INTRODUCTION
In tropical regions, termites play a major role in ecosystem functioning through litter and organic matter transformation and clay enrichment in natural ecosystems (Jouquet et al. Reference JOUQUET, LEPAGE and VELDE2002, Reference JOUQUET, BOTTINELLI, LATA, MORA and CAQUINEAU2007; Konaté et al. Reference KONATÉ, LE ROUX, TESSIER and LEPAGE1999, Mora et al. Reference MORA, MIAMBI, JIMÉNEZ, DECAËNS and ROULAND2005, Traoré et al. Reference TRAORÉ, TIGABU, OUEDRAOGO, BOUSSIM, GUINKO and LEPAGE2008) and in agro-ecosystems (Choosai et al. Reference CHOOSAI, MATHIEU, HANBOONSONG and JOUQUET2009). While termites are famous for their negative and sometimes spectacular impacts on crops and houses (Akpesse et al. Reference AKPESSE, KOUASSI, TANO and LEPAGE2008, Constantino Reference CONSTANTINO2002, Fowler & Forti Reference FOWLER and FORTI1990, Han Reference HAN2000), very little research has been carried out on their predatory role on trees in natural ecosystems. Studies in natural savannas of northern Australia pointed out the high incidence of termite predation on trees (Fox & Clark Reference FOX and CLARK1972, Werner & Prior Reference WERNER and PRIOR2007): up to 60% of adult trees are hollowed and host a termite nest (Werner et al. Reference WERNER, PRIOR and FORNER2008). These hollow trees are also susceptible to breakage following fires (Lonsdale & Braithwaite Reference LONSDALE and BRAITHWAITE1991, Williams et al. Reference WILLIAMS, COOK, GILL and MOORE1999), wind (Koizumi & Hirai Reference KOIZUMI and HIRAI2006) and animals (Holdo Reference HOLDO2003). It seems unlikely that such a high incidence of termites has no consequence for the demography of trees. If it was the case, termites would add this effect on tree demography to their already established role of soil ecosystem engineers (Jouquet et al. Reference JOUQUET, DAUBER, LAGERLÖF, LAVELLE and LEPAGE2006).
Most savannas are subject to recurrent, frequent, sometimes annual, fires. Fire is usually assumed to have little impact on adult trees in savannas (Bond & Midgley Reference BOND and MIDGLEY2001, Menaut & César Reference MENAUT and CÉSAR1979). But it may harm the tree by debarking the trunk and burning the small branches. The resulting exposed dead wood could constitute entry points into the heartwood for termites, which are usually supposed to avoid consuming living wood (Nogueira & Souza Reference NOGUEIRA and SOUZA1987). In fire-prone forests, fire alone is able to cause the appearance of hollows at the foot of tree trunks on the side opposite the fire's direction of spread (Gill Reference GILL1974). This has not been reported in savannas so far, where fire intensity is much lower than in forests, but the mechanism by which this occurs is purely physical and may occur in any fire (Gill Reference GILL1974, Tunstall et al. Reference TUNSTALL, WALKER and GILL1976). Contrary to the general pattern of the ‘fire trap’ (Bond & Midgley Reference BOND and MIDGLEY2001), where only young trees are vulnerable to fire, in some annually burnt savannas adults are as affected by fire as young individuals (Lonsdale & Braithwaite Reference LONSDALE and BRAITHWAITE1991).
In a typical, tropical humid savanna annually subject to fires and where termites are common, hollowing can have a high incidence and can be caused by either or both of these factors. Our aim in this article is to understand the mechanisms of hollow formation and the relative importance of the two different possible causing agents (fire and termites) in this process. Since the damage caused by fire (Gill Reference GILL1974) and by termites (Apolinario & Martius Reference APOLINARIO and MARTIUS2004, Fox & Clark Reference FOX and CLARK1972, Werner & Prior Reference WERNER and PRIOR2007) are not the same, we will: (1) measure the incidence of trunk hollowing in tree populations, (2) define a typology of hollows, (3) test whether different hollow types are due to termites or to fire, and (4) assess the effect of hollows on population structure. In particular, hypotheses to test in point (3) are: (1) fire tends to form visible basal hollows on the leeward side of tree trunks (Gill Reference GILL1974); (2) termites dig out trunks by creating internal cavities invisible from the outside (Werner & Prior Reference WERNER and PRIOR2007) because (3) they mainly enter trees from the roots and only occasionally from broken branches (Werner & Prior Reference WERNER and PRIOR2007).
METHODS
Study area
Field data were collected at the Lamto Research Station in Côte d'Ivoire (6°13′N, 5°02′W) in savannas growing at the margin of the rain forest (Menaut & César Reference MENAUT and CÉSAR1979). Four seasons are defined based on precipitation. Fire occurs each year during the long dry season (usually mid-January, when the northerly Harmattan dry wind blows from the Sahara). Fire has been proposed as the main explanation for the presence of savanna in this area, where rainfall (1200 mm y−1) is able to sustain rain forests (Lamotte & Tireford Reference LAMOTTE and TIREFORD1988, Monnier Reference MONNIER1968). Three main savanna types can be described based on tree cover (Gautier Reference GAUTIER1990) and main grass species: (1) grass savanna dominated by the perennial grass Loudetia simplex (Nees) C.E. Hubbard (tree cover < 7%); (2) shrubby savanna dominated by Andropogoneae grasses (7% < tree cover < 62%), and (3) savanna woodland (tree cover > 62%). Due to a reduced grass biomass under trees (Mordelet & Menaut Reference MORDELET and MENAUT1995), the savanna woodland is subject to lower-intensity fires than the other savanna types (Vuattoux, Gignoux, N'Dri, Konaté, pers. obs.). The dominant tree species are usually < 10 m tall; trees are considered as adults when they reach 2 m in height, corresponding to the average flame height (Menaut & César Reference MENAUT and CÉSAR1979). Termite biogenic structures are frequently encountered throughout all savanna types (Lepage et al. Reference LEPAGE, ABBADIE, JOSENS, KONATÉ, LAVELLE, Abbadie, Gignoux, Le Roux and Lepage2006): above-ground termitaria; below-ground nests; termite mounds (very large, complex biogenic structures grouping below-ground nests of different termite species: Konaté et al. Reference KONATÉ, LE ROUX, TESSIER and LEPAGE1999); comb chambers; sheetings and galleries.
Study plots
In a first field census (incidence plot, Table 1), we assessed the proportion of trees with cavities for all species. On 2.5 ha, all trees >0.5 m height were tagged and identified to species. Tree height was measured and each tree was checked for the presence of cavities. This experiment showed that the incidence of cavities on young trees was low and that one species, Crossopteryx febrifuga, had a very high incidence. We therefore restricted the next experiments to adult trees, and did more detailed observations on C. febrifuga.
Table 1. Details of the different study plots. SS: shrubby savanna; WS: woody savanna.

In a second census (transects, Table 1), we used different savanna types as a natural experiment on fire intensity: woody savanna (WS) as a mild-fire treatment, and shrubby savanna (SS) as an intense-fire treatment. We focused on the measurement of termite activities and on cavity incidence. Two sites were randomly chosen in each savanna type. On each site, two parallel 200 × 20-m transects, 50 m apart, were delimited. All adult trees were sampled, tagged and identified to species. Termite presence and activities both in trees and in their environment were recorded. For C. febrifuga, height and basal diameter were measured, and a detailed assessment of cavity type, shape and size was performed.
We conducted a third census (fire plots, Table 1) to examine the relationship between tree leaning and the formation of openings on the trunk. Eight 80 × 30-m plots were sampled for adult C. febrifuga only. Trees were considered leaning if more than 30° from the vertical. The orientation of their main openings relative to leaning (i.e. facing downward or upward) was recorded.
Typology of hollows
We defined three categories of tree: (1) undamaged trees, (2) trees with an internal cavity and only a few external openings due to missing branches (Figure 1a), hereafter referred to as piped trees, and (3) trees with an extended external opening, sometimes splitting the trunk open into two or more ‘stems’ (Figure 1b), hereafter referred to as externally damaged trees. Damage was assessed by (1) external observation for external openings and (2) ‘sound check’ (an apparently intact trunk was classified as piped when it made a hollow sound when hit) or presence of round holes due to missing branches for internal cavities. This classification was used on the transects. On the incidence plot, trees were only recorded as damaged or undamaged. On the fire plots, only external openings were recorded. In order to better document and understand internal cavity formation, four piped C. febrifuga (two in each savanna type) were felled using a chainsaw and cut into chunks. We took photographs and measured the dimensions of the internal cavity in every chunk (Figure 2).

Figure 1. Typical cavities found in Crossopteryx febrifuga trees in Lamto. Piped tree in the woody savanna: the internal cavity is visible through three broken branches (a). Externally damaged tree in the shrubby savanna: the external opening carries marks of termite (eroded galleries in the heartwood) and fire (charred exposed wood) (b).

Figure 2. The internal cavity found in one of the felled Crossopteryx febrifuga trees.
Termite sampling methods
To estimate the overall termite diversity in the environment of trees, we searched the whole surface of the transects (Table 1) for termites. We used a standardized method modified from a protocol for rapid assessment of termite diversity by Jones & Eggleton (Reference JONES and EGGLETON2000). Each transect was subdivided into contiguous quadrats of 500 m2 (25 × 20 m) each in order to standardize the sampling effort. In all quadrats, we hand-searched all microhabitats (logs, litter, stumps, twigs, nests, runways sheeting, fallen branches, etc.) up to a height of 2 m above ground level. Twelve samples of surface soil (each about 12 × 12 cm to 10 cm depth) were dug out in the quadrat at random locations. The soil was hand-sorted in situ and a representative sample of the termites was sorted.
To assess whether termites were responsible for tree hollowing, we searched all sampled trees for termites and their biogenic structures. The type of biogenic structure (nests, sheetings and galleries) was recorded.
When termites were found, individuals of the soldier caste were collected, preserved in 75% alcohol and later identified to genus using standard determination keys (Bouillon & Mathot Reference BOUILLON and MATHOT1965, Reference BOUILLON and MATHOT1966, Reference BOUILLON and MATHOT1971, Webb Reference WEBB1961) and species descriptions (Grassé Reference GRASSÉ1986). Following Deligne (Reference DELIGNE1966), Josens (Reference JOSENS1972) and Sands (Reference SANDS1998), termite genera were classified into one of four feeding groups: grass-feeders, soil-feeders, wood-feeders and fungus-growers. The two latter groups were expected to be actively feeding on trees.
In felled trees, we recorded termite species, activities and biogenic structures found inside the internal cavity. Trees were uprooted to check for hints of termite entry, because they are supposed to enter the heartwood through roots (Apolinario & Martius Reference APOLINARIO and MARTIUS2004, Werner & Prior Reference WERNER and PRIOR2007).
Fire impact on tree individuals and populations
Fire is expected to cause external openings at the base of trees on the side opposite the direction of fire propagation (Gill Reference GILL1974). Consequently, we should find a biased orientation of external openings. On the transects, we recorded whether external openings started at the base of the tree. On the incidence plot, we recorded the direction openings were facing. Fire could also harm leaning trees on their downward-facing side, exposed to a higher radiative heat. On the fire plots, we recorded whether trees were leaning and whether external openings were facing downward or upward.
At the population level (transects), we compared the size (height and basal diameter) distribution of trees and the proportion of piped trees and externally damaged trees of C. febrifuga individuals between WS and SS, to assess: (1) which type of damage was more harmful for trees in the long term, and (2) whether one of these two types of damage was more associated with average fire intensity.
Statistical analyses
All analyses were processed using the R software (http://www.r-project.org/). We used linear models (lm) for analysing height and diameter and generalized linear models (glm) for analysing the frequency of trees with termites, or with internal cavities and external openings. Main factors of interest were: savanna type; tree species and cavity type. Block factors were plot identities when working with the transect plots. Since block effects were not significant, they were considered as replicates for final analyses. For a few particular cases, we were able to use Fisher's exact test: (1) to compare the proportion of hollowed young and adult trees; (2) to compare the proportion of trees with a basal external opening to those which had an opening starting higher. χ2 tests were used to test the distribution of opening directions and to test its correlation with the direction of lean.
RESULTS
Incidence of hollows in the overall tree population
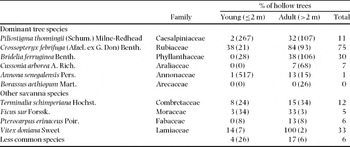
Of the total 1400 trees examined on the incidence plot, 14% were hollowed (Table 2). The proportion of young hollowed trees (3%) was significantly lower than the proportion of hollowed adult trees (36%) (Fisher's exact test, P < 0.001, odds ratio = 0.047). Three dominant species had a high (>30%) hollow incidence for adult trees (P. thonningii, C. febrifuga and B. ferruginea), while the other three had low or very low incidences (<15%). The most frequently affected species was C. febrifuga with 75% of all individuals (84% of adults). On the transects, the proportion of adult C. febrifuga with external openings or external cavities reached up to 97%. Piped trees were more frequent in WS than in SS (deviance = 14.4, df = 1, χ2 = 9.37, P < 0.001), respectively 37% vs 14%. In contrast, externally damaged trees were more frequent in SS than in WS (deviance = 11.0, df = 1, χ2 = 3.31, P < 0.001), respectively 86% vs 63%.
Table 2. Total number of trees >0.5 m of each species and incidence of cavities (%) by size class on the incidence plot. Number of individuals in parentheses.
Correlation between termites and damaged trees
In the transects, 12 termite genera including three wood-feeders were found in WS (Table 3). Most of them were also present in SS. Living termites were encountered on 11% of the 1439 adult trees sampled on the transects. There were slightly more trees with living termites in SS (18%) than in WS (7%). Sixty-four per cent of the biogenic structures in which termites were sampled on trees were galleries, 34% were sheetings and 2% were nests. Arboreal nests were only present in WS.
Table 3. List of termite genera in the woody and shrubby savanna according to their feeding group. 1: presence; 0: absence.
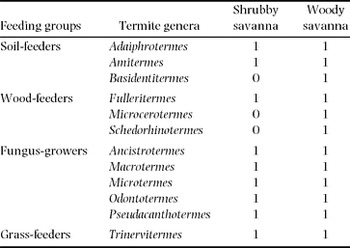
While eight termite genera were found on trees in WS, only two were found on trees in SS (Figure 3). In both savanna types, fungus-growers were dominant, followed by wood-feeders. The overall proportion of these functional groups did not significantly change with savanna type (71% (SS) vs 64% (WS) for fungus growers, 29% (SS) vs 34% (WS) for wood-feeders, Fisher's exact test, P = 0.599, odds ratio = 1.28).

Figure 3. Percentage of adult individual trees bearing termites (presence/absence) of different genera in the shrubby and woody savanna types on the transect plots. Am: Amitermes; Fu: Fulleritermes; Mc: Microcerotermes; Sc: Schedorhinotermes; Mt: Microtermes; An: Ancistrotermes; Ps: Pseudacanthotermes; Od: Odontotermes.
Among the different tree species on which termites were collected on transects, only C. febrifuga and B. ferruginea were in sufficient number for statistical tests to be carried out. The proportion of trees with termites was higher for C. febrifuga (33% of individuals) than for B. ferruginea (11%) (quasibinomial GLM model with logit link; deviance = 31.9, df = 1, χ2 = 21.1, P = 0.006). Of 241 C. febrifuga individuals on the transects, 93% had biogenic structures.
The four felled individuals of C. febrifuga showed intense termite activities inside the internal cavity (Figure 2). For all trees, the bottom end of the cavity was filled with termite soil (a mixture of clay, organic matter and wood debris). Higher up the internal cavity, internal galleries in the heartwood with living termites were observed. The trunk section consumed by termites represented around one third (37% ± 13% at 50 cm height, n = 4) of the trunk diameter (Figure 2). Roots close to the trunk did not show any sign of termites entering the tree from the ground: roots were intact, and the internal cavity did not cut through the bottom end of the trunk.
Indirect evidence of fire action
Openings started at the base of externally damaged trees more frequently in SS (89%) than in WS (77%), (Fisher's exact test, P = 0.023, odds ratio = 0.429). Of the 90 externally damaged C. febrifuga individuals found on the fire plots, most of them (75%) had their opening facing downward at the leaning side (χ2 = 46, df = 1, P < 0.001). The external openings of the 86 individuals of C. febrifuga on the incidence plot were not randomly oriented: most were facing west (χ2 = 40.4; df = 7; P < 0.001) (Figure 4).
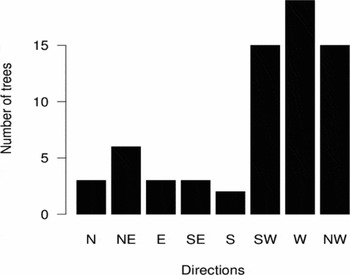
Figure 4. Distribution of the orientation of trunk external openings in Crossopteryx febrifuga trees of the incidence plot.
Population-level effect of external openings and internal cavities
Cavity and savanna types had a significant effect on C. febrifuga height (ANOVA, F1,8 = 16.7; P = 0.003 and F1,8 = 16.0; P = 0.004). Savanna type had a significant effect on basal diameter (F1,8 = 17.6; P = 0.003). Trees were on average taller in WS (height 7.89 ± 1.4 m, n = 83) than in SS (6.06 ± 1.18 m, n = 150, Figure 5), and also had a greater stem diameter (39.3 ± 3.05 cm in WS vs. 33.5 ± 3.81 cm in SS, Figure 5). In SS, piped trees were ~2 m taller than externally damaged trees (ANOVA, F1,4 = 21.5; P = 0.009; height = 7.03 ± 0.75 m vs. 5.09 ± 0.44 m respectively).

Figure 5. The size distribution of Crossopteryx febrifuga (percentage of adult individuals) according to savanna type on the transect plots. Histograms of height (1 m classes) (a) and basal diameter (10 cm classes) (b); x-axis values represent the midpoints of size classes except for the last class.
DISCUSSION
Overall incidence of tree trunk damage
The high incidence of cavities found in the main Lamto tree species (respectively 84%, 32% and 38% of adults of C. febrifuga, P. thonningii and B. ferruginea) are comparable to those of Werner & Prior (Reference WERNER and PRIOR2007) (66% of >1.4 m-height trees) and Fox & Clark (Reference FOX and CLARK1972) in Australian savannas (80% of the >15.24 cm dbh trees), and Apolinario & Martius (Reference APOLINARIO and MARTIUS2004) in an Amazonian rain forest (41% of >50 cm dbh trees). Older (larger) trees were more affected by hollowing in our case, suggesting either that the process takes a long time, or that termites, as main agents, are only attracted by trees with a significant proportion of heartwood. Cowie et al. (Reference COWIE, LOGAN and WOOD1989), Gonçalves et al. (Reference GONÇALVES, DESOUZA, REIS and RIBEIRO2005), Gould et al. (Reference GOULD, LOWE and CLARKE1993), Werner & Prior (Reference WERNER and PRIOR2007) and Whitford & Williams (Reference WHITFORD and WILLIAMS2002) all report a similar size effect, and the figures reported in other savannas increase with the minimal size of the trees they considered.
Contrary to these pioneering studies, we did a separate assessment of internal cavities and external openings, supposing these two different syndromes may derive from different mechanisms. Mechanics suggests that longitudinal external openings are much more likely to cause a breakage of the stem than piping (Mattheck & Kubler Reference MATTHECK and KUBLER1995, Mattheck et al. Reference MATTHECK, BETHGE and WEST1994, Whitford & Williams Reference WHITFORD and WILLIAMS2001). Piping is expected to decrease mechanical resistance only when more than two-thirds of the internal diameter of a trunk is removed (Mattheck & Kubler Reference MATTHECK and KUBLER1995).
Termites as the main piping agents
The internal cavities in C. febrifuga were very similar (extending longitudinally without or with little connection to the outside, sometimes unnoticeable except by sound check or core sampling) to those described in other parts of the world (Bandeira Reference BANDEIRA1993, Fox & Clark Reference FOX and CLARK1972, Gonçalves et al. Reference GONÇALVES, DESOUZA, REIS and RIBEIRO2005, Werner & Prior Reference WERNER and PRIOR2007), suggesting the same origin. It seemed (Figure 2) that only the heartwood was consumed and that termites were not able to attack fresh plant parts, as often reported (Nogueira & Souza Reference NOGUEIRA and SOUZA1987). Heartwood is a biologically dead tissue susceptible to attacks by xylophagous insects (Apolinario & Martius Reference APOLINARIO and MARTIUS2004). Since it is difficult to imagine how fire could hollow out a tree from the inside without a basal hole, wood-feeding termites are the first suspects in this process, but fungus-growers were more frequent in trees than wood-feeders. This group is able to process many types of plant material thanks to its exosymbiosis, and our data show that they can compete for wood with wood-feeders: for example, Ancistrotermes cavithorax is known for digging galleries into dead wood and living trees (Grassé Reference GRASSÉ1937, Josens Reference JOSENS1972, Konaté Reference KONATÉ1998). Another possible explanation is that wood-feeders enter the trees first, and are later replaced by fungus-growers.
Termites were more abundant in SS and more diverse in WS. The shrubby savanna, because of its more intense fire and harsher microclimate, might lead to a higher proportion and exposure of dead wood, favourable to the few species able to withstand this environment. For example, carton arboreal nests of Microcerotermes were observed on tree trunks only in WS – they might burn off too easily in SS. WS might also display a higher diversity because it hosts both savanna and forest species (Dosso et al. Reference DOSSO, KONATÉ, AIDARA and LINSENMAIR2010). Termites are particularly sensitive to moisture: for example, the feeding rate of Microcerotermes crassus is significantly influenced by moisture levels of food environment (Wong & Lee Reference WONG and LEE2010). As a result, we expect them to be more active in pipes than in open cavities where dead wood is exposed to sun and fire. Overall, we found a reverse trend, but this is probably a sampling bias, termites in pipes being more difficult to sample: in all felled piped trees, we found very active termites.
From the tree felling, there was no evidence that termites entered through the root system. As Whitford (Reference WHITFORD2002) and Whitford & Wiliams (2002), we often observed termite galleries built out of clay or stercoral carton climbing the trunk up to a broken dead branch or a very small hole (N'Dri, pers. obs.). According to Werner & Prior (Reference WERNER and PRIOR2007), broken branches are an occasional entry method for termites, but in Lamto they are probably the principal one: from our transect data, almost all piped C. febrifuga trees had a broken branch or hole, 97% of trees had a cavity (internal cavity or external opening), and 93% showed termite biogenic structures.
The low incidence of hollows in young trees may be due to the absence of entry points for termites in those individuals. In the case of Lamto, the majority of small trees are resprouts caught in the fire trap: they produce new shoots every year after the fire. These new shoots are unlikely to bear broken branches, notwithstanding their absence of dead heartwood. Such individuals are unlikely to be attacked by termites. Crossopteryx febrifuga behaves slightly differently: in contrast to other species, it stays in the fire trap only up to 60 cm in height, after which it starts to build up a perennial trunk (Gignoux et al. Reference GIGNOUX, CLOBERT and MENAUT1997). This small trunk is fully exposed to flames and accumulates heartwood much earlier than the other species, which may cause this species to be accessible and attractive to termites earlier in its development and explain its higher incidence of hollowing, including for small individuals (Table 2), in spite of a greater food preference of the dominant fungus-grower species (Ancistrotermes cavithorax and Odontotermes sp.) for other tree species (P. thoningii: Konaté Reference KONATÉ1998).
Fire as the main cavity-opening agent
Gill (Reference GILL1974) put forward the mechanism by which an intense fire is able to cause basal hollows on tree trunks. Fire spread causes a convection column on the leeward side of a bole, which results in a locally increased flame height and more stressful conditions for the tree on this side. This later causes the debarking of the leeward side of the trunk, the killing of the xylem and the exposure of the woody core. Subsequent fires increase the scar and lead to the hollow. There is no reason why this purely physical mechanism should be absent in savannas, although its intensity might be much reduced. In SS, we found more frequent external openings and more basal openings than in WS, and a preferential orientation of openings, all facts consistent with Gill's mechanism.
Another possible mechanism of fire-induced damage, not cited by Gill (Reference GILL1974), is when trunks lean above the flames: in this case, the downward-facing side endures more direct radiative heat than the opposite side. Consistent with this hypothesis, we observed more external openings on the downward-facing side of trunks and branches than on the opposite side. Whitford & Williams (Reference WHITFORD and WILLIAMS2002) similarly found that trees leaning by more than 4° from the vertical had more hollows than vertical trees.
Impact of termite–fire interaction on tree population structure
Our results strongly support a scenario where termites cause tree internal cavities, while fire causes external openings: these two processes are present in both savanna types, in proportions consistent with this scenario. Whereas internal cavities do not seem harmful to trees, the later exposure of these cavities by fire-induced external openings likely weakens the mechanical resistance of the trunk (Mattheck & Kubler Reference MATTHECK and KUBLER1995). The proposed scenario is as follows. (1) Termites may enter trees through naturally broken branches, but apparently not through the roots. On a healthy tree, fire may cause the appearance of broken branches or small areas of exposed dead wood (e.g. due to tree leaning), facilitating termite entry in the trees. Fire thus has a positive effect on later termite action on healthy trees. (2) Once termites have entered the trunk and started piping, they restrict their action to heartwood and do not cause trunk opening. They have no effect on later fire action. (3) Unless the internal cavity is very large, we do not expect a single fire to cause an external opening following Gill's mechanism. But successive fires may cause some areas of the trunk to die, thus becoming attractive to termites, which start to feed on this area (Figure 6). Although termites, as light-avoiding insects, would not expose the internal cavity to full light, a low-intensity fire might be enough to finish the job. Fire and termites would thus interact to open the cavity and form a deep external opening, possibly increasing tree mortality (Lonsdale & Braithwaite Reference LONSDALE and BRAITHWAITE1991, Prior et al. Reference PRIOR, MURPHY and RUSSELL-SMITH2009, Werner et al. Reference WERNER, PRIOR and FORNER2008, Whitford & Williams Reference WHITFORD and WILLIAMS2001, Williams et al. Reference WILLIAMS, COOK, GILL and MOORE1999). (4) Once the external opening is formed and internal dead wood is exposed to full light and hardened by successive fires, the tree becomes less attractive to termites. In contrast to our felled piped trees, externally damaged trees showed less-intense termite activity, possibly due to these reasons. Fire may thus ultimately reduce termite impact by creating a hostile environment for them once the trunk is open.

Figure 6. A felled tree showing a possible mechanism of interaction between fire and termites: the fire causes the death of a sector of the trunk, which then becomes attractive to termites which start excavating it, facilitating later burning and final opening of the internal cavity.
According to this scenario, we expect fire rather than termites to increase the mortality of adult trees, because the impact of fire on trees (external openings) is much more detrimental to trunk mechanical resistance than the impact of termites (internal cavities). We did not directly measure mortality, but the population structure observed in WS and SS strongly support this hypothesis: trees were significantly taller and bigger in WS, where internal cavities were more frequent, than in SS, where external openings were more frequent. Bond & Keeley (Reference BOND and KEELEY2005) also reported that frequent fires tended to reduce tree height. This suggests that external openings are responsible for an increase in tree mortality through mechanical breakage of the trunk, or possibly direct overheating of cambium and vascular tissues from the debarked side of the trunk.
The demographic effects of a piping limited to one-third of the trunk diameter section are probably negligible in comparison with the effects of external openings. From wood mechanics (Mattheck & Kubler Reference MATTHECK and KUBLER1995), a longitudinal crack in a bole is much more detrimental to its resistance than piping – tubes are commonly used in the building industry to produce mechanically resistant and light vertical structures. Consistently, piped trees were significantly taller than externally damaged trees, in both savanna types. Similarly, Werner et al. (Reference WERNER, PRIOR and FORNER2008) also reported that the growth of Eucalyptus miniata was not affected by the tree pipe ratio.
CONCLUSION
While termites primarily cause trunk piping, fire primarily causes lateral openings, which are much more detrimental to trunk mechanical resistance. When both are present, as in the Lamto savanna, their interaction inexorably leads with time to the opening of the tree trunks and their premature breakage. In the long term, this produces tree populations with a lower average individual size in more intensely burnt areas. To confirm this scenario, demographic data linked to the incidence of external openings and internal cavities are needed.
ACKNOWLEDGEMENTS
This work was conducted within the RIPIECSA-Côte d'Ivoire-project funded by the French Ministry of Foreign Affairs. The BDI fellowship program of the CNRS (Centre National de la Recherche Scientifique) funded A. B. N'Dri's scholarship. We thank N'Guessan François, Kounan Honoré, Kouakou Kouadio Hubert, N'Dri Alexis, Kouassi Apolinaire, Brou Bruno, Kouassi Kouassi JB, supporting and technical staff of Lamto, for their efficient assistance during field experiments.











