INTRODUCTION
New Caledonia (south-west Pacific) is a hotspot of plant diversity, with a unique and diverse flora across a range of vegetation types, including rain forest (Ibanez et al. Reference IBANEZ, MUNZINGER, DAGOSTINI, HEQUET, RIGAULT, JAFFRÉ and BIRNBAUM2014, Isnard et al. Reference ISNARD, L'HUILLIER, RIGAULT and JAFFRÉ2016, Jaffré et al. Reference JAFFRÉ, RIGAULT, MUNZINGER, Bonvallot, Gay and Habert2012, Morat et al. Reference MORAT, JAFFRÉ, TRONCHET, MUNZINGER, PILLON, VEILLON and CHALOPIN2012). Despite the floristic diversity of these rain forests, it is not unusual to find stands where a single species forms 50–100% of canopy trees (i.e. monodominant forests: Connell & Lowman Reference CONNELL and LOWMAN1989). Species that form monodominant forests include Nothofagus spp. (Nothofagaceae), Arillastrum gummiferum (Myrtaceae), Cerberiopsis candelabra (Apocynaceae) and Codia mackeeana (Cunoniaceae) (Demenois et al. Reference DEMENOIS, IBANEZ, READ and CARRICONDE2017, Ibanez & Birnbaum Reference IBANEZ and BIRNBAUM2014, Read et al. Reference READ, JAFFRÉ, GODRIE, HOPE and VEILLON2000, Reference READ, SANSON, BURD and JAFFRÉ2008). These dominants are long-lived, relatively shade-intolerant trees that establish after large disturbances, but appear unable to maintain dominance without further disturbance at low to mid-altitudes (Demenois et al. Reference DEMENOIS, IBANEZ, READ and CARRICONDE2017, Ibanez & Birnbaum Reference IBANEZ and BIRNBAUM2014, Read & Jaffré Reference READ and JAFFRÉ2013, Read et al. Reference READ, MCCOY and JAFFRÉ2015). Progression to mixed-canopy forest is predicted in the absence of disturbance (Demenois et al. Reference DEMENOIS, IBANEZ, READ and CARRICONDE2017, Read & Jaffré Reference READ and JAFFRÉ2013), for which there is some evidence in the Quaternary pollen record (Hope & Pask Reference HOPE and PASK1998).
Both persistent (Connell & Lowman Reference CONNELL and LOWMAN1989, Peh et al. Reference PEH, LEWIS and LLOYD2011a, Torti et al. Reference TORTI, COLEY and KURSAR2001) and non-persistent monodominance (Connell & Lowman Reference CONNELL and LOWMAN1989, Newbery et al. Reference NEWBERY, VAN DER BURGT, WORBES and CHUYONG2013) have been described in tropical forests elsewhere. In both cases, monodominant forests commonly occur on similar soil parent material to that of nearby mixed-canopy forests (McGuire et al. Reference MCGUIRE, ZAK, EDWARDS, BLACKWOOD and UPCHURCH2010, Peh et al. Reference PEH, SONKÉ, LLOYD, QUESADA and LEWIS2011b, Read et al. Reference READ, JAFFRÉ, FERRIS, MCCOY and HOPE2006). However, although soil characteristics do not necessarily explain local boundaries between these forests, nutrient relations may partly explain the capacity of some species to achieve dominance after disturbance, or to maintain dominance in the case of persistent monodominance. For example, decomposition/nutrient cycling is slow in some persistent (Brookshire & Thomas Reference BROOKSHIRE and THOMAS2013, Corrales et al. Reference CORRALES, MANGAN, TURNER and DALLING2016, McGuire et al. Reference MCGUIRE, ZAK, EDWARDS, BLACKWOOD and UPCHURCH2010, Peh et al. Reference PEH, SONKE, TAEDOUNG, SENE, LLOYD and LEWIS2012) and non-persistent (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009, McCoy Reference MCCOY1991) monodominant species and forests, potentially benefitting dominants by drawing down nutrients below critical levels for competitors. The ectomycorrhizal association (EM) common to many monodominants is likely to enhance nutrient-uptake efficiency (Connell & Lowman Reference CONNELL and LOWMAN1989, Newbery et al. Reference NEWBERY, ALEXANDER and ROTHER1997), but data for nutrient-uptake and -use efficiency are lacking for seedlings in these systems.
Rapid growth to pre-empt resources after disturbance is predicted to be a key trait of non-persistent monodominants (Connell & Lowman Reference CONNELL and LOWMAN1989, Hart Reference HART1990). In New Caledonia, monodominant forests commonly occur on ultramafic soils, which are very infertile, with low levels of P, Ca and K, low cation exchange capacity and high levels of phytotoxic metals (Isnard et al. Reference ISNARD, L'HUILLIER, RIGAULT and JAFFRÉ2016, McCoy et al. Reference MCCOY, JAFFRÉ, RIGAULT and ASH1999, Read et al. Reference READ, JAFFRÉ, FERRIS, MCCOY and HOPE2006). Hence, efficient nutrient uptake and use, and possibly metal tolerance, may contribute to superior growth rates and consequent dominance (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009, Read et al. Reference READ, FERRIS and JAFFRÉ2002). A pot trial showed that sun-grown seedlings of some New Caledonian monodominants had growth rates that were high, but nonetheless similar to those of some shade-intolerant subordinates that also regenerate episodically (Read et al. Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017). A unique allocation trait of seedlings of monodominants was a low root mass fraction (RMF, root dry mass per unit total plant dry mass) (Read et al. Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017); pairing of this trait with fast growth suggests high efficiency of nutrient uptake and/or use.
Hence, we investigate nutrient-uptake and -use efficiency, and Ni uptake, to understand determinants of potential growth advantage in these monodominants, focusing on seedlings since dominance is likely to establish early on disturbed sites. We test the following predictions: (1) Monodominants have higher nutrient-uptake efficiency, and/or photosynthetic and plant-level nutrient-use efficiency in sunny conditions than subordinates; (2) In shade, any efficiency advantage for monodominants declines (if nutrients become less limiting than light); (3) Monodominants have lower foliar Ni concentrations than subordinates; we focus on Ni since it is potentially phytotoxic (Krämer Reference KRÄMER2010, L'Huillier & Edighoffer Reference L'HUILLIER and EDIGHOFFER1996). Monodominants are not the only trees that regenerate episodically. Therefore, we test whether these predictions apply more broadly to shade-intolerant species that regenerate episodically (ER species) versus shade-tolerant species that regenerate continuously (CR species).
METHODS
Species selection and growth conditions
Nineteen species were selected from monodominant and adjacent mixed-canopy forests on ultramafic soils (predominantly ferritic ferrallitic soils: Read et al. Reference READ, JAFFRÉ, FERRIS, MCCOY and HOPE2006). One species was collected per family, except for three Nothofagus species, with species categorized as monodominant vs subordinate and ER vs CR (Table 1). Regeneration mode was categorized based on the shape parameter, c, from fitting population size structures to the Weibull probability density function (Read & Jaffré Reference READ and JAFFRÉ2013): c ≤1 indicates reverse-J and exponential curves, suggesting continuous regeneration; c >1 suggests increasingly synchronous establishment to c ≅ 3.6 where the curve is bell-shaped; higher values indicate negative skewing (Bailey & Dell Reference BAILEY and DELL1973). We used c ≤ 1.2 to indicate actual or potential continuous regeneration (Read et al. Reference READ, MCCOY and JAFFRÉ2015). The regeneration mode of Planchonella wakere could not be categorized from our data (Read & Jaffré Reference READ and JAFFRÉ2013), so was not included in analyses of trait differences between ER and CR species.
Table 1. Rain-forest canopy species from New Caledonia used in this study, with their dominance and regeneration categories (modified after Read et al. Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017). The codes used in figures are listed after species’ names. Species are categorized as typically monodominant (M) or subordinate (S), and episodic (ER) or continuous regenerators (CR) (Read et al. Reference READ, MCCOY and JAFFRÉ2015). Weibull c is averaged from values of 1–5 sites, excluding disjunct population size structures. We note, however, that these data were obtained from less than three sites for about half the species, and that sites vary in history and canopy cover (Read & Jaffré Reference READ and JAFFRÉ2013). Species nomenclature follows Florical vers. 22.IV.2016 (http://www.botanique.nc/herbier/florical), including retaining Nothofagus rather than Trisyngyne as reinstated by Heenan & Smissen (Reference HEENAN and SMISSEN2013).
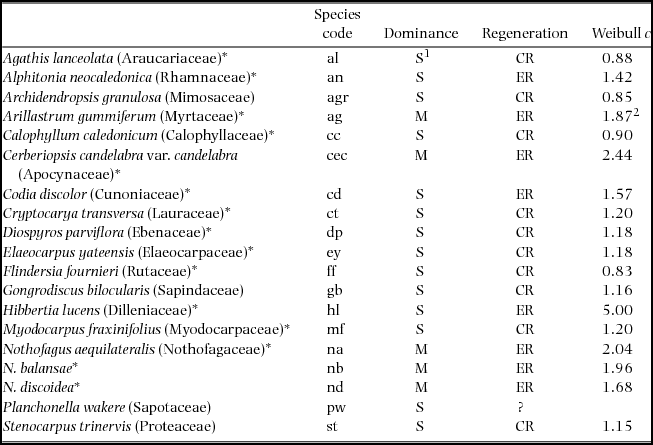
1 Agathis lanceolata can dominate small stands (Manauté et al. Reference MANAUTÉ, JAFFRÉ, VEILLON, KRANITZ, Bieleski and Wilcox2009); 2updated from Demenois et al. (Reference DEMENOIS, IBANEZ, READ and CARRICONDE2017). Species with asterisks were included in comparisons of Ni concentration of canopy foliage and seedling foliage.
The experiment was undertaken at the Vale-New Caledonia Environmental Conservation Service nursery facility in the Plaine des Lacs (22.27°S, 166.91°E, 260 m asl). Photosynthesis, growth rates and biomass allocation patterns have been reported from this experiment (Read et al. Reference READ, MCCOY and JAFFRÉ2015, Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017). Seedlings were collected from forests, when possible, to increase the likelihood of mycorrhizal inoculation, or grown from seed, and were estimated to be ~6–18 mo old (10–30 cm high) at the start of the experiment. They were transplanted into 3-L planter bags of soil from Nothofagus-dominated rainforest in the Plaine des Lacs (collected at ~20–25 cm depth across about eight locations) (Appendix 1). The soil was sieved, then mixed with perlite and coconut fibre in the ratio of 75:15:10 to assist drainage and reduce compaction. A small amount of mixed-forest litter and humus was added to pots to facilitate mycorrhizal inoculation. Seedlings were acclimated in a nursery house with shade-cloth walls (~70% transmission of photosynthetic photon flux density (PPFD)) and a clear plastic roof (~50% transmission of PPFD) for at least 10 wk prior to the experiment.
For the experiment, four replicate blocks of sun and shade (total of eight blocks) were established in a nursery house. Shade cloth formed the roof and sides of each block to 30 cm above mesh tables holding the pots. Transmitted PPFD was ~50% in the sun blocks and ~20% in the shade blocks, with further shade cloth added after 10 wk to reduce transmitted PPFD to ~30% and 10% respectively. PPFD was measured once per week at midday in the centre of each replicate block with a Li-Cor (Li-190R) quantum sensor. Midday PPFD averaged 473 (94–1361) µmol m−2 s−1 in the sun treatment and 175 (31–441) µmol m−2 s−1 in the shade treatment over the growth period. The replicates of each light treatment were positioned randomly (drawn blind) in a row. Up to four seedlings per species were positioned randomly (drawn blind) in a grid in each replicate block, i.e. up to 16 seedlings per species in each light treatment. Ongoing litterfall contributes significant nutrient input in rain-forest soils (Brearley et al. Reference BREARLEY, PRESS and SCHOLES2003). This may be particularly important for these infertile ultramafic soils and therefore a low dosage of slow-release fertilizer (2 g 270-day Nutricote® Hot Aussie Blend (Yates Australia): N, 18.1%; P, 2.6%; K, 6.7%) was added to pots at the start of the experiment to simulate ongoing nutrient input from leaf litter. This dosage was one-quarter strength of the maximum used in a comparable study of some Australian tropical rain-forest trees (Gleason et al. Reference GLEASON, READ and ARES2011). An additional treatment was included for four species: unfertilized Archidendropsis granulosa, Diospyros parviflora, N. aequilateralis and N. balansae seedlings were randomly positioned in each replicate block of each light treatment (four seedlings per block). The watering regime varied across seasons, most commonly light watering for 10 min three times daily, with 5 min of misting per hour between 0930 and 1530. Maximum daily temperature averaged 25.3°C (18.1–31.6°C) in the sun treatment and 23.9°C (18.1–31.1°C) in the shade treatment. Minimum daily relative humidity averaged 70% and 75% in sun and shade treatments respectively (Thermocron® iButtons: Maxim Integrated, San Jose, Ca, USA).
Seedling harvests
At the start of the experiment (July 2011), 7–15 seedlings per species were harvested to determine initial biomass and element concentration. Seedlings were washed, partitioned into leaves, stems and roots, then dried to constant mass at 60°C and weighed. Replicates were then combined to form a single sample per species for chemical assays. There were insufficient seedlings of N. discoidea for an initial harvest. The final harvest was undertaken after 21–23 wk (December 2011). Up to five seedlings per species per light treatment, used previously for measurements of photosynthesis (Read et al. Reference READ, MCCOY and JAFFRÉ2015) (at least one seedling per replicate block where possible), were washed and partitioned into roots, stems and leaves. They were dried at 60°C, weighed, then prepared for chemical analysis as either leaf tissue or stem-plus-root tissue. The exception was sun plants of Gongrodiscus bilocularis, for which there was only enough tissue mass for whole-plant analysis.
Nutrient and heavy metal assays
Element analysis was undertaken by the Environmental Analysis Laboratory, Southern Cross University, Lismore, Australia. Samples were dried at 70°C for 24 h prior to fine grinding. Tissue N concentration was measured by combustion using a LECO CNS2000 Analyser, and P, K and Ni concentrations were measured by ICP-MS following microwave digestion with nitric acid, with concentrations expressed on a dry mass basis.
Estimation of nutrient-uptake and -use efficiency
Although there is considerable evidence for P-limitation (Paoli & Curran Reference PAOLI and CURRAN2007, Vitousek et al. Reference VITOUSEK, PORDER, HOULTON and CHADWICK2010), some lowland tropical rain forests are not limited by P alone (Alvarez-Clare et al. Reference ALVAREZ-CLARE, MACK and BROOKS2013, Neba et al. Reference NEBA, NEWBERY and CHUYONG2016, Newbery et al. Reference NEWBERY, CHUYONG, GREEN, SONGWE, TCHUENTEU and ZIMMERMANN2002, Wright et al. Reference WRIGHT, YAVITT, WURZBURGER, TURNER, TANNER, SAYER, SANTIAGO, KASPARI, HEDIN, HARMS, GARCIA and CORRE2011). Foliar N:P is high in these New Caledonian rain-forest trees on ultramafic soils (18–49, across 28 species), and foliar P concentration is relatively low (0.18–0.60 mg g−1), suggesting P-limitation (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009). However, interpreting limiting nutrients can be complex, with variation according to growth conditions, species and plant size and age (Alvarez-Clare et al. Reference ALVAREZ-CLARE, MACK and BROOKS2013, Güsewell Reference GÜSEWELL2004, Vitousek et al. Reference VITOUSEK, PORDER, HOULTON and CHADWICK2010), as well as co-limitation (Canham et al. Reference CANHAM, BERKOWITZ, KELLY, LOVETT, OLLINGER and SCHNURR1996, Olde Venterink et al. Reference OLDE VENTERINK, WASSEN, VERKROOST and DE RUITER2003). Levels of K, Ca and Ca:Mg are also low in New Caledonian ultramafic soils (Becquer et al. Reference BECQUER, RIGAULT and JAFFRÉ2002, Read et al. Reference READ, JAFFRÉ, FERRIS, MCCOY and HOPE2006), and foliar levels of N, P, K and to a lesser extent Ca are at the low end of ranges reported in tropical rainforest trees (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009). Here we focus on N, P and K.
Nutrition and nutrient-uptake and -use efficiency were estimated by the following methods. (1) Foliar N, P and K concentrations were measured, and mass ratios of N:P, N:K and K:P (g g−1) were calculated. Critical values of N:P < 14 and N:P > 16 of above-ground biomass have been used to indicate N-limitation and P-limitation respectively (Aerts & Chapin Reference AERTS and CHAPIN2000) (modified to N:P < 10 and N:P > 20 respectively by Güsewell Reference GÜSEWELL2004). An additional condition of foliar P < 1.0 mg g−1 has been suggested for P-limitation alone (Güsewell Reference GÜSEWELL2004), with N:K < 2.1 and K:P > 3.4 to preclude K-limitation (Olde Venterink et al. Reference OLDE VENTERINK, WASSEN, VERKROOST and DE RUITER2003). Ni concentration was measured to assess possible toxicity: 10–50 µg g−1 may cause toxicity (Krämer Reference KRÄMER2010). (2) Instantaneous potential photosynthetic nutrient-use efficiency (PNUE) was calculated as Amax-mass/(leaf nutrient concentration per dry mass) (Reich et al. Reference REICH, UHL, WALTERS and ELLSWORTH1991), where Amax-mass is the maximum rate of net assimilation (light-saturated) per leaf dry mass (assimilation data from Read et al. Reference READ, MCCOY and JAFFRÉ2015). (3) Nutrient-uptake efficiency of roots (UpE) (specific absorption rate: Hunt Reference HUNT, Thomas, Murphy and Murray2003) was estimated as (nutrient uptake/time) × [(ln final root dry mass – ln initial root dry mass)/(final root dry mass – initial root dry mass)] (Gleason et al. Reference GLEASON, READ and ARES2011, Hunt Reference HUNT, Thomas, Murphy and Murray2003). (4) Plant-level nutrient-use efficiency was estimated as nutrient productivity (Prod), calculated as [(final total dry mass – initial total dry mass) / time] × [(ln final nutrient content – ln initial nutrient content)/(final nutrient content – initial nutrient content)], where nutrient content refers to mass in total plant dry mass (Gleason et al. Reference GLEASON, READ and ARES2011, Hunt Reference HUNT, Thomas, Murphy and Murray2003).
Data analysis
Data were analysed using the four block values as replicates for each species per light treatment. When more than one plant per species was sampled per block, the average value was used. Effects of light treatment and species on nutrient traits were examined by a linear mixed-effects model (LMM) (Pinheiro & Bates Reference PINHEIRO and BATES2000) that incorporated block as a random effect to account for the spatial dependency structure. Models included the additive effects of light treatment (sun and shade) and species (18–19 species per light treatment) after initial multiplicative models found no evidence of interactions (P > 0.25). Subsequent contrasts (with Holm P-value adjustments for multiple comparisons) were used to investigate specific comparisons (monodominants vs subordinates, and episodic vs continuous regenerators) within each light treatment. The same mixed-effects model was used to compare light and nutrient treatments within species. LMM and contrasts were fitted using the nlme (https://CRAN.R-project.org/package=nlme>) and multcomp (Hothorn et al. Reference HOTHORN, BRETZ and WESTFALL2008) packages respectively in R 3.2.2 (R CORE TEAM, https://www.R-project.org/). Data assumptions were checked and log-transformation was used for some traits. To better understand the trends in nutrient-uptake and -use efficiency between species groups, associations among traits were tested by Pearson correlation. Species regeneration responses to light occur along a continuum, so associations of Weibull c with efficiency traits were tested by Pearson correlation. In addition, Pearson correlation was used to test the potential contribution of specific root length (SRL, root length per root dry mass) (data from Read et al. Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017) to UpE, and the potential effects of nutrient-uptake and -use efficiency on relative growth rate (RGR) (data from Read et al. Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017). Correlation analysis was undertaken with SYSTAT v. 13.
RESULTS
Leaf and whole-plant nutrients and Ni
Significant differences in foliar concentration were recorded among species for all elements, with no effect of light treatment (Table 2). Nleaf, Pleaf and Kleaf varied 4–7-fold across species and light treatments, with high Nleaf in the N-fixing A. granulosa (Figure 1a). Nleaf, Pleaf and Kleaf were low in Nothofagus spp. compared with most species, but high in C. candelabra (Figure 1a–c). N:Pleaf varied 3-fold (Figure 1d), generally ≥ 20. N:Kleaf averaged 1.6 across species and light treatments (0.8–3.0), and K:Pleaf averaged 19 (5.9–38.6). Nileaf varied 14-fold, with high levels in C. cerberiopsis, D. parviflora and H. lucens (Figure 1e). Whole-plant Ni concentration (NiWP) varied 9-fold, from 0.13 mg g−1 to 1.1 mg g−1. Ratios of Ni concentration in leaf:stem + root tissue were commonly ~0.2 (11 species), ranging to 0.6 in C. candelabra.
Table 2. Tests of light treatment and species effects on foliar element concentrations in seedlings of 19 New Caledonian rain-forest tree species. Results of the linear mixed-effects model are given. Additive models, where appropriate (P > 0.25 for the interaction term), did not alter the conclusions of tests of main effects, so results of initial multiplicative models are shown. L, data log-transformed for analysis.
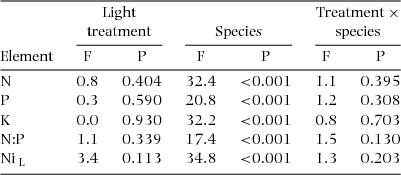
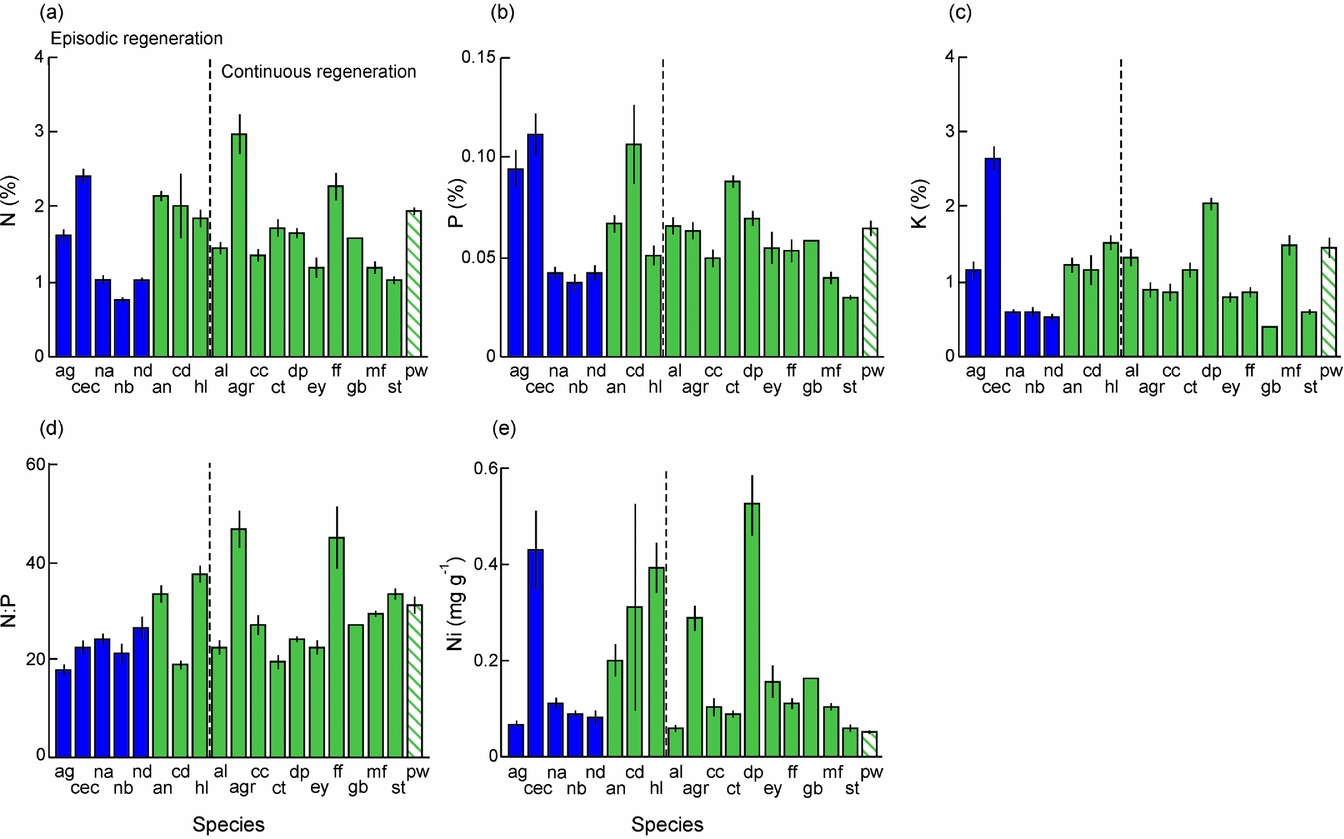
Figure 1. Foliar nutrient and Ni concentrations in seedlings of 19 rain-forest tree species from New Caledonia. The data presented are the means of block averages with SE, pooling seedlings from both light treatments. N (a), P (b), K (c), N:P (d) and Ni (e). Species codes are given in Table 1. Species status with respect to monodominant vs subordinate, and continuous vs episodic regeneration, is shown: monodominants, blue bars; subordinates, green bars; ER species to the left of the dashed line; CR species to the right of the dashed line. The regeneration pattern of Planchonella wakere, shown by hatched fill, is uncertain, but its traits seem to be aligned with those of continuously regenerating species (Read et al. Reference READ, MCCOY and JAFFRÉ2015, Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017).
In four species grown with and without fertilizer (Table 3), RGR was 1.5–4.5-fold higher in the fertilized treatment, with the greatest effect in the N-fixer A. granulosa (Table 3). PWP concentration did not differ between nutrient treatments, except for higher values in A. granulosa in unfertilized plants (Table 3). NWP was 1.3–2-fold higher in fertilized than unfertilized plants for each species, and KWP was 1.1–2.2-fold higher in fertilized seedlings of A. granulosa and D. parviflora. N:PWP was low in unfertilized plants, 7–17 (highest in A. granulosa), compared with 15–43 in fertilized plants.
Table 3. Whole-plant nutrient concentrations and relative growth rates (RGR) in seedlings of four New Caledonian rain-forest tree species grown with and without a low dosage of slow-release fertilizer. The data presented are the means of block averages with SE, with element concentrations given on a dry mass basis. RGR for the fertilized treatment is taken from Read et al. (Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017). Results of the linear mixed-effects model are given.
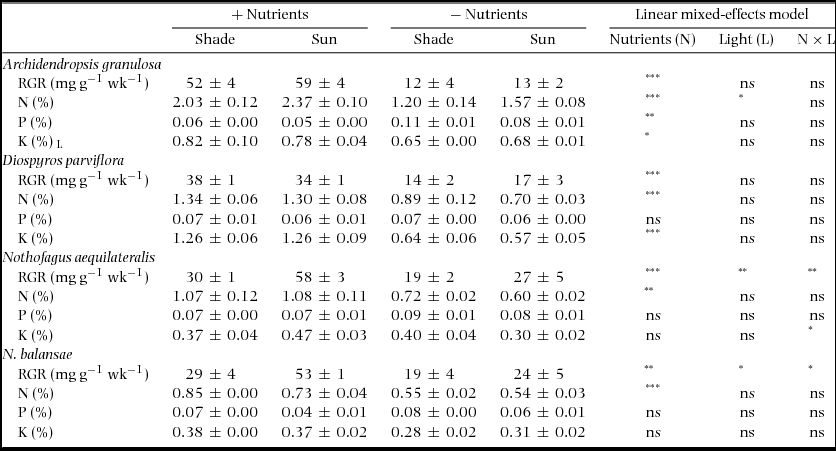
* P < 0.05; ** P < 0.01; *** P < 0.001; ns, not significant. L, data log-transformed for analysis.
Nutrient-uptake and -use efficiency
Amax-mass correlated positively with Nleaf in both sun (R = 0.63, P = 0.005, n = 18) and shade plants (R = 0.65, P = 0.003, n = 19) and with Pleaf in shade plants (R = 0.63, P = 0.004) (data log-transformed). PNUE differed among species and light treatments for all nutrients, but effects of light varied across species (Table 4). PNUE-N, PNUE-P and PNUE-K varied 4–6-fold across species and light treatments (Table 4, Figure 2a–c). PNUE for all nutrients was higher on average in sun plants (PNUE-N: 0.095 ± 0.007 mmol mol−1 s−1; PNUE-P: 5.86 ± 0.58 mmol mol−1 s−1; PNUE-K: 0.42 ± 0.04 mmol mol−1 s−1) than shade plants (PNUE-N: 0.082 ± 0.006 mmol mol−1 s−1; PNUE-P: 4.78 ± 0.41 mmol mol−1 s−1; PNUE-K: 0.35 ± 0.04 mmol mol−1 s−1)) (Figure 2a–c).
Table 4. Effects of light treatment and species on nutrient-uptake and -use efficiency in seedlings of 18–19 New Caledonian rain-forest tree species. Results of the linear mixed-effects model are presented. Additive models, where appropriate (P > 0.25 for the interaction term), generally did not alter the conclusions of tests of main effects, so results of initial multiplicative models are shown.
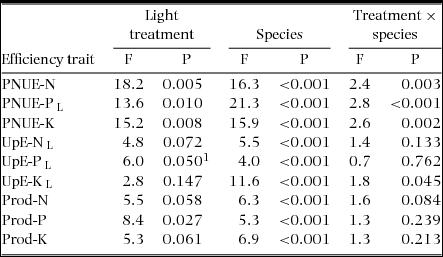
PNUE, photosynthetic nutrient-use efficiency; UpE, nutrient-uptake efficiency; Prod, nutrient productivity. 1Using the additive model: F = 6.2, P = 0.047. L, data log-transformed for analysis.
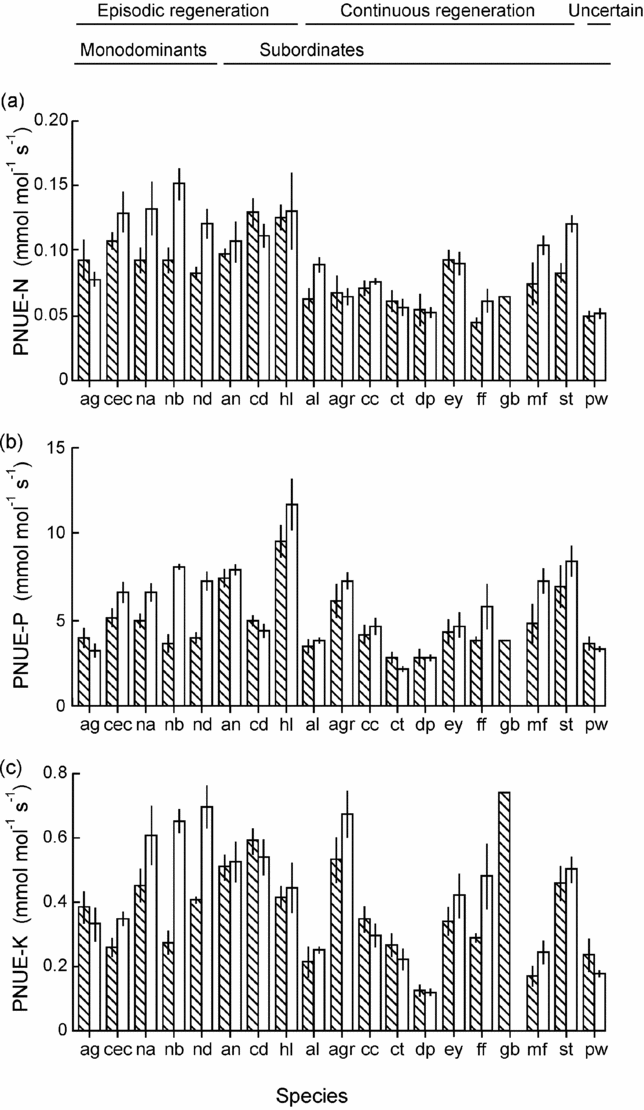
Figure 2. Photosynthetic nutrient-use efficiency (PNUE) in seedlings of 19 rain-forest tree species from New Caledonia. The data presented are the means of block averages with SE for PNUE-N (a), PNUE-P (b) and PNUE-K (c). The left hand hatched column is the shade treatment for each species, and the right hand open column is the sun treatment. Species codes are given in Table 1. Species status with respect to monodominant vs subordinate, and continuous vs episodic regeneration, is shown.
Uptake efficiency differed among species for all nutrients (Table 4), varying 9–17-fold for N and K respectively, but 32-fold for P (Figure 3a–c). UpE-N and UpE-P were particularly high in A. gummiferum in sun plants compared with other species. UpE-P was weakly higher in sun (0.107 ± 0.025 mg g−1 wk−1) than shade plants (0.072 ± 0.133 mg g−1 wk−1) (Table 4), and effects of light treatment on UpE-K differed across species (Table 4). No correlation was recorded between UpE for any nutrient and SRL in either sun (P = 0.39–0.90) or shade (P = 0.40–0.83) treatment (all data log-transformed). Nutrient productivity differed significantly across species, varying 5–10-fold across species and light treatments, higher in sun for Prod-P (65.7 ± 6.2 g g−1 wk−1) than shade plants (52.2 ± 5.1 g g−1 wk−1) (Table 4, Figure 4a–c).

Figure 3. Nutrient-uptake efficiency (UpE) in seedlings of 18 rain-forest tree species from New Caledonia. The data presented are the means of block averages with SE for UpE-N (a), UpE-P (b) and UpE-K (c). The left hand hatched column is the shade treatment for each species, and the right hand open column is the sun treatment. Species codes are given in Table 1. Species status with respect to monodominant vs subordinate, and continuous vs episodic regeneration, is shown.

Figure 4. Nutrient productivity (Prod) in seedlings of 18 rain-forest tree species from New Caledonia. The data presented are the means of block averages with SE for Prod-N (a), Prod-P (b) and Prod-K (c). The left hand hatched column is the shade treatment for each species, and the right hand open column is the sun treatment. Species codes are given in Table 1. The status of each species with respect to monodominant vs subordinate, and continuous vs episodic regeneration, is shown.
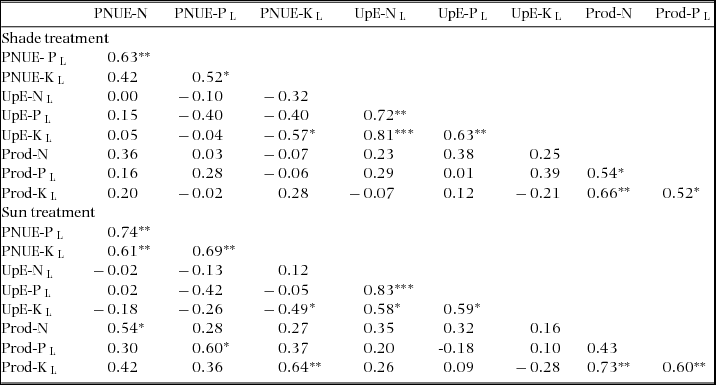
Trait values in sun plants were moderately to strongly positively correlated with values of the same trait in shade plants (R = 0.618–0.820; P = <0.001–0.006; n = 17–18). Correlations among efficiency traits across all species are given separately for sun and shade treatments in Appendix 2. Correlations were generally stronger in the sun treatment. The main patterns were (1) generally positive correlations among PNUE for all nutrients and among UpE for all nutrients; (2) PNUE was positively correlated with Prod for the same nutrient in sun plants; (3) UpE and Prod were not correlated for any nutrient, but Nothofagus spp., A. gummiferum and C. candelabra combined high levels of both traits for N, and Nothofagus spp. and A. gummiferum combined moderate to high levels for P in sun plants (Figure 5).
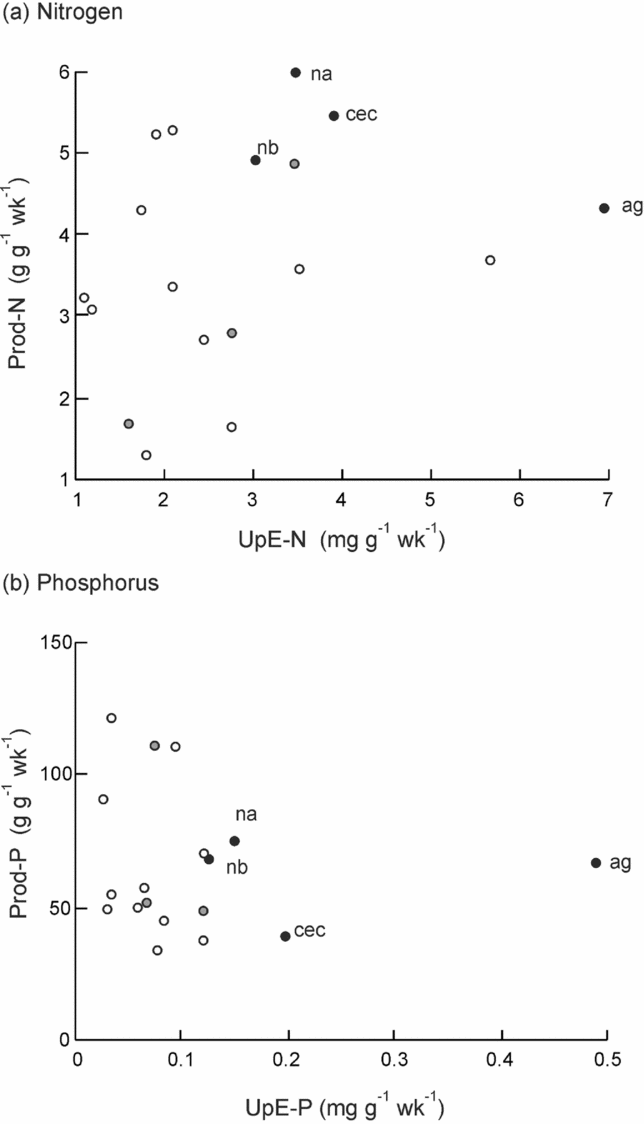
Figure 5. Association of nutrient productivity (Prod) with uptake efficiency (UpE) in seedlings of 18 rain-forest tree species from New Caledonia. The data presented are the means of block averages of species in the sun treatment for nitrogen (a) and phosphorus (b). Species codes are given in Table 1. Filled black symbols, monodominants; filled grey symbols, subordinate ER species; open symbols, subordinate CR species.
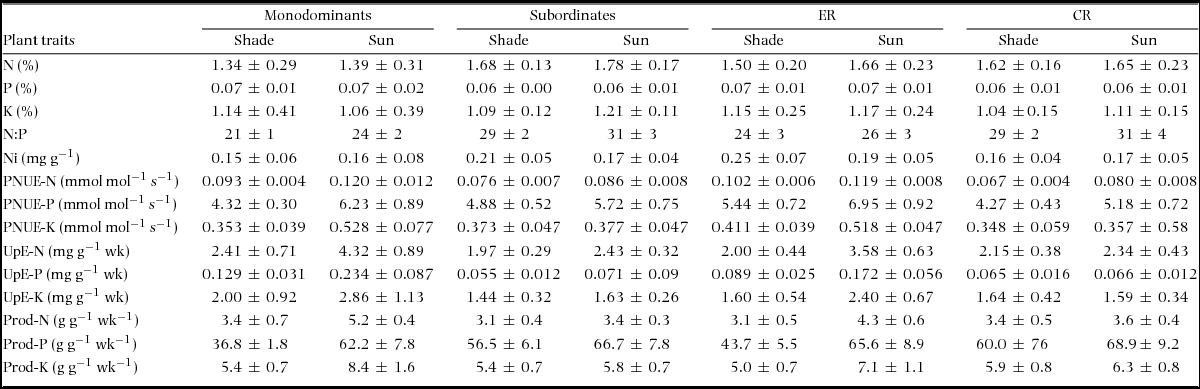
Trait differences between monodominants vs subordinates and CR vs ER species
Element concentrations were generally variable within each species group, but some trends emerged (mean ± SE values are given for all traits by group in Appendix 3). Nleaf was lower in monodominants than subordinates, despite high values in C. candelabra (Table 5, Figure 1a). There was no difference between ER and CR species. Pleaf did not differ between monodominants and subordinates, with high values in A. gummiferum and C. candelabra contrasting with low values in Nothofagus spp. (Table 5, Figure 1b), but was higher in ER than CR species (Table 5, Figure 1b). No difference in Kleaf was reported for either contrast (Table 5, Figure 1c). N:Pleaf was lower in monodominants than subordinates and in ER than CR species in both light treatments (Table 5, Figure 1d). In the shade treatment, Nileaf was lower in monodominants than subordinates, but higher in ER than CR species (Table 5, Figure 1e).
Table 5. Planned contrasts of foliar element concentrations and efficiency traits in seedlings of 17–19 New Caledonian rain-forest tree species: monodominants (M) vs subordinates (S) and episodically (ER) vs continuously regenerating (CR) species. Additive models of light treatment and species were used if initial multiplicative models found no evidence of interactions (P > 0.25), with Holm P-value adjustments. Acronyms are explained in Table 4. Mean ± SE are given in Appendix 3.
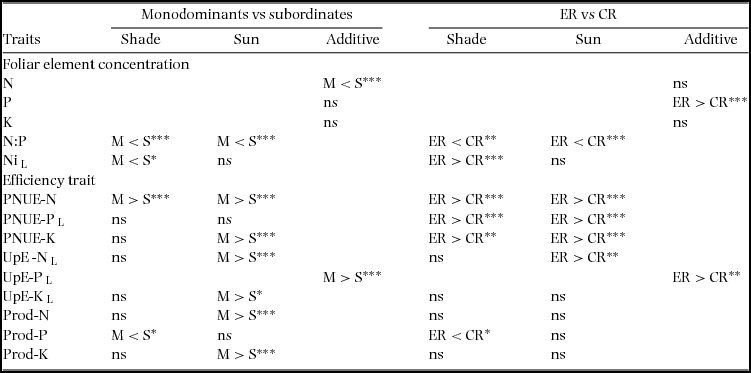
L, data log-transformed for analysis. * P < 0.05; ** P < 0.01; *** P < 0.001; ns, not significant.
PNUE-N was higher in monodominants than subordinates and PNUE-K was higher in monodominants in sun plants; PNUE was higher in ER than CR species for all nutrients in both sun and shade plants (Table 5, Figure 2a–c). Of the monodominants, PNUE was consistently high for all nutrients in sun plants of Nothofagus spp., but lower in A. gummiferum, and also low in C. candelabra for K (Figure 2a–c). UpE was higher in monodominants than subordinates for all nutrients, in sun or pooled across light treatments, and higher in ER than CR species for N and P (Table 5, Figure 3a–c). Plant-level nutrient-use efficiency was more variable across nutrients: Prod-N and Prod-K were higher in monodominants than subordinates in sun plants, with no difference in Prod-P (Table 5, Figure 4a, c); for shade plants, Prod-P was lower in monodominants than subordinates and in ER than CR species (Table 5, Figure 4b). No differences in productivity were recorded in sun plants of ER vs CR species for any nutrients (Table 5, Figure 4a–c).
Weibull c correlated strongly and positively across species with PNUE-N in both sun and shade plants, and weakly with Prod-N in sun plants and PNUE-P in shade plants (Table 6). RGR correlated positively with PNUE for all nutrients and UpE-N and UpE-P in sun plants, and with UpE-N and Prod-P in shade plants (Table 6).
Table 6. Correlations of nutrient-uptake and -use efficiency traits with Weibull c and relative growth rate (RGR) in seedlings of 17–18 New Caledonian rain-forest tree species. The data presented are Pearson correlation coefficients (R) using species’ means (means of block averages). Acronyms are explained in Table 4.
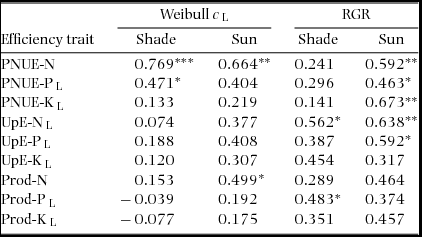
* P <0.05; ** P <0.01; *** P <0.001. L, data log-transformed for analysis.
DISCUSSION
In general, predicted differences in efficiency between monodominants and subordinates, and between ER and CR species, were supported (detailed below). In addition, any differences between groups were usually stronger in the sun than the shade treatment, as predicted. However, there was often considerable within-group variation, and high efficiency did not occur in monodominants and ER species for all traits in the sun treatment.
Foliar nutrients and Ni in monodominants vs subordinates and ER vs CR species
There was no consistent pattern in foliar nutrient concentrations between groups. Monodominants had lower mean Nleaf than subordinates, largely due to lower levels in Nothofagus spp. than most other species. N:Pleaf was lower in monodominants than subordinates, and in ER species more generally, largely due to high values in a small number of subordinates. Of the species with high N:P, suggesting strong P-limitation, A. granulosa is a nodulated legume, and A. neocaledonica is non-nodulated but attracts and supports high levels of Frankia in its rhizosphere (Gauthier et al. Reference GAUTHIER, JAFFRÉ and PRIN2000). The higher NWP (and N:PWP) in fertilized than unfertilized plants for the four species tested, with no increase in PWP, suggests luxury uptake of N (and K in two species) in the main experiment (fertilized plants). The combination of N:Pleaf > 20, low Pleaf, low N:K and high K:P, together with similar PWP in the two nutrient treatments, is consistent with P limiting growth in the main experiment. Kleaf did not differ between fertilizer treatments in Nothofagus species, suggesting possible P-K co-limitation. Burslem et al. (Reference BURSLEM, TURNER and GRUBB1994, Reference BURSLEM, GRUBB and TURNER1995) found that seedlings of rain-forest species missing their mycorrhizal partner, or which are non-mycorrhizal, responded to P-addition, but inoculated seedlings appeared to be limited by cations. We cannot be sure that all seedlings in our experiment were optimally inoculated. Furthermore, although rain-forest soils were used in this study, they have been altered by pot conditions. In particular, the degree to which the added fertilizer matched the ongoing nutrient input through litterfall under natural conditions is uncertain. Therefore, these results do not indicate that P is necessarily limiting in the rain-forest understorey, although N:P ratios of tree foliage suggests P-limitation in the canopy (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009).
There was no evidence of hyperaccumulation of Ni (>1 mg g−1 foliar dry mass) or of Mn (details not presented here), as recorded in some New Caledonian species (Jaffré Reference JAFFRÉ1977, Jaffré et al. Reference JAFFRÉ, PILLON, THOMINE and MERLOT2013). Nevertheless, Nileaf was commonly in the range considered toxic to non-adapted plants, indicating likely adaptive detoxification and sequestration mechanisms (Baker Reference BAKER1987, Krämer Reference KRÄMER2010). Notably, Ni levels were 2–20 times higher in leaves of sun-grown seedlings than sun leaves of mature trees (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009) of the same species collected across four forests (averaging 8 times higher, but strongly correlated: RP = 073, P = 0.003; n = 15 species, shown in Table 1). This difference between seedlings and adults may relate to possible higher Ni availability in the potting soils due to a high content of fine organic material (Becquer et al. Reference BECQUER, BOURDON and PETARD1995, Perrier et al. Reference PERRIER, COLIN, JAFFRÉ, AMBROSI, ROSE and BOTTERO2004), reducing conditions (Becquer et al. Reference BECQUER, BOURDON and PETARD1995, Reference BECQUER, RIGAULT and JAFFRÉ2002), P-fertilization (as found for Cr by Becquer et al. Reference BECQUER, RIGAULT and JAFFRÉ2002), or to less intense mycorrhizal inoculation in these seedlings compared with trees (Amir et al. Reference AMIR, LAGRANGE, HASSAÏNE and CAVALOC2013).
Nevertheless, NiWP and partitioning varied considerably among species. Variation among species in metal uptake may arise in part from differential effects of mycorrhizas (Amir et al. Reference AMIR, LAGRANGE, HASSAÏNE and CAVALOC2013, Baker Reference BAKER1987). Of the ER species, Nileaf was particularly low in A. gummiferum and Nothofagus spp. Both species have EM associations (Carriconde et al. unpubl. data, Gourmelon et al. Reference GOURMELON, MAGGIA, POWELL, GIGANTE, HORTAL, GUEUNIER, LETELLIER and CARRICONDE2016, Perrier et al. Reference PERRIER, AMIR and COLIN2006), which are often associated with reduced metal uptake (Baker Reference BAKER1987, Jourand et al. Reference JOURAND, HANNIBAL, MAJOREL, MENGANT, DUCOUSSO and LEBRUND2014). Most associated species belong to families that typically only have AM relationships (Read & Hope 1996). Low Nileaf has also been recorded in Nothofagus trees compared with co-occurring species (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009), similar to levels in related species growing on non-ultramafic soil (Read et al. Reference READ, FERRIS and JAFFRÉ2002). Although there is no clear evidence of metal toxicity in naturally occurring species on these ultramafic soils, tolerance may incur a cost in productivity, depending on the mechanisms involved. If low Nileaf is achieved efficiently, e.g. less resources devoted to sequestration due to efficient reduction in metal uptake by mycorrhizas, productivity may be enhanced in taxa such as Nothofagus relative to co-occurring species, particularly if supplying carbon to an EM fungus does not incur a carbon cost to the plant host, except in the heaviest shade (Corrêa et al. Reference CORRÊA, GUREVITCH, MARTINS-LOUÇÃO and CRUZ2012).
Nutrient-uptake and -use efficiency in monodominants vs subordinates and ER vs CR species
The range of values of uptake efficiency of roots and nutrient productivity reported in this study is similar to that from comparable studies (Gleason et al. Reference GLEASON, READ and ARES2011). Despite variation among species in the various efficiency traits, there were some clear trends between species groups, particularly in the sun treatment. In particular, UpE for all nutrients was higher in monodominants than subordinates, and also in ER than CR species for N and P. High UpE was achieved in part by high SRL in some monodominants (Read et al. Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017). However, UpE was not correlated with SRL for any nutrient across all species, so additional factors must be contributing to variation in UpE across species, e.g. mycorrhizal uptake (especially for immobile nutrients such as P) and uptake kinetics. In some forests, the trend for higher uptake efficiency on low-nutrient soils might be more pronounced than that for use efficiency, due in part to EM relationships of canopy dominants (Paoli et al. Reference PAOLI, CURRAN and ZAK2005). There is evidence of higher efficiency of P uptake in EM than AM fungi (Jones et al. Reference JONES, DURALL and TINKER1998), with utilization of insoluble mineral P and organic forms, and rapid exploration and nutrient acquisition of resource-rich patches before nutrients become depleted or inaccessible (Chuyong et al. Reference CHUYONG, NEWBERY and SONGWE2000, Plassard & Dell Reference PLASSARD and DELL2010, Tibbett & Sanders Reference TIBBETT and SANDERS2002).
Notably, litter decomposition and nutrient cycling are often slow in monodominant species and forests (Brookshire & Thomas Reference BROOKSHIRE and THOMAS2013, Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009, Corrales et al. Reference CORRALES, MANGAN, TURNER and DALLING2016, McGuire et al. Reference MCGUIRE, ZAK, EDWARDS, BLACKWOOD and UPCHURCH2010). This may lower nutrient availability and pH, potentially leading to positive feedback, with suggested draw-down of resources below levels that are critical for competitors (Brookshire & Thomas Reference BROOKSHIRE and THOMAS2013, Torti et al. Reference TORTI, COLEY and KURSAR2001), including at the seedling stage. Slow decomposition may also create a more physically difficult and drought-prone establishment environment, and a higher fuel load for fires, which may affect seedling establishment and population dynamics. If reduced nutrient availability is to benefit seedlings of monodominants, they must have strategies of nutrient-uptake or -use efficiency that enhance their performance under these conditions relative to competitors. Notably, EM associations occur in many tropical monodominants, despite their general infrequency in the tropics compared with AM associations (Connell & Lowman Reference CONNELL and LOWMAN1989), and differences in microbial communities (including those of EM fungi) have been recorded between monodominant and mixed-canopy rain forests (Carriconde et al. unpubl. data, Gourmelon et al. Reference GOURMELON, MAGGIA, POWELL, GIGANTE, HORTAL, GUEUNIER, LETELLIER and CARRICONDE2016, McGuire et al. Reference MCGUIRE, ZAK, EDWARDS, BLACKWOOD and UPCHURCH2010). Non-persistent monodominance is common in New Caledonia, and furthermore, occurs in several taxa across multiple families. This is probably partly due to the historical frequency of large-scale disturbances, particularly cyclones and wildfires, together with plant adaptations that enhance post-disturbance regeneration (Demenois et al. Reference DEMENOIS, IBANEZ, READ and CARRICONDE2017, Hope & Pask Reference HOPE and PASK1998, McCoy et al. Reference MCCOY, JAFFRÉ, RIGAULT and ASH1999, Read et al. Reference READ, EVANS, SANSON, KERR and JAFFRÉ2011). However, the apparently higher frequency of monodominance in forests on ultramafic soils than those on volcano-sedimentary soils in this region is not explained. Notably, monodominance is also prominent in open secondary formations on ultramafic soils in New Caledonia, typically dominated by species with EM and/or root nodule associations (McCoy et al. Reference MCCOY, JAFFRÉ, RIGAULT and ASH1999), providing further evidence for the potentially prominent role of microbial symbioses in determining dominance on these soils.
Our study suggests that efficiency in sun-grown seedlings of these New Caledonian non-persistent monodominants extends beyond nutrient uptake, to include photosynthetic and, significantly, plant-level nutrient-use efficiency. The relatively high PNUE recorded in monodominants must contribute to high growth rates recorded by Read et al. (Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017) in seedlings of monodominants, and of ER species more broadly compared with CR species. Indeed, in the sun treatment, PNUE was positively correlated with Prod and RGR, but not in the shade treatment. Notably, while there was no relationship between uptake efficiency and nutrient productivity across all species, Nothofagus spp., A. gummiferum and C. candelabra combined high UpE-N with high Prod-N, and Nothofagus spp. and A. gummiferum combined high UpE-P with moderate Prod-P. This combination of high uptake efficiency with moderate to high plant-level use efficiency is likely to contribute significantly to competitiveness and consequently dominance on these infertile soils. On nutrient-limited soils, nutrient conservation is expected to play an important role in determining competitiveness (Aerts & Chapin Reference AERTS and CHAPIN2000, Berendse Reference BERENDSE1994) and may be inversely related to nutrient productivity due to evolutionary trade-offs (Berendse & Aerts Reference BERENDSE and AERTS1987). We have only limited data relating to nutrient conservation, with no difference in resorption efficiency or proficiency found between mature trees of Nothofagus spp. and co-occurring species (Chatain et al. Reference CHATAIN, READ and JAFFRÉ2009), and no information on leaf lifespan across these species.
Non-persistent monodominants are likely to establish in relatively open conditions created by cyclones, wildfire or drought (Newbery et al. Reference NEWBERY, VAN DER BURGT and MORAVIE2004, Read & Jaffré Reference READ and JAFFRÉ2013), where nutrient availability may be greater than in the undisturbed forest. Nevertheless, on these very infertile ultramafic soils, nutrient-uptake and -use efficiency may be critical to achieving dominance, even after disturbance. It is uncertain whether these traits are as important to achieving early dominance after disturbance in monodominant forests occurring on more fertile soils. It is also uncertain whether these traits are critical for seedlings of persistent monodominants, which are likely to establish in less disturbed conditions (Connell & Lowman Reference CONNELL and LOWMAN1989), with strong competition for nutrients, but also for light. However, slow decomposition/nutrient cycling in stands of some persistent monodominants, often with EM associations (Brookshire & Thomas Reference BROOKSHIRE and THOMAS2013, Corrales et al. Reference CORRALES, MANGAN, TURNER and DALLING2016, McGuire et al. Reference MCGUIRE, ZAK, EDWARDS, BLACKWOOD and UPCHURCH2010, Torti et al. Reference TORTI, COLEY and KURSAR2001), suggest that nutrient-uptake and/or -use efficiency may be similarly critical for establishing, or at least maintaining, dominance (Connell & Lowman Reference CONNELL and LOWMAN1989).
In the field, it is rare to find seedlings of a wide range of canopy species growing under common conditions that allows effective comparison of traits or responses to fertilization. Seedling performance may differ under field conditions from that in pot trials for various reasons, including facilitation of transfer of mineral nutrients and carbon between plants by EM mycelial networks (Finlay & Read Reference FINLAY and READ1986, McGuire Reference MCGUIRE2007). Furthermore, nutrient-uptake and -use efficiency may vary ontogenetically. For example, in older juveniles and adults, high use efficiency might be achieved by low nutrient allocation to wood increment while maintaining canopy productivity (Gleason et al. Reference GLEASON, READ, ARES and METCALFE2009). In addition, efficiency traits may respond to site microheterogeneity and competition in ways that are difficult to predict from transplant and pot experiments. Nevertheless, the trends shown here and previously (Read et al. Reference READ, MCCOY, JAFFRÉ, SANSON and LOGAN2017) suggest that these monodominant species combine high mass allocation efficiency (low root mass fraction, and sometimes high specific root length) with high uptake efficiency, and moderate to high productivity per nutrient mass, although with some variation in efficiency traits among species. The benefits of this trait combination may be enhanced under field conditions, with seedlings of monodominants pre-empting limiting nutrients. However, other traits not yet investigated may also contribute to post-disturbance dominance, including resistance to stresses such as seasonal drought and herbivory, and reproductive traits such as mast seeding, the latter, at least, also potentially influenced by the EM symbiosis (Henkel Reference HENKEL, MAYOR and WOOLLEY2005, Newbery Reference NEWBERY2005, Newbery et al. Reference NEWBERY, ALEXANDER and ROTHER1997).
CONCLUSIONS
Whereas some previous studies have suggested that monodominants may have high nutrient-uptake efficiency via EM associations, our data suggest high nutrient-uptake efficiency combined with high photosynthetic nutrient-use efficiency and moderate to high nutrient productivity in sun-raised seedlings of monodominant species, the level varying according to nutrient and species. In addition, A. gummiferum and Nothofagus spp. possibly have efficient tolerance of high soil Ni levels via EM associations. In the sun treatment, ER species showed high photosynthetic nutrient-use efficiency, and high uptake efficiency for N and P, but no difference in nutrient productivity, compared with CR species. This study was conducted in modified forest soil in pots in a nursery house, and extrapolation to field performance should be cautious. Nevertheless, the trait combination recorded in monodominants, together with high biomass allocation efficiency, is likely to facilitate superior growth rates on infertile soils, but only in the open conditions that occur after large disturbances such as cyclones and wildfire. This effect may be even stronger in the field than in pot experiments, due to competitive interactions, with seedlings of monodominants potentially pre-empting limiting soil nutrients. However, other plant traits may contribute to post-disturbance dominance by enhancing juvenile survival directly (including reproductive traits such as mast seeding), or indirectly by effects on growth rates. Field studies of differential establishment and early growth and survival of monodominants relative to subordinates should shed light on these issues.
ACKNOWLEDGEMENTS
We are grateful to Vale New Caledonia for hosting this experiment at their Environmental Conservation Service nursery facility, and to their staff for invaluable assistance in preparing and maintaining the seedling experiment. We thank Graham Lancaster and staff of the Environmental Analysis Laboratory, Southern Cross University, Lismore, Australia, for elemental analysis, G. Sanson and A. Warren for assistance during the experiment, J. Demenois for providing population data for A. gummiferum, and S. Gleason for helpful comments on this paper. We gratefully acknowledge permission from Direction de l'Environnement, Province Sud, New Caledonia, to undertake this research, and the Committee for Research and Exploration of the National Geographic Society for support as part of a larger project (Grant #8798-10).
Appendix 1. Soil chemistry of sites where soil was collected for potting the experimental seedlings. Data are means of the average values from two sites, with SE. Ten samples were analysed per site, on two dates (June/July 2009 and March/April 2011), except pH which was measured only in 2011 and total N reported only from 2009. Total N was measured by a CHNS-analyser and the remaining total elements were measured by ICP-AES after alkaline fusion and dissolution in HCl. NH4Cl was used to extract exchangeable (exch.) cations. Analyses were undertaken at the Institut de recherche pour le developpement (IRD) in Noumea.

Appendix 2. Associations among nutrient-uptake and -use efficiency traits, separately for sun and shade treatments, in seedlings of 17–19 New Caledonian rain-forest tree species. Data presented are Pearson correlation coefficients (R) based on species’ means (means of block averages). *, P < 0.05; **, P < 0.01; ***, P < 0.001. PNUE, photosynthetic nutrient-use efficiency; UpE, nutrient-uptake efficiency; Prod, nutrient productivity; L, log-transformed for analysis.
Appendix 3. Foliar element concentrations and efficiency traits in seedlings of 17–19 New Caledonian rain-forest tree species (mean ± SE): monodominants vs subordinates and episodically (ER) vs continuously regenerating (CR) species. Acronyms are explained in Appendix 2.