Natural history observations provide a basis for meaningful models and empirical studies that put results into an appropriate context. This is especially true for antagonistic interactions between predatory species that have significant impacts in their native and introduced habitats. Despite the fact that top predation has been steadily expanding as a research topic (Sergio et al. Reference SERGIO, SCHMITZ, KREBS, HOLT, HEITHAUS, WIRSING, RIPPLE, RITCHIE, AINLEY, ORO and JHALA2014), actual quantification of the effect of vertebrate predation on ecologically important predatory invertebrate species in unmanipulated systems is rare (Gunnarsson Reference GUNNARSSON2007, Redford Reference REDFORD and Genoways1987).
The cane toad (Rhinella marina Linnaeus, 1758; Figure 1) is a good candidate for studying interactions between top predator species because of its toxicity, large size and generalist feeding habit. Paraponera clavata (Fabricus, 1775; Figure 2), commonly known as the bullet ant, is sympatric with the cane toad across most of its native range, and is a common, top predatory invertebrate with few known natural enemies (Dyer Reference DYER2002). Here I present observations of cane toad predation on bullet ant nests located in a lowland wet forest along Pipeline Road (PLR), Parque Nacional Soberania, Panama. Several descriptive measures of cane toad predation effect on ant nests, and nest density are provided. I conclude this communication with a brief discussion of how significant this interaction may be for bullet ant and sympatric herbivore populations.
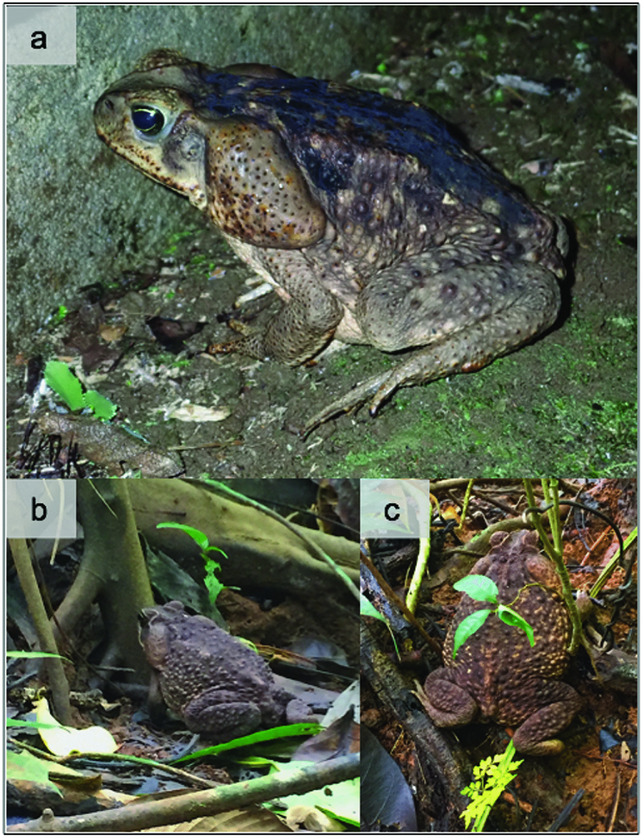
Figure 1. Cane toad (Rhinella marina) situated next to a building at La Selva Biological Station, Costa Rica in July 2018 (a). Cane toad positioned next an entrance hole to a bullet ant nest on PLR (b); the same toad positioned on top of earthworks directly adjacent another nest entrance (c).

Figure 2. Bullet ant (Paraponera clavata) patrolling outside of the entrance to its nest on PLR in August 2016 (a). Several adults antennating each other along primary trunk trail ~1.5 m above nest entrance at La Selva Biological Station, Costa Rica in June 2018 (b).
The cane toad is the world's largest toad with an average length of 10–15 cm. It is distributed from southern Texas to central Brazil, reaching its greatest density in tropical latitudes (Zug & Zug Reference ZUG and ZUG1979). It is most commonly found in open areas around human settlements (Figure 1a; Heatwole Reference HEATWOLE1966) in its native range, although it also inhabits transitional areas in and around secondary forest (Zug & Zug Reference ZUG and ZUG1979). All life stages have toxic, alkaloid-containing compounds called bufadienolides (Mailho-Fontana et al. Reference MAILHO‐FONTANA, ANTONIAZZI, TOLEDO, VERDADE, SCIANI, BARBARO, PIMENTA, RODRIGUES and JARED2014) that vary in type and quantity throughout ontogeny (Hayes et al. Reference HAYES, CROSSLAND, HAGMAN, CAPON and SHINE2009). This toxicity makes cane toad skin highly lethal to most animals if ingested (Allen & Neill Reference ALLEN and NEILL1956). The cane toad has an extremely generalized diet (Zug & Zug Reference ZUG and ZUG1979), including plants, detritus, rodents, bats, birds, reptiles, other amphibians, and many groups of invertebrates, including bullet ants (Isaacs & Hoyos Reference ISAACS and HOYOS2010).
The bullet ant is found in lowland tropical forests from Nicaragua to the central Amazon between sea level and ~750 m (Janzen & Carroll Reference JANZEN, CARROLL and Janzen1983, Murphy & Breed Reference MURPHY and BREED2007). Nests are most frequent in late-successional secondary and primary forests where annual levels of precipitation are high (Belk et al. Reference BELK, BLACK, JORGENSEN, HUBBELL and FOSTER1989, Bennett & Breed Reference BENNETT and BREED1985, Murphy & Breed Reference MURPHY and BREED2007). They are known for their large body size (~2.5 cm in length), neurotoxicity (Piek et al. Reference PIEK, HUE, MANTEL, TERUMI and SCHMIDT1991) and severe sting. These ants build subterranean nests at the base of trees that can be recognized as a mound of upturned earth with a series of vertical openings that lead into the subterranean sections of the nest. Workers ascend the tree adjacent to their nest to various levels of the canopy where they forage for plant material, extra-floral nectar and small arthropods, most of which are herbivorous (Figure 2b; Dyer Reference DYER2002, Tillberg & Breed Reference TILLBERG and BREED2004). The bullet ant is a major consumer of arthropods compared to predatory bugs, paper wasps (Dyer Reference DYER1997), army ants (Tillberg & Breed Reference TILLBERG and BREED2004) and predatory mammals (Glanz Reference GLANZ and Gentry1990). Colony size varies from 200–3000 adults (Breed & Harrison Reference BREED and HARRISON1988, Janzen & Carroll Reference JANZEN, CARROLL and Janzen1983) with 400 adults suggested as an average (Fewell et al. Reference FEWELL, HARRISON, STILLER and BREED1992).
Twelve bullet ant nests were observed along PLR, ~8 km north-west of Gamboa, over 81 non-consecutive days from May 2015 to October 2016. The bullet ant nest census was done by locating nest entrances while walking East-West transects across forest trails. The census area spanned 104 ha. Mean nest density was calculated as 0.12 nests ha−1. All nests were observed between 08h00–11h00 or 13h00–19h00.
Cane toad predation events (N = 5) were observed during a survey to find bullet ant nests for a separate study in which choice test bioassays were conducted to determine the palatability of plant and animal tissue extracts. Upon observation of a cane toad eating bullet ants the total number of ants consumed by the cane toad was recorded as either the total ants consumed in 3 h, or the total consumed before the toad stopped eating ants and left the vicinity (Supplemental Video 1). The number of cane toad predation events that resulted in the elimination of all adult ants in a nest was divided by the total number of nests censused to calculate the extirpation rate of bullet ant nests. Nest activity was always checked the morning after a cane toad predation event and then weekly for the duration of the study. Nest activity was assessed by aggressively inserting a stick into the nest entrance and agitating the earthworks to uncover adults, cocoons, larvae or eggs. Nests were determined to be completely consumed, or abandoned by the colony, if no adult ants were visible upon agitation of the nest. Ants in active nests will aggressively respond in force to disturbances (Belk et al. Reference BELK, BLACK, JORGENSEN, HUBBELL and FOSTER1989) such as dropping pebbles on the nest entrance from chest height or gently brushing the earthworks with fresh leaves (C. Morrison, pers. obs.).
All adult bullet ants were consumed by cane toads when they exited or entered the nest (Figure 1b–c; Supplemental Video 2). Cane toads under observation consumed an average ± SD of 109 ± 56.4 adult ants. The maximum number of individuals consumed by a toad during an observation was 211. The average duration of feeding by toads was 1.75 ± 0.58 h following initial observation of the predation event. Cane toads consumed 0.99 ± 0.19 ants min−1 across all predation events. This cane toad blitz feeding is similar to strategic positioning behaviour previously observed in insectivorous Australian herpetofauna (Mo Reference MO2015, Pianka & Pianka Reference PIANKA and PIANKA1970). Six of the 12 bullet ant nests (50%) observed during this study were completely consumed by cane toads, other natural enemies, or abandoned. A toad was observed preying upon ants from five of the six nests (83%) that ceased to display activity during this study. I cannot account for what led to the destruction, or relocation, of the sixth nest. These calculations demonstrate that 42% of nests (5/12) censused were completely consumed by cane toads or abandoned by the remaining adult nestmates following a predation event. I did not observe ant activity recommencing in any of the nests that were preyed on for the duration of the study. I do not know if one, or several, cane toads preyed on the nests that were consumed or abandoned by the ants. Each nest was observed for an average of 79.5 ± 43.5 h.
This study provides the first quantification of how vertebrate predation affects the viability of a dominant tropical ant species. The bullet ant nest density observed here (0.12 nests ha−1) is an order of magnitude lower than nest densities reported from Costa Rica (4–18 nests ha−1; Belk et al. Reference BELK, BLACK, JORGENSEN, HUBBELL and FOSTER1989, Bennett & Breed Reference BENNETT and BREED1985, Dyer Reference DYER2002) and Barro Colorado Island, Panama (6.5 ha−1; Perez et al. Reference PEREZ, CONDIT and LAO1999). The low density of bullet ant nests may be the result of intense and ongoing predation by cane toads on PLR. If we assume an average colony size of 400 adult ants on PLR that is consistent with previously reported colony sizes (Fewell et al. Reference FEWELL, HARRISON, STILLER and BREED1992) the theoretical amount of time that it would take an individual cane toad to consume all adults in a colony would be 404 min at the consumption rate of 1 adult ant min−1. This estimation of latency to consumption of all adults in a colony is consistent with observations of bullet ant consumption reported here.
It is important to recognize that bullet ants tend to be associated with late-successional and primary wet forests (Belk et al. Reference BELK, BLACK, JORGENSEN, HUBBELL and FOSTER1989, Bennett & Breed Reference BENNETT and BREED1985, Murphy & Breed Reference MURPHY and BREED2007) while cane toads are more abundant in open areas associated with human settlement (Heatwole Reference HEATWOLE1966, Isaacs & Hoyos Reference ISAACS and HOYOS2010). This pattern of habitat use suggests that the interaction that I observed may not be pervasive across these species’ ranges. Rather, intense predation pressure from cane toads may be illustrative of an ecotonal interaction which exemplifies forces that keep these top predators in their respective habitats. While this is an important alternative to consider, several experienced tropical researchers have observed cane toads preying upon bullet ant nests in the manner reported here within mature secondary and primary forest at La Selva Biological Station, Costa Rica (K. Kuhn, C. Robledo-Garcia, O. Vargas, pers. comm.). Either scenario is likely to result in incidence of significant and variable trophic cascades (Hunter & Price Reference HUNTER and PRICE1992) given the omnivorous diet of bullet ants (Tillberg & Breed Reference TILLBERG and BREED2004).
High rates of bullet ant predation could result in broader consequences for local communities. PLR is representative of many tropical forests that are bordered and intersected by marginal habitat that have been subject to human disturbance. It has recently been shown that Costa Rican sites with contemporary human disturbance correlate with diminished ability for bullet ant colonies to forage optimally compared to those inhabiting late-successional or primary forest (McGee & Eaton Reference MCGEE and EATON2014). Negative fitness effects associated with disturbed habitats could make bullet ant colonies more susceptible to predation (Kneitel & Chase Reference KNEITEL and CHASE2004). It follows that significant reductions in the number of healthy colonies sourcing foragers to the forest canopy could present herbivorous arthropods that normally suffer high predation from bullet ants with a greater degree of enemy-free space (Price et al. Reference PRICE, BOUTON, GROSS, MCPHERON, THOMPSON and WEIS1980). Censuses of cane toads and bullet ant nests of primary and secondary forest across these species’ ranges would reveal whether interactions between these species are truly common and if they alter normally occurring trophic cascades or are the result of an already modified trophic cascade (Dyer Reference DYER2002, Letourneau & Dyer Reference LETOURNEAU and DYER1998).
Perhaps the most outstanding question that this investigation compels is how are cane toads able to bypass bullet ant protections to consume copious numbers of ants? Bullet ants rapidly exit the nest to inspect disturbances to the earthworks, even minor perturbations (Figure 2a) and cane toads are heavy, lumbering animals that do not move around a habitat with great fluidity. Cane toad movements suggest that they are unlikely to mount the earthworks to take up an attack position in such a fluid manner as to go unnoticed by the ants. However, they clearly do. Apparently bullet ants do not possess the cognitive ability to perceive toads as a threat and modify their behaviours in response to it. Regardless, it is remarkable that bullet ants do not confront cane toads and attempt to drive them from the entrance of their nests given their typical vigilance in defending a nest entrance. Cane toads must possess an effective capacity to manoeuvre themselves into position to so effectively prey upon the nest. Are bullet ants unable to detect the cane toads positioned outside their nests? Are the toads producing a chemical or physical signal that bullet ants perceive as benign or familiar? What evolutionary innovations do the toads possess to thwart well-developed protections of bullet ants? Future investigation of these questions will expand our understanding of how predators leverage their behavioural capabilities to consume aggressive, well-defended prey, and how antagonistic interactions between top predator species affect community structure.
ACKNOWLEDGEMENTS
I would like to thank C. Aubert for assistance conducting bullet ant chemical palatability assay tests during the period in which these observations were made. Thoughtful reviews of this manuscript were provided to me by several colleagues and two peer reviewers. Support was provided to me via a Smithsonian Tropical Research Institute Short Term Fellowship and a Smithsonian Predoctoral Fellowship. The Panamanian environmental ministry, MiAmbiente, provided research permits: SE/AP-37-15 and SE/AP-23-15.
SUPPLEMENTARY MATERIALS
For supplementary material for this article, please visit https://doi.org/10.1017/S0266467418000342
Supplemental Video 1. Cane toad leaving bullet ant nest entrance vicinity at 08h36 after preying on bullet ants for 69 minutes on 3 November 2015.
Supplemental Video 2. Cane toad preying on bullet ants that were leaving and entering nest on 3 November 2015 at 07h45. This toad consumed 75 bullet ants in total.