas.
Though camera traps were effective in recording temporal patterns, there were certain limitations in our study. Firstly our results did not account for species variation in detection probability. Further, the placement of cameras mainly focused on large carnivore signs which would have consequently reduced the capture probabilities of ungulates and smaller carnivores in our study. Moreover, some herbivores have specialized habitat niche (Belovsky & Slade Reference BELOVSKY and SLADE1986, Ramesh et al. Reference RAMESH, SANKAR, QURESHI and KALLE2012c), thus the camera placement would have affected their temporal activity patterns (Ramesh et al. Reference RAMESH, KALLE, SANKAR and QURESHI2012a).
Despite the limitations the present study is the first to prove the role of body size in activity budgets of mammals in India. The rules governing mammal community structure (Jetz et al. Reference JETZ, CARBONE, FULFORD and BROWN2004) have led us to believe that this could be a predictable structure arising from body size and activity. Resource exploitation at different spatial scales predicts temporal niche separation among species of different sizes offering an additional mechanism for coexistence between herbivores and carnivores on shared resources. This work contributes to our understanding of how species of differing body size can influence patterns of activity, and constitutes a dimension for niche differentiation in a spatially heterogeneous environment.
ACKNOWLEDGEMENTS
We thank the Director and Dean, Wildlife Institute of India and the Chief Wildlife Warden, Tamil Nadu for granting us permission to work in Mudumalai. This research was undertaken as a part of ‘Sympatric carnivore studies’, funded by the Wildlife Institute of India. We thank our field assistants C. James, M. Kethan, M. Mathan and forest department staff for their assistance and support during fieldwork.






