INTRODUCTION
Habitat alteration (i.e. fragmentation, degradation and conversion) can have detrimental effects on avian populations as it may reduce the conditions appropriate for reproduction and survival of individuals, and result in altered population dynamics and life-history traits (Lens et al. Reference LENS, VAN DONGEN, NORRIS, GITHIRU and MATTHYSEN2002, Newton Reference NEWTON1998, Parker et al. Reference PARKER, BECKER, SANDERCOCK and AGREDA2006, Sutherland Reference SUTHERLAND1998). Habitat alteration in forested habitats can occur through changes in vegetation structure and composition (Brawn et al. Reference BRAWN, ROBINSON and Thompson2001, Martin & Martin Reference MARTIN and MARTIN2001, Sæther & Engen Reference SÆTHER, ENGEN, Sibbly, Hone and Clutton-Brock2003). If those changes are clumped within patches, variation in habitat quality can establish habitat-specific variation in demographic parameters (Kristan Reference KRISTAN2007).
The theoretical foundation for considering the relationship between habitat quality and demographic parameters (i.e. per capita birth, death, emigration and immigration) is well developed for source-sink population dynamics (Donovan & Thompson Reference DONOVAN and THOMPSON2001, Kawecki Reference KAWECKI, Hanski and Gaggiotti2004, Pulliam Reference PULLIAM1988). This approach has been applied to an increasing number of species living in fragmented landscapes with habitats differing in quality and connectivity by dispersal (Opdam & Wiens Reference OPDAM, WIENS, Norris and Pain2002, Smith & Hellman Reference SMITH and HELLMAN2002). The source-sink model distinguishes between habitat patches according to their capacity to sustain stable or growing populations in high-quality source patches (population growth rate, Lambda, λ ≥ 1), and decreasing populations in low-quality sink patches (λ < 1) that depend on immigrants from source patches to maintain patch occupancy (Pulliam Reference PULLIAM, Rhodes, Chesser and Smith1996, Thomas & Kunin Reference THOMAS and KUNIN1999). However, source-sink dynamics may be masked if patch quality varies temporally (Jonzén et al. Reference JONZÉN, RHODES and POSSINGHAM2005, Kawecki Reference KAWECKI, Hanski and Gaggiotti2004, Watkinson & Sutherland Reference WATKINSON and SUTHERLAND1995). Hence, estimating demographic parameters to identify whether habitat patches act as either source or sink is key to understanding the influence of human activities on the availability and quality of habitats (Johnson Reference JOHNSON2007, Smith & Hellman Reference SMITH and HELLMAN2002, With & King Reference WITH and KING2001).
Studying variation in population growth rate (λ) due to changes in demographic parameters will help us to better understand the consequences of altering habitat quality on population persistence (Holt et al. Reference HOLT, BARFIELD and GONZÁLEZ2003, Knutson et al. Reference KNUTSON, POWELL, HINES, FRIBERG and NIEMI2006, Nichols & Hines Reference NICHOLS and HINES2002). However, there are very few demographic parameter estimates for neotropical forest bird species, and some of these have shown large variation (Ricklefs Reference RICKLEFS1997, Sandercock et al. Reference SANDERCOCK, BEISSINGER, STOLSON, MELLAND and HUGHES2000). Nesting success as a potential surrogate of seasonal productivity, varied from 8% in tropical lowland forests (Robinson et al. Reference ROBINSON, ROBINSON, ROBINSON and BRAWN2000, Styrsky et al. Reference STYRSKY, BRAWN and ROBINSON2005) to more than 60% in montane cloud forests (Skutch Reference SKUTCH1985). Conversely, estimates of annual adult survival varied from less than 50% to over 92% in tropical primary forest, and 85% in montane cloud forests (Blake & Loiselle Reference BLAKE and LOISELLE2008, Karr et al. Reference KARR, NICHOLS, KLIMKIEWICZ and BRAWN1990, Johnston et al. Reference JOHNSTON, PEACH, GREGORY and WHITE1997, Parker et al. Reference PARKER, BECKER, SANDERCOCK and AGREDA2006, Ricklefs Reference RICKLEFS1997, Sandercock et al. Reference SANDERCOCK, BEISSINGER, STOLSON, MELLAND and HUGHES2000). Overall, demography and life-history traits for most tropical bird species remain poorly known (Martin Reference MARTIN2004, Sæther & Engen Reference SÆTHER, ENGEN, Sibbly, Hone and Clutton-Brock2003) and there have been few attempts to relate life-history estimates, such as productivity and annual survival, to population growth rates for tropical forest birds (Githiru & Lens Reference GITHIRU and LENS2006, Morton & Stutchbury Reference MORTON and STUCHBURY2000, Willson Reference WILLSON2004).
Here, we present productivity (the number of female offspring produced per female per breeding season) and apparent annual survival (φ) estimates for a population of Catharus frantzii Cabanis (ruddy-capped nightingale thrush; Aves: Turdidae) in the Central Highlands of Chiapas, southern Mexico. We evaluated habitat-specific variation in productivity and apparent annual survival in two contiguous habitat types (primary and secondary forests). Pair density and nesting success of C. frantzii were higher in the primary forest with undisturbed understorey vegetation than in secondary forest (Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008). Higher levels of nesting success and daily nest survival in primary forest represent higher habitat quality than in secondary forest. We assessed population persistence by determining the effects of population density, productivity and annual adult survival on the population growth rate (λ). Our objectives were to: (1) compare habitat-specific variation in productivity, (2) estimate apparent annual survival for adults and juveniles, and (3) model population growth rate (λ) and compare the estimated values of λ between habitats. We predicted that a reduction of 50% of understorey vegetation would negatively affect productivity, annual survival and population growth rate. Our hypothesis was that primary forest habitats would be more likely to support higher vital rates and realize a stable or increasing population growth rate (λ ≥ 1) than secondary forest.
METHODS
Study species
Catharus frantzii is a small (15–18 cm) highly sedentary forest dwelling passerine, monomorphic in size and colour. It is found in the understorey and dense edge undergrowth of humid montane forests, with a discontinuous distribution from Central Mexico to Panama (Clement et al. Reference CLEMENT, HATHWAY, BYERS and WILCZUR2000). Catharus frantzii is a cup nesting bird that lays a clutch of two eggs. A successful breeding attempt lasts 30–33 d. Parents feed fledglings up to 4 wk after they leave the nest and allow juveniles to remain on the natal territory up to 4 mo (Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008). Although there is little information on the population structure and dynamics of C. frantzii, the species is listed as endangered by the Mexican government due to the loss of tropical montane forests in Mexico. Variation in abundance of C. frantzii has been attributed to the availability of suitable forest habitat, and interactions with other Catharus species may further limit its distribution to primary forest habitats (Raitt & Hardy Reference RAITT and HARDY1970, Tejeda-Cruz & Sutherland Reference TEJEDA-CRUZ and SUTHERLAND2004, Young et al. Reference YOUNG, DEROSIER and POWELL1998). In the northern and central highlands of Chiapas, C. frantzii occurs in a wide range of forest habitats (Raitt & Hardy Reference RAITT and HARDY1970), and coexists with the migratory C. ustulatus (Swainson's thrush) during autumn and spring migration (Hiron et al. Reference HIRON, RANGEL-SALAZAR and CHRISTENSEN2006). The lack of year-round interactions with other Catharus thrushes may allow C. frantzii to exploit a variety of habitats in the Central Highlands of Chiapas.
Study site
The study site was the Cerro Huitepec Biological Reserve (hereafter Huitepec Reserve), a partially isolated reserve in the Central Highlands of Chiapas, 4.5 km north-west of San Cristóbal de Las Casas, Chiapas, Mexico (16°44′38″N; 92°40′15″W). The mean annual temperature was 14.5°C with a mean annual precipitation of 1300 mm. Rainfall was highly variable throughout the year, with a dry season (December–March), two transitional months (November and April) and a wet season (May–October). Firewood gathering was prevalent in the Huitepec Reserve, and the surrounding land was used for housing, agriculture and exotic pine plantations. Open and secondary habitats around the reserve have increased recently. We studied C. frantzii from 1995 to 2003 in five forest types in the Reserve varying in seral stage and aspect: montane cloud forest (∼35 ha), wet oak forest (∼32 ha), riparian forest (∼10 ha), dry-oak forest (∼40 ha) and second-growth forest (∼30 ha) (Ramírez-Marcial et al. Reference RAMÍREZ-MARCIAL, OCHOA-GAONA, GONZÁLEZ-ESPINOSA and QUINTANA-ASCENCIO1998). We combined montane cloud, wet oak and riparian forests as primary, mature forest habitat (≈77 ha, 2100–2700 m elevation), and dry oak forest and second-growth forest as secondary, young forest habitat (≈70 ha, 2100–2350 m) based on vegetation composition and structure, and variation in humidity, habitat productivity, and disturbance history (Rangel-Salazar Reference RANGEL-SALAZAR2006). For a more detailed description of the vegetation see Ramírez-Marcial et al. (Reference RAMÍREZ-MARCIAL, OCHOA-GAONA, GONZÁLEZ-ESPINOSA and QUINTANA-ASCENCIO1998).
Productivity
Reproductive data were collected from April to August, 2000 to 2003 (Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008). We identified territories by spot-mapping and nests were located by following territorial individuals showing breeding behaviour. Nests were monitored every 3–4 d, and 2–3 d near the predicted fledging date. Observation of fledglings or parents carrying food near the nest area within 5–8 d after the predicted fledging date was used as evidence for fledging success. A nest that fledged at least one young was considered successful.
Variation in reproductive rates across habitats, expressed as productivity (β), was estimated from nesting-success data and inferred from the number of female fledglings produced from successful nests. We assumed a 1:1 sex ratio in the brood. Fledglings from successful females were the number of chicks recorded during the last visit before fledging. In most cases, we were able to confirm this number by observing offspring after they fledged (Rangel-Salazar Reference RANGEL-SALAZAR2006). The number of nesting attempts also influences productivity (Anders & Marshall Reference ANDERS and MARSHALL2005). We calculated the annual productivity per habitat type as:
where βi was the number of female offspring produced per female per season in i habitat, n was the number of female offspring fledged from a successful nest, m was the Mayfield estimate of overall nesting success (Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008), and a was the number of nesting attempts per female (Anders & Marshall Reference ANDERS and MARSHALL2005). We estimated 1.5 nesting attempts per breeding season for females at Huitepec by considering the length of the breeding season (mid-April–mid-August, ≈110 d), the length of one nesting period (≈33 d), and the period between nest loss and re-nesting (≈29 d), which suggests a limited number of re-nesting attempts (i.e. 0.5) after nest failures. We considered C. frantzii to produce only a single brood per season as we recorded a fledgling parental care period of more than 3 mo (Rangel-Salazar Reference RANGEL-SALAZAR2006).
Adult and juvenile survival
We used the recapture and re-sight data collected over 9 y to estimate the overall return rate. This rate was separated into apparent or local annual survival (φ) and encounter (ρ) rates (Sandercock Reference SANDERCOCK2006, White & Burnham Reference WHITE and BURNHAM1999). Birds were caught over a 2-wk period during each spring, summer, fall and winter of 1995–1999, and spring and late summer of 2000–2003. In each plot, 10 to 12 mist-nets (2.5 × 12.5 m, 36-mm mesh) were placed in groups of one to three along trails and cut lanes. Mist-nets were opened just before sunrise (06h15–06h30) and checked every 30–40 min for a 5-h period. Three of five mist-netting plots were the same throughout the 9-y study and an additional plot was added for each habitat type in 2000. Mist-netting plots were 500–900 m apart. Some individuals were targeted with one to three mist-nets when nesting or in random sites combined with broadcasting of pre-recorded calls. Males were identified by the presence of cloacal protuberance and females by the presence of an incubation patch. In some individuals, gender was not identified because of the lack of breeding evidence. Juveniles were considered within their first year of life and identified by the presence of spots on the wing covers. All captured birds were given one metal leg ring, and from 2000 to 2003 three additional coloured plastic rings.
Apparent (local) survival probability (φ hereafter annual survival; the probability that a marked individual was still alive and present within the sampling area after a year as unit of time), and encounter probability (ρ, probability that an individual was encountered, conditional of being alive within the sampling area) were estimated using the Cormack–Jolly–Seber (CJS) mark–recapture re-sighting models (Hastings Reference HASTINGS1997, Lebreton et al. Reference LEBRETON, BURNHAM, CLOBERT and ANDERSON1992, Minta & Mengel Reference MINTA and MENGEL1989). By using the individual capture history, we considered three global models to estimate annual survival and hypothesized that annual survival would vary individually or with the independent or combined effects of habitat (h), year (time, t), and gender (g; Franklin et al. Reference FRANKLIN, ANDERSON, GUTIERREZ and BURNHAM2000). The first global model included 196 birds captured as adults (individuals ≥1 y old upon their first capture). Since we were unable to assign gender for 50 of these individuals, for this model we only considered constant (φ., ρ.) and time-dependent habitat-effects (φt×h, ρt×h). The second global model was limited to 146 individuals with gender identified. Thus, annual adult survival and encounter rate models included constant (φ., ρ.) and time-, habitat- and gender-effects, with habitat and gender interaction terms (φt×h×g, ρt×h×g; Sandercock et al. Reference SANDERCOCK, BEISSINGER, STOLSON, MELLAND and HUGHES2000, Willson Reference WILLSON2004). The third global model was restricted to estimate juvenile annual survival (n = 25 individuals), with constant survival and encounter rates (φ., ρ.).
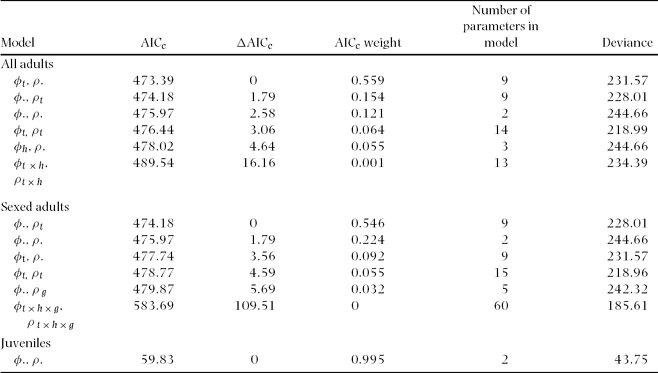
Models for annual survival and encounter rates were selected for parsimony on the basis of the number of parameters, and the maximum likelihood approach with the lowest value for Akaike's Information Criterion (Burnham & Anderson Reference BURNHAM and ANDERSON2002, Turchin Reference TURCHIN1998). Over-dispersion of data (i.e. lack of independence) was evaluated with goodness-of-fit tests (GOF) for the global completely parameterized, time-dependent models using the program RELEASE (Version 3.0; Burnham et al. Reference BURNHAM, ANDERSON, WHITE, BROWNIE and POLLOCK1987). Encounter histories of individuals were analysed using program MARK (White & Burnham Reference WHITE and BURNHAM1999), following the approach by Lebreton et al. (Reference LEBRETON, BURNHAM, CLOBERT and ANDERSON1992). Normalized Akaike weights (w i) were used to evaluate the relative support for different models in the candidate model set (Burnham & Anderson Reference BURNHAM and ANDERSON2002). We considered model averaging when estimating time variation in annual survival and encounter rates (1995–2003) from the different models listed in Table 2, and assumed that models differing by <2 AIC units provided equivalent fit of the data (Doncaster et al. Reference DONCASTER, CLOBERT, DOLIGEZ, GUSTAFSSON and DANCHIN1997).
Population modelling
Demographic parameters were calculated using Pulliam's model (Pulliam Reference PULLIAM1988, Reference PULLIAM, Rhodes, Chesser and Smith1996; Pulliam & Danielson Reference PULLIAM and DANIELSON1991) to estimate discrete population growth (λ):
where φadult represented the annual adult survival rate, φjuvenile the annual juvenile survival rate and β represented annual productivity.
Statistical analysis
We used general linear models (GLM) with Type III sum of squares to test the effects of habitat (primary vs. secondary), year (2000–2003), and their interactions on pair density (pairs ha−1), productivity and annual adult survival (Underwood Reference UNDERWOOD1997). We used habitat (n = 2) and year as units of replication for pair density and productivity (n = 4), and annual survival (n = 9). Thus, some individuals in the population may have been represented in the data set more than once. Regression analyses were performed to examine the explanatory variation of breeding pair density, productivity and annual adult survival on annual variation for λ. Breeding-pair densities were obtained from Rangel-Salazar et al. (Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008). Models containing two-way interaction terms among pairs of explanatory variables on λ were reduced by sequentially discarding non-significant interaction terms (P > 0.05). Prior to analyses, variables were checked for normality with Shapiro-Wilks W test and for equality of variance with Bartlett's test (Gotelli & Ellison Reference GOTELLI and ELLISON2004). When required, data were square root-transformed (distances) or arcsine-transformed (ratios) to meet assumptions of normality. Statistical analyses were performed with SAS-JMP IN 5.1. All means are presented ±1 SE and considered significant at P ≤ 0.05.
RESULTS
Productivity
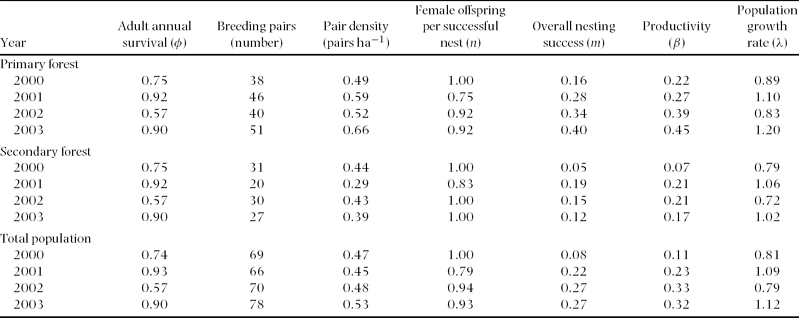
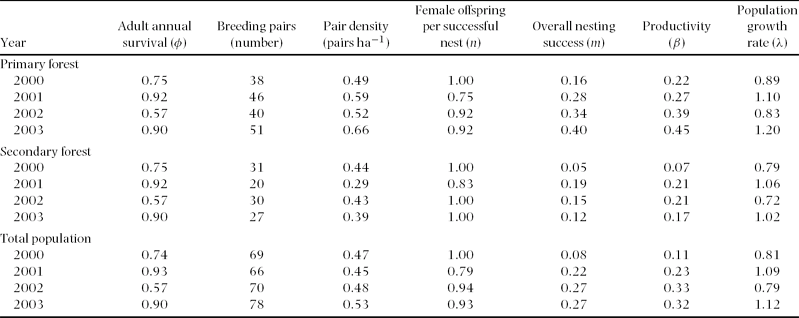
The breeding population at the Huitepec Reserve averaged 71 pairs per year over the 4-y period, with an annual range of 66 to 78 pairs (Table 1). The annual productivity estimates did not overlap between habitats, and varied from 0.22 to 0.45 (female offspring per female y−1) in primary forest, and from 0.07 to 0.21 in secondary forest (Table 1). Thus, overall productivity was significantly higher in primary than in secondary forest (mean ± SE; 0.33 ± 0.04 vs. 0.17 ± 0.04; F 1,6 = 7.2, P = 0.04).
Table 1. Annual demographic parameter estimates for Catharus frantzii (ruddy-capped nightingale thrush) in relation to habitat at the Huitepec Reserve, Chiapas, Mexico. m is the Mayfield estimate of overall nesting success. Productivity (β) represents the number of female offspring produced per female per year. The population growth rate (λ) > 1 indicates a source population, λ< 1 indicates a sink population, and λ ≈1 indicates a stable population. Data on breeding pairs and pair density were obtained from Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008.
Adult and juvenile survival
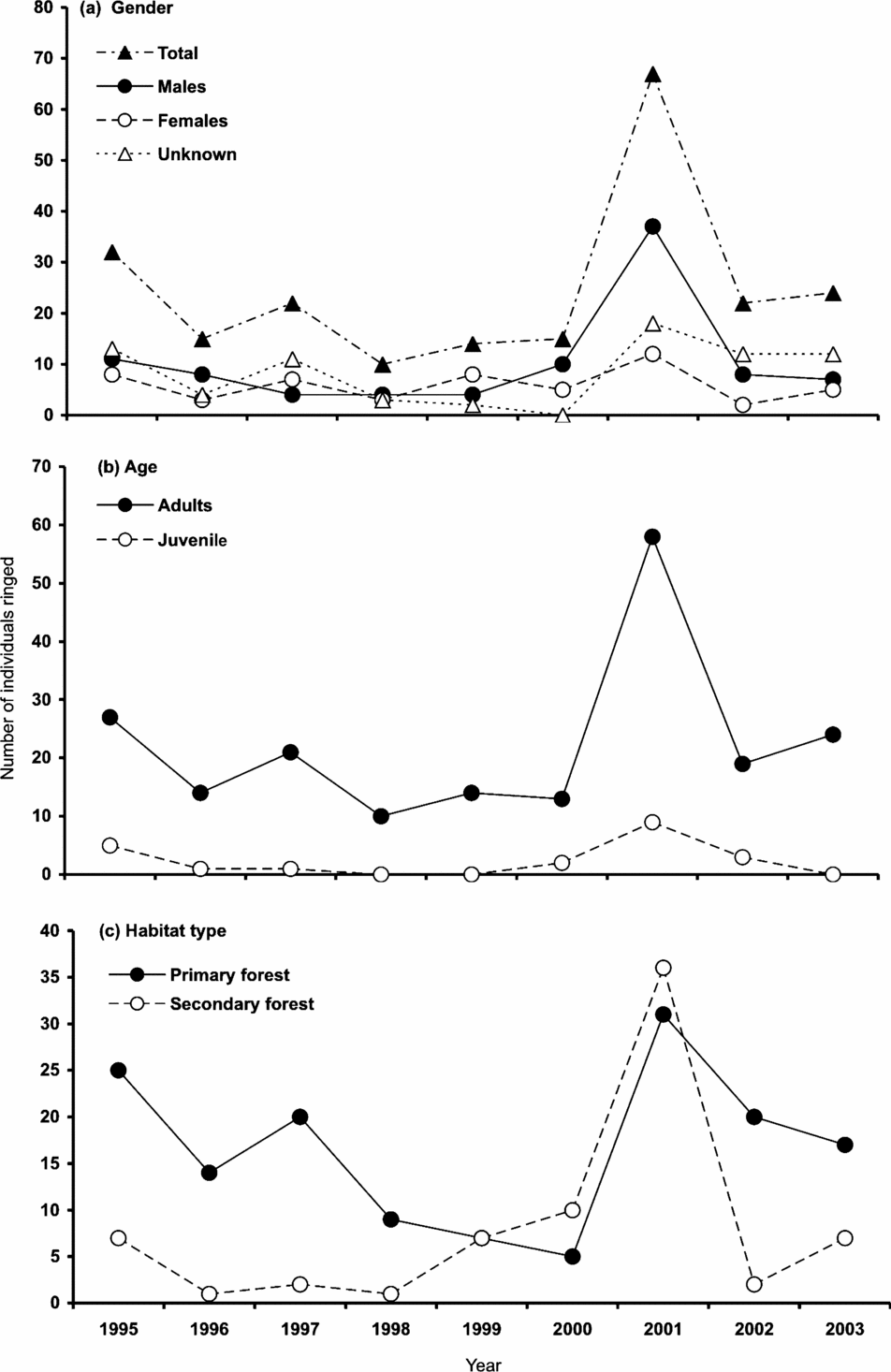
Between 1995 and 2003, 221 individuals were captured: 93 males, 53 females and 75 of unknown gender (50 adults, 25 juveniles; Figure 1a). In all years, adults comprised the majority of the population (Figure 1b). We captured more birds in the primary forest, particularly from 1995–1998 and 2002–2003 (Figure 1c).
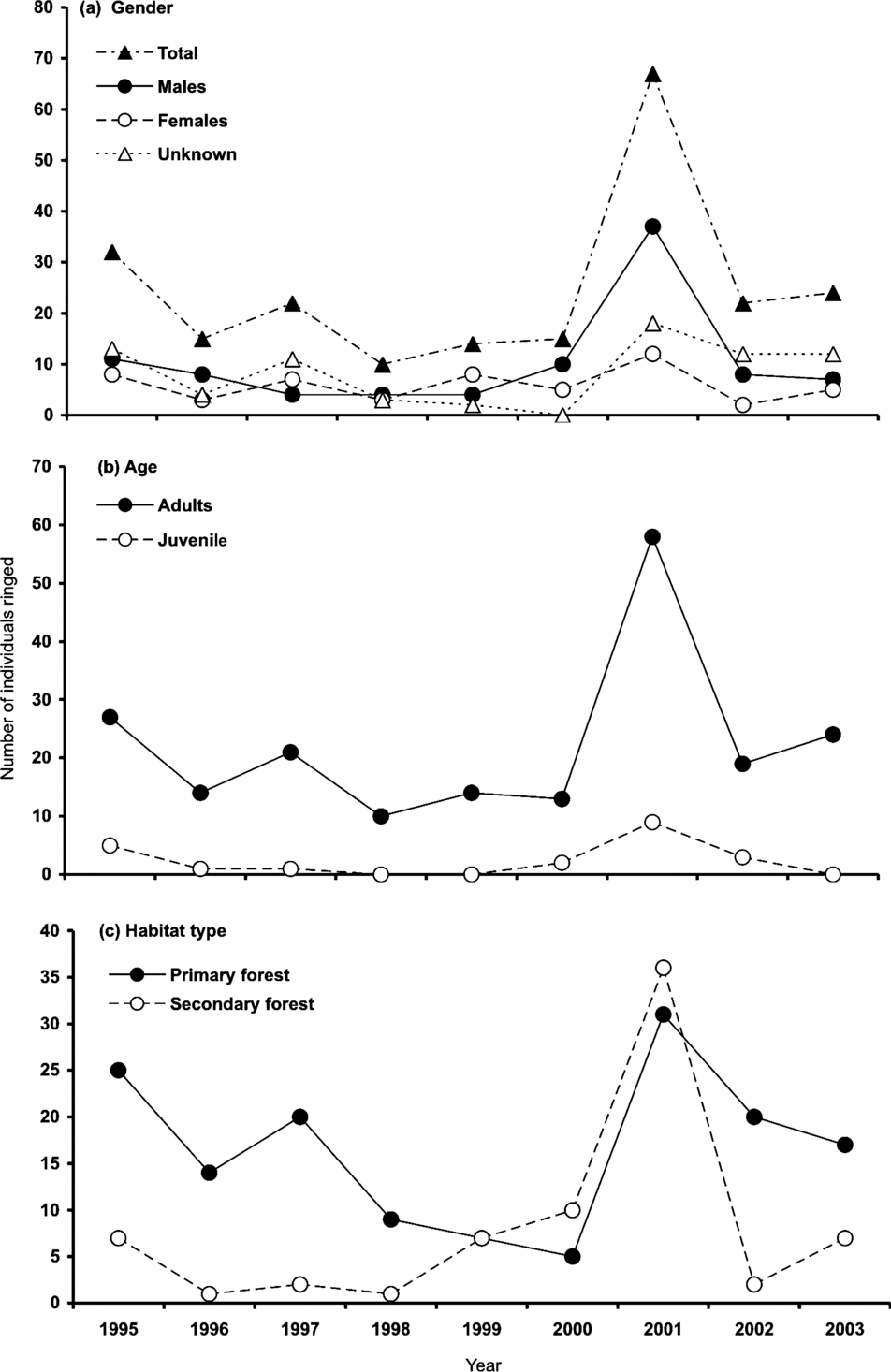
Figure 1. Annual number of individuals (total n = 221) of Catharus frantzii (ruddy-capped nightingale thrush) ringed by gender (a), age (b), and habitat (c) in the Huitepec Reserve, Chiapas, Mexico, 1995–2003.
Sixty-six per cent (n = 146) of the 221 individuals captured were never re-encountered. The mean proportion of once-captured individuals (potential floaters or transients) over total captures did not vary annually between primary and secondary forests (0.45 ± 0.06 vs. 0.33 ± 0.06; F 1,16 = 1.9, P = 0.18), or between survey intervals (1995–1999 vs. 2000–2003; 0.34 ± 0.06 vs. 0.45 ± 0.07; F 1,16 = 1.36, P = 0.27). No interaction between habitat and survey intervals was detected for the mean proportion of once-captured individuals (GLM, R 2 = 0.19, F 3,14 = 0.01, P = 0.90). Sixty-seven (89%) of the 75 individuals encountered more than once, showed high site tenacity (i.e. individuals re-captured in the same net line in plots), with just nine birds recaptured in a different plot from where they were originally banded. Four of these nine individuals moved within the same habitat, and five individuals changed habitat (one individual was originally captured in primary and recaptured in secondary forest, the remaining four individuals moved from secondary to primary forest).
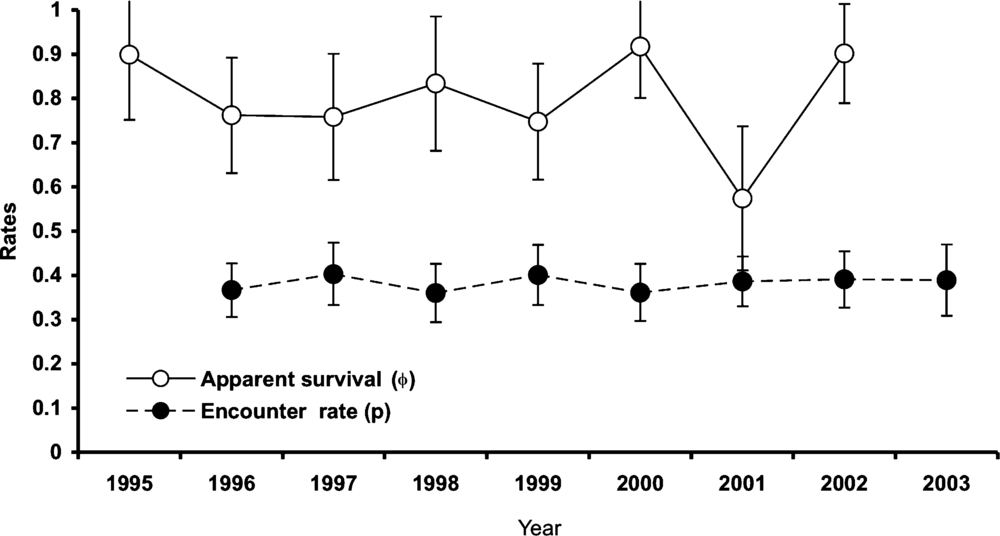
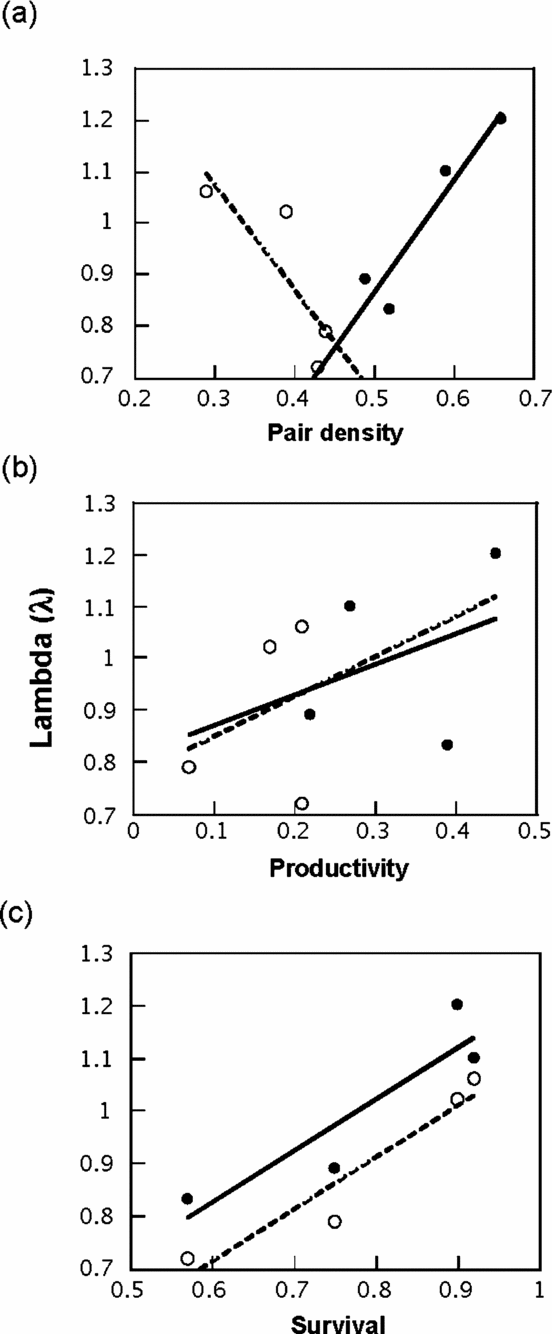
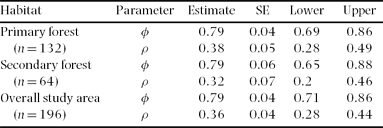
The completely parameterized models to estimate annual survival and encounter rates met the assumptions of mark-recapture methods based on goodness of fit tests (χ2 ≤ 1.64, P ≥ 0.19). The best model for annual adult survival had constant and time-dependent survival and encounter rates (Table 2). Annual adult survival and encounter rates appeared relatively stable across the study (Figure 2), and neither varied between primary and secondary forest (Table 3). Overall annual adult survival in the reserve was 0.79, and it did not vary across habitats (F 1,4 = 0.03, P = 0.87). Males and females exhibited similar annual survival in both primary forest (φMales = 0.80, φFemales = 0.77) and secondary forest (φMales = 0.83, φFemales = 0.79). However, annual survival for males (0.79 ± 0.05) and females (0.82 ± 0.05) were higher than for unknown sex individuals (0.32 ± 0.05)(F 2,3 = 27.0, P < 0.05). Encounter rates did not differ by gender between primary (ρMales = 0.42, ρFemales = 0.35) and secondary forest (ρMales = 0.31, ρFemales = 0.25). Annual survival over their first winter was 0.67 for juvenile birds (only 12% lower than for adults), with an encounter rate of 0.39 (model with constant survival and encounter rate; Table 2).
Table 2. Model selection results from program MARK for annual adult survival (φ) and encounter rates (ρ) for Catharus frantzii in the Huitepec Reserve, Chiapas, from 1995 to 2003. Only the five best models and the first, fully parameterized global models are presented (t = year, h = habitat, g = gender, AICc = Aikaike's Information Criterion, Deviance = model fit for all adults (n = 196), sexed adults (n = 146) and juveniles (n = 25)).
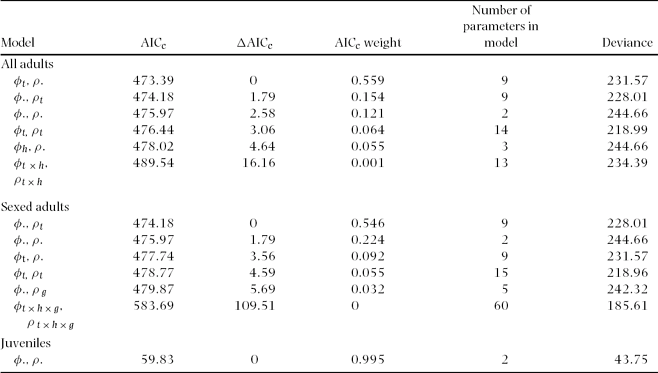
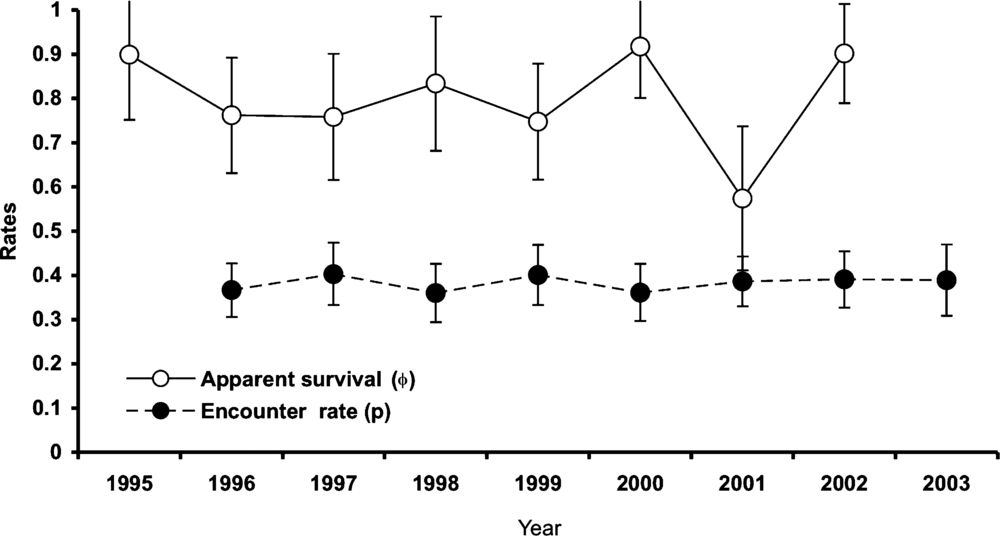
Figure 2. Estimates of annual adult survival (φ) and encounter rates (ρ) for Catharus frantzii at the Huitepec Reserve, Chiapas, Mexico (1995–2003). Point estimates were derived from the fully time-dependent Cormack-Jolly-Seber (CJS) Model (φhabitat × t, phabitat × t).
Table 3. Annual adult survival (φ) and encounter rates (p) for Catharus frantzii in the Huitepec Reserve, Chiapas, 1995 to 2003. Parameter estimates are presented with standard error (SE) and lower and upper confidence limits. Number of individuals (n) is given in parentheses.
Population modelling
Estimates of population growth (λ = 0.97 ± 0.09 (mean ± SE), 95% Confidence Intervals (CI) = 0.88–1.03) indicated a gradually declining population for C. frantzii at the Huitepec Reserve, when considering annual adult survival of φ = 0.79, annual juvenile survival of φ = 0.67 and an annual productivity of 0.27 female offspring per female. Lambda fluctuated annually within and across habitats, with a range of 0.83–1.20 in primary forest and 0.72–1.06 in secondary forest (Table 1). Overall, λ from the primary forest did not vary from secondary forest (1.01 ± 0.09 vs. 0.89 ± 0.09, F 1,6 = 0.79, P = 0.41). However, λ did vary across years when habitat was fixed (F 3,4 = 8.1, P < 0.05), being stable or increasing in 2001 and 2003, and declining in 2000 and 2002 (Table 1).
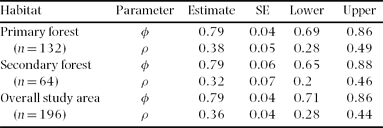
We performed general linear models (GLM) to evaluate if annual variation in λ was associated with pair density, productivity and annual survival for the Huitepec population. We did not find two-way interactions between density, productivity or annual survival with λ (F 1,4 ≤ 1.15, P ≥ 0.29). When habitat type was considered, only pair density showed interaction with habitat (F 1,4 = 15.27, P = 0.02; Figure 3a). Lambda showed a positive, but non-significant correlation with productivity (r = 0.68, F 1,4 = 0.45, P = 0.53; Figure 3b), and a strong, positive correlation with annual adult survival (r = 0.98, F 1,4 = 26.0, P < 0.01; Figure 3c).
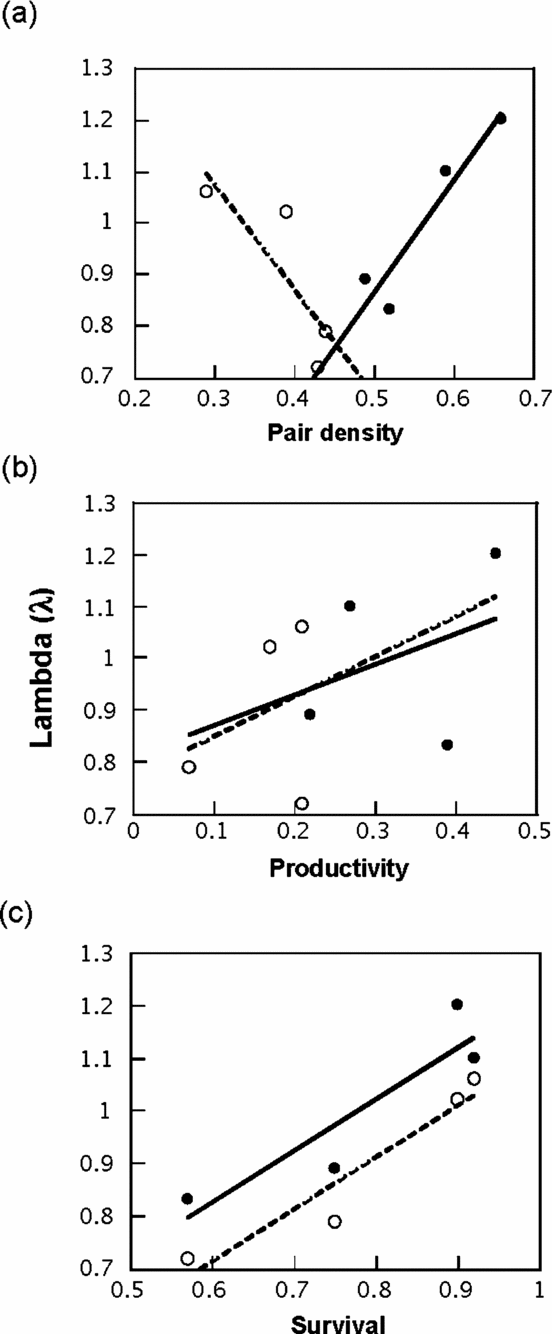
Figure 3. Finite population growth rate (λ) vs. pair density (pairs ha−1) (a), productivity (β, female offspring per female per season) (b) and annual adult survival (c) of Catharus frantzii at the Huitepec Reserve, Chiapas, Mexico, 2000–2003. λ is plotted for each habitat as a residual for the three independent variables. Interaction pair density × habitat: F 1,4 = 15.3, P = 0.02; productivity: r = 0.68, F 1,4 = 0.45, P = 0.53; adult survival: r = 0.98, F 1,4 = 26.0, P < 0.01. Lines represent fitting lines. Dark lines and black circles represent primary forest and dashed lines and white circles secondary forest.
DISCUSSION
We predicted that the primary forest habitat with more understorey vegetation and less habitat disturbance would support higher productivity, annual survival, and population growth rates for C. frantzii than the secondary forest habitat. However, we found that only productivity differed significantly between forest habitats. Comparable annual survival rates of juveniles and adults between habitats were sufficient to negate deficits in offspring production, and thus achieve relative parity in population growth rates between forest habitats.
Catharus frantzii in primary forest experienced higher pair density, daily nest survival and overall fledgling production than in secondary forest, and despite strong differences in availability of habitat attributes, birds appeared to be using similar general criteria for nest-site selection in both habitats (Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008). There appeared to be more suitable nesting sites in primary forests and stronger habitat selection in secondary forests. These components of reproductive success may reflect variation in habitat quality on productivity (Donovan & Thompson Reference DONOVAN and THOMPSON2001), since selection of primary forest habitats significantly increased productivity. In contrast, in a mixed deciduous upland forest in eastern North America, there was no significant association between nesting success and productivity in a wood thrush (Hylocychla mustelina) population (Underwood & Roth Reference UNDERWOOD and ROTH2002). Our results were in accord with other studies that found inconsistent effects of habitat variation on demographic rates of bird populations in temperate (Brown & Roth Reference BROWN and ROTH2002, Holmes et al. Reference HOLMES, MARRA and SHERRY1996, Murphy Reference MURPHY2001, Zanette Reference ZANETTE2000), and tropical habitats (Morton & Stutchbury Reference MORTON and STUCHBURY2000, Parker et al. Reference PARKER, BECKER, SANDERCOCK and AGREDA2006, Sandercock et al. Reference SANDERCOCK, BEISSINGER, STOLSON, MELLAND and HUGHES2000, Willson Reference WILLSON2004). Thus, if a single vital rate is not a reliable indicator of fitness, it would be a misleading indicator of habitat quality (Johnson Reference JOHNSON2007).
Alternative explanations of why productivity varied between habitats may involve resource availability, predation risk and parental behaviour (Ghalambor & Martin Reference GHALAMBOR and MARTIN2001). Catharus frantzii may avoid secondary forests because the lower vegetation density of secondary forest provides relatively poorer feeding and breeding opportunities (González-Espinosa et al. Reference GONZÁLEZ-ESPINOSA, OCHOA-GAONA, RAMÍREZ-MARCIAL, QUINTANA-ASCENCIO, Wilson and Sader1995, Morón-Ríos & Huerta-Lwanga Reference MORÓN-RÍOS and HUERTA-LWANGA2006), resulting in a lower carrying capacity. However, although habitat structure may provide a proximate measure of avian habitat quality (Maguire Reference MAGUIRE2006), there may be a threshold level of habitat degradation (e.g. ≥50% reduction of understorey vegetation) in secondary forests beyond which productivity becomes negatively impacted. For temperate forest birds, thresholds of reduction in vegetation cover detrimental to population persistence in fragmented landscapes can vary from 20% to 80% (Hannon & Schmiegelow Reference HANNON and SCHMIEGELOW2002, Zanette & Jenkins Reference ZANETTE and JENKINS2000). Despite the lower carrying capacity of the secondary forest compared with the primary forest in our study site, territories with suitable habitat were available, allowing C. frantzii pairs to successfully rear fledglings. Catharus franzii may have been able to compensate for the higher nest depredation levels in the secondary forest through re-nesting attempts (Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008). Re-nesting after nest failure could be an important life-history strategy for bird species from montane tropical forests, and the enhanced reproductive success achieved through re-nesting attempts after failures has been hypothesized as critical for maintaining bird populations in isolated ecosystems and elsewhere (Grzybowski & Pease Reference GRZYBOWSKI and PEASE2005, Martin et al. Reference MARTIN, HANNON and ROCKWELL1989, Sieving & Karr Reference SIEVING, KARR, Bierregaard and Laurance1997).
The overall annual adult survival for C. frantzii was high (φ = 0.79) with a moderate encounter rate (ρ = 0.36). Although high rates of annual survival have been reported for other tropical birds (Morton & Stutchbury Reference MORTON and STUCHBURY2000, Parker et al. Reference PARKER, BECKER, SANDERCOCK and AGREDA2006, Willson Reference WILLSON2004), most of these estimates were limited by small sample size and large confidence limits (Sandercock et al. Reference SANDERCOCK, BEISSINGER, STOLSON, MELLAND and HUGHES2000). We consider our estimates of annual survival and encounter rates to be robust, given our moderate sample size of 221 individuals, and relatively small confidence intervals. Annual adult survival and encounter rates did not vary across habitats or genders, and were stable through most of our study, except for adult survival in 2001 and for unsexed individuals. In 2001 we had an apparent unusual increase of captures that may negatively affect annual survival estimates. Unfavourable environmental conditions affecting foraging behaviour on American Robins (Turdus migratorius) have shown that individuals may congregate in areas to increase their foraging success (Vanderhoff & Eason Reference VANDERHOFF and EASON2008). Differences in apparent annual survival between sex-known and sex-unknown individuals may reflect important differences in true annual survival or site fidelity between them. However, these differences may have resulted from an artefact of the data or analytical methodology (Blake & Loiselle Reference BLAKE and LOISELLE2008).
We recorded high levels of site tenacity as most recaptured birds were found in the same sampling plot and habitat type. In contrast, birds captured only once complicated estimates of annual survival and encounter rates, and it is not clear why they comprised such a large proportion of the sample (Nichols & Hines Reference NICHOLS and HINES2002). These individuals may have been non-territorial individuals or floaters from the local population, or transients present briefly in the study area (Newton Reference NEWTON1998, Stutchbury & Morton Reference STUTCHBURY and MORTON2001). A proportion of the population (possibly the residents) had strong sedentary behaviour, while the larger proportion of individuals (visitors or vagrants) moved larger distances. We note that site tenacity did not vary with habitat type.
Our data were in accord with previous findings that most tropical forest birds have high adult annual survival and low reproductive rates (Morton & Stutchbury Reference MORTON and STUCHBURY2000, Stiles Reference STILES1992, Willson Reference WILLSON2004). Our estimates of productivity and annual survival suggested that both primary and secondary forests were able to support stable populations of C. frantzii in the Huitepec Reserve. However, in some years the reserve acted as a sink, since λ in 2002 declined by 21% (17% in primary and 28% in secondary forest). Habitat factors did not appear to affect population stability of C. frantzii in the Reserve (Opdam & Wiens Reference OPDAM, WIENS, Norris and Pain2002). Although both productivity and annual survival were demographic parameters that significantly influenced population growth (λ) of C. frantzii at the Huitepec Reserve, our results indicated that variation in λ was explained best by changes in annual adult survival. Thus, it is important to determine what types of habitat change might negatively affect or improve annual survival in C. frantzii.
Overall, we did not find that persistence of C. frantzii populations in a montane forest reserve of Chiapas were affected by habitat type or disturbance. Reduction of understorey vegetation in the secondary forest may not have been modified sufficiently to observe significant negative impacts on the critical vital rates. In an earlier study, we found that secondary forest had lower pair density, nesting success, and number of fledglings produced (Rangel-Salazar et al. Reference RANGEL-SALAZAR, MARTIN, MARSHALL and ELNER2008). However in this study, we found that annual survival did not vary between habitats, and annual survival was the primary demographic trait maintaining population growth in this relatively long-lived tropical passerine, although productivity was also important. Given the relatively high rate of transient birds, further studies to understand individual movements within the reserve and patterns of patch occupancy are warranted. Despite their apparent deteriorated condition, secondary forests in the Huitepec Reserve appeared able to support persistent avian populations in the understorey such as C. frantzii, in spite of loss and degradation of this montane forest habitat throughout the region (Cayuela et al. Reference CAYUELA, REY-BENAYAS and ECHEVERRÍA2006).
ACKNOWLEDGEMENTS
We would like to thank T. Will, P. Enríquez, F. Bolom, E. Pineda, L. Rubio, W. Amos, E. Sántiz, M. Hiron, C. Chavez-Zichinelli, K. Elliot, D. Bradley and J. Martínez for assistance in the field. B. Sandercock, M. Drever, S. Wilson, N. Mahony, L. Mahon, M. González and members of K. Martin's Lab provided helpful comments and suggestions to improve this study. We also thank M. Mossop for his editorial help. Our research was supported by El Colegio de la Frontera Sur, Consejo Nacional de Ciencia y Tecnología (Grant No. 84108 to JLRS), The Canadian Wildlife Service-Latin American Program, Environment Canada and The Centre for Applied Conservation Research from the University of British Columbia. Two anonymous reviewers provided insightful comments to improve an early version of this manuscript. We dedicate this paper to the memory of Jamie N.M. Smith.