INTRODUCTION
Populations of herbivorous insects are controlled by food resources (bottom-up control) and natural enemies (top-down control) (Hairston et al. Reference HAIRSTON, SMITH and SLOBODKIN1960). Since the introduction of this concept, the relative importance of these two factors has received considerable attention (Lewinsohn et al. Reference LEWINSOHN, NOVOTNY and BASSET2005, Richards & Coley Reference RICHARDS and COLEY2007, Walker & Jones Reference WALKER and JONES2001).
In externally feeding caterpillars (Lepidoptera), predation is the main cause of mortality (Dempster Reference DEMPSTER1983, Feeny et al. Reference FEENY, BLAU and KAREIVA1985). The pressure from natural enemies depends on forest type and the degree of disturbance, which can impact predator abundance (Barlow et al. Reference BARLOW, PERES, HENRIQUES, STOUFFER and WUNDERLE2006, Perfecto & Vandemeer Reference PERFECTO and VANDERMEER1996, Trollope et al. Reference TROLLOPE, WHITE and COOKE2009, Zanette et al. Reference ZANETTE, DOYLE and TREMONT2000) or their ability to find prey (Philpott et al. Reference PHILPOTT, PERFECTO and VANDERMEER2006, Richards & Coley Reference RICHARDS and COLEY2007). In particular, abrupt edges in disturbed areas are associated with higher predator (Didham et al. Reference DIDHAM, GHAZOUL, STORK and DAVIS1996, Faveri et al. Reference FAVERI, VASCONCELOS and DIRZO2008, González-Gómez et al. Reference GONZÁLEZ-GÓMEZ, ESTADES and SIMONETTI2006, Kareiva Reference KAREIVA1987, Saab Reference SAAB1999, Sieving & Willson Reference SIEVING and WILLSON1998) or parasite abundances (Doak Reference DOAK2000, but see Kruess Reference KRUESS2003) in fragmented forests. Altitudinal trends in predation pressure are poorly known since most of the studies in the tropics have focused on the lowland forests (Novotny & Basset Reference NOVOTNY and BASSET2005), however, there are studies on how predators, parasitoids and prey vary with altitude (Hodkinson Reference HODKINSON1999, Rodríguez-Castañeda et al. Reference RODRÍGUEZ-CASTAÑEDA, DYER, BREHM, CONNAHS, FORKNER and WALLA2010, Reference RODRÍGUEZ-CASTAÑEDA, FORKNER, DYER, TEPE and GENTRY2011; Samson et al. Reference SAMSON, RICKART and GONZALES1997, Sanders Reference SANDERS2002, Sivinski et al. Reference SIVINSKI, PIÑERO and ALUJA2000).
Caterpillars use a range of defences to protect them against attacks. Free-living caterpillars often rely on chemical protection, warning coloration or hairs (reviewed by Witz Reference WITZ1990). Leaf rolls, folds and ties also protect caterpillars from predators, particularly birds, ants and wasps (Atlegrim Reference ATLEGRIM1992, Cappuccino Reference CAPPUCCINO1993, Loeffler Reference LOEFFLER1996). However, leaf refuges can also serve as cues to predators and thus have negative effects on survival of their inhabitants (Nakamura & Ohgushi Reference NAKAMURA and OHGUSHI2003), as demonstrated for birds (Murakami Reference MURAKAMI1999, Robinson & Holmes Reference ROBINSON and HOLMES1982), and predatory and parasitic wasps (Gentry & Dyer Reference GENTRY and DYER2002, Weiss et al. Reference WEISS, WILSON and CASTELLANOS2004).
Predation events, in contrast to parasitism or herbivory, can only be rarely observed in nature as they happen fast. The impact of predators on prey may be estimated accurately by experimental removal of predators or by direct measurement of mortality rates. The use of artificial models of prey have already proved suitable for the assessment of relative predation pressure by different predators – birds, mammals and ants – as each group leaves recognizable marks on the attacked caterpillar (Faveri et al. Reference FAVERI, VASCONCELOS and DIRZO2008, Koh & Menge Reference KOH and MENGE2006, Loiselle & Farji-Brener Reference LOISELLE and FARJI-BRENER2002, Posa et al. Reference POSA, SODHI and KOH2007, Richards & Coley Reference RICHARDS and COLEY2007).
In this study, we use artificial caterpillars exposed in tropical rain forests of New Guinea to test the following hypotheses: (1) incidence of attacks will be higher on exposed than on semi-concealed caterpillars, (2) predation by ants will decrease and that by birds will increase with altitude, and (3) incidence of attacks will increase with the intensity of forest disturbance.
METHODS
We conducted our study at four tropical forest sites in Papua New Guinea: (1) Wanang 3 (5°13.5′S, 145°04.9′E, 120 m asl), situated within > 10 000 ha of contiguous lowland primary forest in the Wanang Conservation Area; (2) Wanang 1 (5°14.2′S, 145°10.9′E, 125 m asl), a mosaic of primary and secondary lowland rain forest near Wanang 1 village situated on the border of the Wanang Conservation Area; (3) Ohu (5°16.2′S, 145°41.1′E, 170 m asl), a 300-ha fragment of lowland primary forest near Ohu village, surrounded by secondary forest created by slash-and-burn agriculture; (4) Kotet (6°9.77′S, 146°50.37′E, 1700 m asl), a montane primary rain forest in the Finisterre Mountains close to Kotet village.
The lowland study sites have a humid climate with a mild dry season from July to September; the average annual rainfall is 3600 mm and the annual average temperature is 26.5 °C (McAlpine et al. Reference MCALPINE, KEIG and FALLS1983). The Kotet area has a lower montane humid climate with a mild dry season from April to September. Average annual rainfall is 4000 mm (McAlpine et al. Reference MCALPINE, KEIG and FALLS1983) and average temperature 17 °C (Tvardikova & Novotny, unpubl. data).
We used artificial caterpillars exposed on vegetation to monitor attack by predators and parasitoids. They were made from modelling clay (Koh-I-Noor Hardtmuth brand), which is malleable, oil-based and non-toxic. Artificial caterpillars were modelled by pressing the plasticine through a syringe. The syringe was used to ensure that each caterpillar had an absolutely smooth surface. Artificial caterpillars were 15 mm long and 3 mm in diameter, matching in body size locally common crambid and tortricid caterpillars, as well as median size in the entire caterpillar community (Novotny & Basset Reference NOVOTNY and BASSET1999). A mixture of brown and green modelling clay was used to create a natural-looking dark green colour. Models of free-living caterpillars resembled in size and appearance several locally common caterpillars including those from the genus Imma (Immidae). Leaf folds of semi-concealed caterpillars resembled natural leaf folds of Choreutis species. The real and artificial caterpillars and folds are shown in Figure 1.

Figure 1. Attack marks by individual predator groups of natural enemies and semi-concealed and exposed caterpillars, and their plasticine models: leaf fold created by a real caterpillar of genus Choreutis (a), model of a leaf fold with a plasticine caterpillar hidden inside (b), a real caterpillar of genus Imma and a model of a free living caterpillar (c), bite marks by a small rodent (d), bird's beak mark on a roll of semi-concealed caterpillar (e), beak marks by a bird (f), caterpillar predated by a wasp (upper part) and by an ant (lower part) (g), bite marks by an ant (upper part) and ovipositor marks by a parasitoid (lower part) (h).
We conducted our experiments only on selected, phylogenetically related tree species, mimicking a possible host plant range of a caterpillar species and thus its natural spatial distribution on the vegetation. In this way we also controlled for the effects of tree species between sites and habitats, including the size, shape and surface of leaves of the experimental trees. Our focal species were Ficus congesta Corner, F. conocephalifolia Ridley, F. badiopurpurea Diels and F. bernaysii King. They were selected because of their broad distribution along disturbance and altitudinal gradients; at least three of these species were common at each experimental site (F. congesta was rare in Ohu, and F. bernaysii was nearly absent in Kotet and Wanang 1 and 3). The studied forests, particularly in the lowlands, include also numerous other Ficus species with species-rich herbivore communities (Basset & Novotny Reference BASSET and NOVOTNY1999, Novotny et al. Reference NOVOTNY, MILLER, BASSET, CIZEK, DARROW, KAUPA, KUA and WEIBLEN2005).
Each experiment was conducted along a single 2175-m-long transect at each study site. Thirty sampling points (about 75 m apart) were established along each transect. Within a radius of 20 m from each sampling point, all suitable saplings of the four focal species (2–5 m high) were marked with flagging tape at their base. Twenty (10 exposed and 10 semi-concealed) artificial caterpillars were placed on the focal Ficus trees at each sampling point, between 3 and 5 m above the ground. All experiments were completed between December 2010 and March 2011.
Exposed caterpillars were pinned on the distal half of leaf so that the head of the pin was hidden in modelling clay. Artificial semi-concealed caterpillars were pinned on the basal half of a leaf and the leaf was then folded over it and fixed by a drop of Super Glue. Artificial caterpillars were at least 30 cm apart from one another. We used 10 exposed and 10 semi-concealed caterpillars per site, i.e. a total of 600 artificial caterpillars along each transect.
Each caterpillar was inspected at 24-h intervals for six consecutive days and carefully examined for characteristic bite marks (Figure 1). Missing caterpillars were excluded from the analyses as their status could not be ascertained. All missing caterpillars were replaced by new individuals. Caterpillars with some marks were collected and replaced by new caterpillars in the same locations. Predated caterpillars were transported to the laboratory where they were examined for signs of predation or parasitism under a stereomicroscope.
Markings on the plasticine models were compared with images in the literature (Howe et al. Reference HOWE, LÖVEI and NACHMAN2009, Posa et al. Reference POSA, SODHI and KOH2007) and our own reference collection of plasticine models offered to common predators. Damage of uncertain origin was photographed and identified later. Detailed investigations of mandible marks on smooth surfaces aided differentiation of many potential predators, and enabled us to recognize attacks by ants of different sizes (Formicidae), birds (Aves), rodents (Rodentia), predatory wasps (Hymenoptera: Vespidae) and parasitoid wasps (Hymenoptera: Ichneumonoidae), which left ovipositor marks (Howe et al. Reference HOWE, LÖVEI and NACHMAN2009). Various types of marks by natural enemies are shown in Figure 1. We also tested whether predators were attracted to plasticine material by comparing attacks on 10 exposed caterpillars and 10 plasticine balls (5 mm in diameter) exposed on five trees for 24 h at every experimental site.
We estimated canopy openness from canopy photos (Canon 450D, same settings for all photos; Pekin & Macfarlane Reference PEKIN and MACFARLANE2009) taken at every sampling point (30 pictures per transect). Pictures were analysed in a Gap Light Analyzer (GLA_v.2) with the threshold at 150.
Statistical analyses
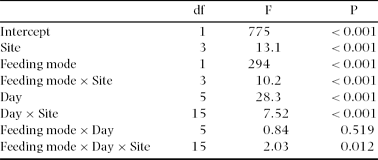
We tested the effect of site and caterpillar feeding mode on incidence of attacks by repeated-measures ANOVA with nested design and two within-category effects. All 30 sampling points were nested in each of the four experimental sites. Percentages of attacked caterpillars were arcsine transformed. We excluded all unidentified attack attempts or lost caterpillars from the analysis. The day of experiment (from 1 to 6) was used as the first within-sampling-point effect, and feeding mode of caterpillar (semi-concealed or exposed) as the second within-sampling-point effect.
The attacks of individual predator taxa were also analysed using repeated-measures ANOVA. Repeated observations from each observation point were summed together, as the time did not show any significant impact in previous analyses. Total numbers of attacked caterpillars were logarithmically transformed (log x + 0.1). Type of predator (ant, bird, wasp, mammal, parasitoid) was used as the first effect and feeding mode of caterpillar (semi-concealed, exposed) as the second effect. Tukey post hoc tests were performed to test differences between study sites and predator taxa. Statistica 9 for Windows (Statsoft Inc., http://www.statsoft.com) was used for the analyses and graphs.
RESULTS
We exposed a total of 14 400 caterpillars, and identified 2443 attack attempts. We excluded 432 (3%) missing caterpillars from analyses, because we were not able to identify the predator. Overall percentage of attacked caterpillars was significantly lower on semi-concealed (5.44%) than on exposed (11.4%) caterpillars (Table 1, Figure 2). This was true in all types of habitat and at all times after initial exposure (Table 1). Although the number of attacks changed with time after exposure, it did not show any trends, and did not correlate with the length of experiment in any habitat (Pearson r < 0.17, P > 0.05).
Table 1. Effects of site (Wanang 1, Wanang 3, Oho, Kotet), feeding mode (semi-concealed or exposed), and day (from the start of experiment) on the incidences of attack on caterpillars. Repeated-measures ANOVA.
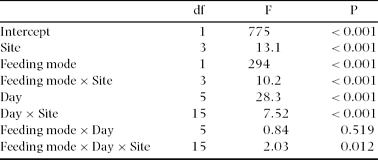

Figure 2. The number of attacks recorded after 24 h on 300 semi-concealed and 300 exposed caterpillars at different sites. Sites with significantly different rates of attack (P < 0.05) are denoted by different letters; capital letters = differences within semi-concealed caterpillars, lowercase letters = differences within exposed caterpillars. Differences between semi-concealed and exposed caterpillars within one site are marked with stars (*** P < 0.001).
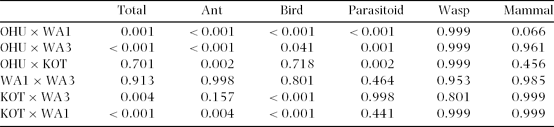
Total percentage of attacked caterpillars during the whole experiment was highest (5.7%) in the lowland forest fragment in Ohu and in the montane primary forest in Kotet (4.7%), and significantly lower in the partly disturbed and primary lowland forests in Wanang (3.2%) (Figure 2, Table 2). The majority of all recorded attacks on caterpillars was by birds (6.6%) and ants (6.8%), followed by predatory wasps (1.86%), parasitoids (0.77%) and small mammals (0.77%). Individual enemies showed different numbers of attacks across sites and between semi-concealed and exposed caterpillars (Table 3).
Table 2. Comparison of incidence of attack at different sites. Sites: Ohu (lowland forest fragment), Wanang 3 (WA3, lowland primary forest), Wanang 1 (WA1, lowland secondary forest), Kotet (KOT, primary forest at high elevation). Tukey post hoc test results for total number of attacks, and attack by individual enemies are shown.
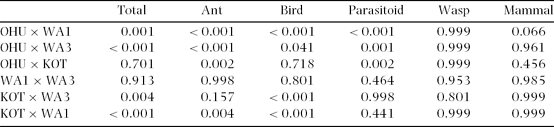
Table 3. Effects of natural enemy, site (four habitats), and feeding mode (semi-concealed or exposed) on the number of attacks on caterpillars (repeated measures ANOVA).

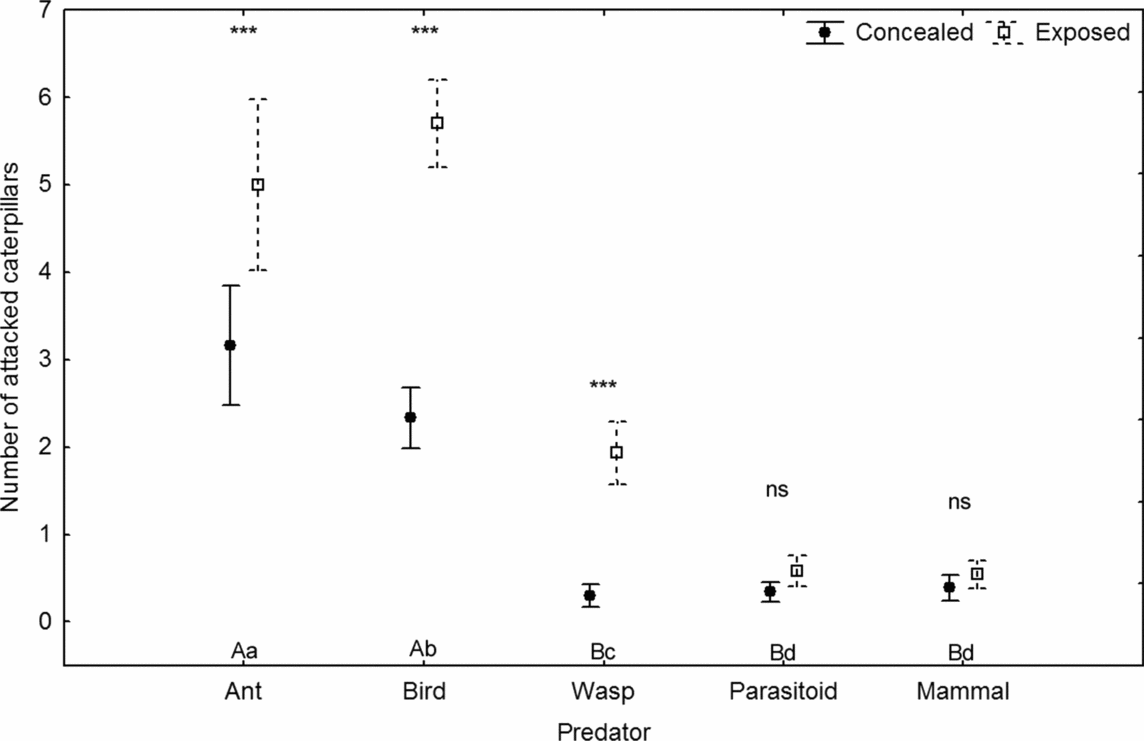
Ants, birds and wasps attacked exposed caterpillars significantly more than semi-concealed ones (P < 0.001 for all predator groups), while mammals (P = 0.615) and parasitoids (P = 0.354) attacked the two types of caterpillars with similar, and low, frequencies (Figure 3). For exposed caterpillars, different groups ranked by attack frequency were: birds > ants > wasps > (parasitoids = mammals) while in semi-concealed caterpillars, the incidence of attacks by (ants = birds) > (wasps = parasitoids = mammals).

Figure 3. The number of attacks by different natural enemies recorded after 24 h on 300 on semi-concealed and 300 exposed caterpillars. Sites with significantly different incidence of attack (P < 0.001) are denoted by different letters; capital letters = semi-concealed caterpillars, lower-case letters = exposed caterpillars. Differences between incidence of attack of individual natural enemies on semi-concealed and exposed caterpillars are marked in stars (*** P < 0.01). Current effect: F(4, 464) = 31.3, P < 0.001. Vertical bars denote 95% confidence intervals.
Ants attacked the largest number of caterpillars in the lowland forest fragment in Ohu (11.3% = 399 ind.) and the lowest number in montane forest in Kotet (5.1% = 167 ind.). Birds were significantly more frequent predators of caterpillars in montane Kotet (12.8% = 420 ind.) than in lowland forests, where the forest fragment in Ohu (7.1% = 252 ind.) had significantly higher incidence of attacks by birds than both disturbed and primary Wanang forests (4.5% = 162 and 3.5% = 127 ind.). Parasitoids attacked significantly more caterpillars in Ohu (1.2% = 45 ind.) than anywhere else (maximum 0.7% = 27 ind. in Wanang 3, Figure 4).

Figure 4. The number of attacks by different natural enemies recorded after 24 h on 600 caterpillars at the four experimental sites. Enemy groups with significantly different incidence of attack (P < 0.05) are denoted by different letters or numbers; capital letters = ants, small letters = birds, numbers = parasitoids.
In the lowlands, the canopy openness was significantly higher in disturbed forest (Wanang 1, mean ± SD = 22.5% ± 2.69%) and the primary forest fragment (Ohu, 25.3% ± 5.25%) than in the undisturbed primary forest (Wanang 3, 9.74% ± 1.49%) (ANOVA, Tukey post hoc test: P = 0.03). Canopy openness in the montane forest in Kotet was mid-way between that found in the disturbed and undisturbed forests (16.0% ± 2.46%).
The experiment comparing plasticine balls and plasticine caterpillars showed significant preference for caterpillars by all natural enemies (Site: P = 0.012, F3 = 21.3; Site × Predator: P = 0.071, F12 = 0.63, ANOVA) as they attacked caterpillars 8.6 ± 0.5 times more than balls (0.9%) at all sites.
DISCUSSION
The results of experiments with artificial caterpillars have to be interpreted with caution. These caterpillars provide only visual cues to their natural enemies whilst lacking chemical signals which may be important for prey recognition by some predators (Gentry & Dyer Reference GENTRY and DYER2002, Murakami Reference MURAKAMI1999, Vet & Dicke Reference VET and DICKE1992, Weiss et al. Reference WEISS, WILSON and CASTELLANOS2004). It should be emphasized that our method samples only a tiny fraction of the parasitoid community, since many parasitoid Hymenoptera locate hosts through chemical cues (Wölfling & Rostás Reference WÖLFLING and ROSTÁS2009), and numerically dominant Tachinidae would not leave any markings on the models as they deposit eggs onto the skin of the host insect (Stireman et al. Reference STIREMAN, O'HARA and WOOD2006). The absence of motion can also eliminate attacks by some natural enemies (parasitoids: Vinson Reference VINSON1984; spiders: Nyffeler Reference NYFFELER1999). On the other hand, moving prey may be more vulnerable (Lima & Dill Reference LIMA and DILL1990). Further, the experiments measure only attack incidence whilst ignoring differential ability of prey to escape (Lima Reference LIMA1992) and defend itself against predators (Dyer Reference DYER1997). Levels of predation by different predators exhibit significant variation (Dyer Reference DYER1997, Reference DYER2002; Hölldobler & Wilson Reference HÖLLDOBLER and WILSON1990), and different enemy taxa also handle live insects very differently from dead or fake prey.
The incidences of attack on our exposed artificial caterpillars were similar to incidences of attack measured on genuine exposed caterpillars in exclosure experiments (7.5% ± 6.7%, median = 5.8%, nine studies from both tropical and temperate habitats; Remmel et al. Reference REMMEL, DAVISON and TAMMARU2011).
In contrast, other manipulative studies with real larvae provided higher estimates of daily attack incidence (78% in lowland forest, Brazil – Jeanne Reference JEANNE1979, 75.5% in Costa Rica – Dyer Reference DYER2002). Daily attack incidence on artificial caterpillars in a lowland seasonal forest in Barro Colorado Island in Panama was 11.1% (Richards & Coley Reference RICHARDS and COLEY2007) and 42.0% (Koh & Menge Reference KOH and MENGE2006), 13.7% in semi-evergreen lowland dipterocarp forest in the Philippines (Posa et al. Reference POSA, SODHI and KOH2007) and 29.1% ± 23.3% d−1; median = 26.6% in three studies from different tropical areas (Remmel et al. Reference REMMEL, DAVISON and TAMMARU2011). An extremely low predation rate of 0.03% d−1 was recorded on cotton fields in Uganda (Howe et al. Reference HOWE, LÖVEI and NACHMAN2009). It is worth noting in this context that a constant daily mortality rate of 1%, 5% and 20% would produce overall mortality of respectively 19%, 66% and 99% over 3 wk of caterpillar life span.
Overall share of attacks by arthropods (ants, wasps, parasitoids) in our study (46%) was lower than that found in some other forest studies on artificial caterpillars: 90% or greater in Koh & Menge (2006), and Loiselle & Farji-Brener (2002), but higher than 39% found in a similar study in understorey (Posa et al. Reference POSA, SODHI and KOH2007).
Our experiments using models of caterpillars do not necessarily provide an estimate of natural predation rates, but the relative number of predation incidents may be comparable among habitats (Brodie Reference BRODIE1993). We believe that artificial caterpillar experiments serve well as a relative measure of number of attacks (Howe et al. Reference HOWE, LÖVEI and NACHMAN2009). Likewise, Richards & Coley (2007) found no differences between number of attacks on artificial and real undefended caterpillars.
The lower incidence of attacks on semi-concealed than exposed caterpillars by birds, ants and wasps is the strongest pattern revealed in our study, as it is consistent across all sites. At the same time the abundance of semi-concealed caterpillars in herbivore communities is high. In lowland New Guinea forest, they are more than twice as abundant as exposed caterpillars (Novotny et al. Reference NOVOTNY, MILLER, HRCEK, BAJE, BASSET, LEWIS, STEWART and WEIBLEN2012). Although there are studies showing that leaf rolls can decrease risk of predation (Atlegrim Reference ATLEGRIM1992, Cappuccino Reference CAPPUCCINO1993, Loeffler Reference LOEFFLER1996), their role in avoiding predation has not been quantified. Here we report that leaf refuges protect caterpillars against predation and they improve caterpillar survivorship.
The expectation was that mainly visually oriented birds prey more on exposed caterpillars, although at least some bird species specialize on leaf-rollers (Robinson & Holmes Reference ROBINSON and HOLMES1982). We confirmed that difference in incidence of attack between semi-concealed and exposed caterpillars was greater for birds than for other predators. This is not self-evident as equally plausibly, birds could use leaf rolls as visual cues for finding caterpillars.
The preference by ants and wasps for exposed caterpillars is in concordance with the study of Krombein (Reference KROMBEIN1967) of abundant social wasps (Klein et al. Reference KLEIN, DEWENTER, BUCHORI and TSCHARNTKE2002) which had bigger impact on exposed than semi-concealed caterpillars, while less abundant solitary wasps fed chiefly on leaf rollers and leaf tiers in lowland tropical forest (Klein et al. Reference KLEIN, DEWENTER, BUCHORI and TSCHARNTKE2002). Fowler & Macgarvin (Reference FOWLER and MACGARVIN1985) showed that free-living caterpillars were reduced in abundance more than leaf tiers on birch trees with Formica ants. In contrast, Ito & Higashi (Reference ITO and HIGASHI1991) found similar impact of ants on both free-living and semi-concealed caterpillars.
Mammals and parasitoids did not show any significant differences in attacks on the two types of caterpillar. In terms of invertebrate biomass, caterpillars represent an important food source for rodents (Posa et al. Reference POSA, SODHI and KOH2007, Roux et al. Reference ROUX, CHAPUIS, FRENOT and VERNON2002), however we observed very few predatory attacks by rodents on both semi-concealed and exposed caterpillars. It is well established that parasitism is much higher on concealed larvae, such as miner and gallers, than on exposed caterpillars, with semi-concealed caterpillars probably experiencing incidence of parasitism somewhere in between (Hawkins et al. Reference HAWKINS, CORNELL and HOCHBERG1997). Gentry & Dyer (Reference DYER2002) showed higher incidence of parasitism for semi-concealed feeders than for free-living caterpillars. Our results do not conform to this expectation, although this might be due to the low number of attacks we recorded, and hence low statistical power.
The effects of habitat alternation on predation are not well understood (Koh & Menge Reference KOH and MENGE2006) although there is some evidence of higher predation and parasitism in disturbed areas (Doak Reference DOAK2000, González-Gómez et al. Reference GONZÁLEZ-GÓMEZ, ESTADES and SIMONETTI2006, Posa et al. Reference POSA, SODHI and KOH2007) and forest gaps (Faveri et al. Reference FAVERI, VASCONCELOS and DIRZO2008). Correspondingly, our forest fragment had incidence of attack twice those of primary and secondary forests (Wanang 1 and 3). However, our results on differences between forest types should be considered as preliminary since we did not study replicated sites for each habitat. The lack of a difference between incidence of attack in primary and secondary forests is surprising, but could be explained by the conditions of our secondary forest at the site. We were working in a selectively logged area which also included old food gardens, and some patches of primary forest.
Externally feeding insect herbivores are exposed to intense predation pressure by ants (Hölldobler & Wilson Reference HÖLLDOBLER and WILSON1990, Rodewald et al. Reference RODEWALD, YAHNER and BRAWN2001, Stamp & Bowers Reference STAMP and BOWERS1991), birds (Koh & Menge Reference KOH and MENGE2006, Posa et al. Reference POSA, SODHI and KOH2007) and wasps (Shelly Reference SHELLY1986, Stamp & Bowers Reference STAMP and BOWERS1988) in tropical lowland rain forests. This was also true at our study sites. Predation at our sites increased with disturbance. A higher intensity of attack by ants in forest fragments could be caused by higher abundances of some invasive species or by different composition of communities (Klimes et al. Reference KLIMES, JANDA, IBALIM, KUA and NOVOTNY2011, Peters et al. Reference PETERS, FISCHER, SCHAAB and KRAEMER2009) and by higher levels of aggression of some invasive ant species (Human & Gordon Reference HUMAN and GORDON1999). Another reason could be facilitated access to forest interior in anthropogenically changed landscapes (Rodewald et al. Reference RODEWALD, YAHNER and BRAWN2001), greater insolation of the forest understorey causing higher activity of arthropods (Faynor et al. Reference FAYNOR, MEHMOOD and POTDAR1996, Klimes et al. Reference KLIMES, JANDA, IBALIM, KUA and NOVOTNY2011, Louda & Rodman Reference LOUDA and RODMAN1996) or improved visibility facilitating caterpillar location by visually oriented enemies (Martin & Karr Reference MARTIN and KARR1986, Valladares et al. Reference VALLADARES, SALVO and CAGNOLO2006). Richards & Coley (2007) showed that differences in light availability could lead to dramatic changes in trophic interactions between caterpillars and their predators. Posa et al. (Reference POSA, SODHI and KOH2007) found different incidence of predation in habitats with a range of canopy openness (closed-canopy forest, open-canopy forest, rural areas) and Richards & Coley (2007) found differences in predation under continuous forest canopy and in gaps. This could be a possible explanation in our case as the canopy openness was significantly higher in the forest fragment in Ohu and the secondary forest in Wanang 1 compared with the primary forest in Wanang 3, and marginally (non-significantly) higher in forest fragment than in secondary forest. Also, parasitoids attacked more caterpillars in the forest fragment than in the other habitats. This pattern mirrors other results (Valladares et al. Reference VALLADARES, SALVO and CAGNOLO2006), and again can be explained by higher efficiency resulting from improved visibility facilitating host location, which in parasitoids seems driven also by visual clues (Salvo & Valladares Reference SALVO and VALLADARES2004).
The incidence of attack at higher altitudes was higher than in lowlands, a similar result to that from temperate forests (Jeanne Reference JEANNE1979, Mäntylä et al. Reference MÄNTYLÄ, ALESSIO, BLANDE, HEIJARI, HOLOPAINEN, LAAKSONEN, PIIRTOLA and KLEMOLA2008). This could also be caused by increased light availability in montane forest which facilitates predation (Martin & Karr Reference MARTIN and KARR1986), by higher abundances of predators (Kessler et al. 2011), or by lower abundance of potential prey (Kessler et al. Reference KESSLER, HERZOG, FJELDSÅ and BACH2001, McCoy Reference MCCOY1990). The highest predation caused by birds was in the montane forest, and this could be the result of a higher proportion of insectivorous birds living in the understorey of primary forest at higher elevations than in lowlands (insectivores represented 63% of all montane and 47% of all lowland species in our quantitative survey; Tvardikova unpubl. data). In contrast, Schwenk et al. (Reference SCHWENK, STRONG and SILLETT2010) did not find any effect of bird predation on arthropod abundance across an altitudinal gradient (290–780 m asl). The abundance of predatory wasps was also unrelated to altitude (Banko et al. Reference BANKO, OBOYSKI, SLOTTERBACK, DOUGILL, GOLTZ, JOHNSON, LAUT and MURRAY2002). We expected lower predation by ants in montane areas, since this group become progressively less abundant with increasing altitude and ant are already very rare at 1800 m asl (Samson et al. Reference SAMSON, RICKART and GONZALES1997). Surprisingly, predation by ants was not lower in montane forest than in some lowland forests in our study.
In conclusion, our study detected fewer attacks on semi-concealed than exposed caterpillars. We were able to identify predation attempts of birds, ants, wasps, rodents and also attacks of parasitoids on our artificial caterpillars, demonstrating the potential of this method for predation studies. The differences in relative attack pressure among the habitats from this study show that human disturbance can affect the biotic interactions between caterpillars and predators. In addition, we showed that the differences in potential predator assemblages across elevation could lead to dramatic changes in trophic interactions, with herbivores being limited by different kinds of predators in different habitats and at different elevations.
ACKNOWLEDGEMENTS
We are thankful to field assistants (Taju Hais, Stafford Brus, Cliffson Philip, Benson Philip and John Minje) for help in the field, and to Bonny Koane for their supervising in the field. We thank Jan Hrček, Petr Klimeš, Tom Fayle and Legi Sam for critical comments on the manuscript, and to Jan Lepš for comments on statistical analyses. The project was financially supported by the Czech Science Foundation Grants 206/09/0115 and 206/08/H044, Czech Ministry of Education ME09082, US National Science Foundation DEB-0841885, and was also created as a part of Center of Excellence for Global Study of Biodiversity and Function of Forest Ecosystems, reg. n. CZ.1.07/2.3.00/20.0064 co-financed by the European Social Fund and the state budget of the Czech Republic.