INTRODUCTION
Local ecosystems are rarely isolated from neighbouring ecosystems and subsidies from one ecosystem to another are important in supporting biodiversity and productivity in the receiving ecosystem (Meyer & Schultz Reference MEYER and SCHULTZ1985, Polis et al. Reference POLIS, ANDERSON and HOLT1997). Theoretical works (Gravel et al. Reference GRAVEL, GUICHARD, LOREAU and MOUQUET2010, Loreau & Holt Reference LOREAU and HOLT2004) indicate that transfers can provide important subsidies for organisms in the receiving ecosystem that may enhance primary and secondary production.
The movement of fauna can provide an important avenue for movement of materials across habitat boundaries (Polis et al. Reference POLIS, ANDERSON and HOLT1997), including the marine terrestrial boundary (Anderson & Polis Reference ANDERSON and POLIS1998, Gende et al. Reference GENDE, EDWARDS, WILLSON and WIPFLI2002, Polis & Hurd Reference POLIS and HURD1996, Rose & Polis Reference ROSE and POLIS1998). There are many examples for biotic vectors moving material from the marine to the terrestrial habitat, for example anadromous fish that breed and die in freshwater streams (Cederholm et al. Reference CEDERHOLM, KUNZE, MUROTA and SIBATANI1999, Hocking & Reynolds Reference HOCKING and REYNOLDS2011, Naiman et al. Reference NAIMAN, BILBY, SCHINDLER and HELFIELD2002) and insects that feed on marine food sources and in turn are eaten by terrestrial spiders (Polis & Hurd Reference POLIS and HURD1996). However, very few studies have shown fauna-driven transfer of material from land to sea.
Transfer of material from terrestrial to marine habitats is strongly influenced by physical/hydrological connectivity, e.g. riverine outflows, which enhance primary and secondary production in marine ecosystems (Baisre & Arboleya Reference BAISRE and ARBOLEYA2006, Dunton et al. Reference DUNTON, WEINGARTNER and CARMACK2006, Loneragan & Bunn Reference LONERAGAN and BUNN1999, Lovelock et al. Reference LOVELOCK, FELLER, ELLIS, SCHWARZ, HANCOCK, NICHOLS and SORRELL2007, Paerl Reference PAERL1997, Smith et al. Reference SMITH, TILMAN and NEKOLA1999). Here we investigate whether mammalian herbivores can play a role as significant biotic vectors for transferring terrestrial material into a marine environment.
In the tropics and subtropics mangrove trees form forests in the intertidal zone. These forests are accessible to both marine and terrestrial fauna. Although much of the fauna does not directly feed on the trees (but see Feller Reference FELLER1995, Reef et al. Reference REEF, BALL and LOVELOCK2012, Robertson & Duke Reference ROBERTSON and DUKE1987), the trees provide habitat for a range of invertebrates, algae and microphytobenthos, which support consumers. The role of mangroves in supporting fish and crustaceans is well established (Faunce & Serafy Reference FAUNCE and SERAFY2006, Fry & Ewel Reference FRY and EWEL2003, Mumby et al. Reference MUMBY, EDWARDS, ERNESTO ARIAS-GONZALEZ, LINDEMAN, BLACKWELL, GALL, GORCZYNSKA, HARBORNE, PESCOD, RENKEN, WABNITZ and LLEWELLYN2004). They also provide key roosting habitats for birds (Nagelkerken et al. Reference NAGELKERKEN, BLABER, BOUILLON, GREEN, HAYWOOD, KIRTON, MEYNECKE, PAWLIK, PENROSE, SASEKUMAR and SOMERFIELD2008) and flying fox species (Pierson & Rainey Reference PIERSON, RAINEY, Wilson and Graham1990) and provide shade and shelter for other terrestrial mammal species (Hutchings & Recher Reference HUTCHINGS, RECHER and Teas1983, Odum et al. Reference ODUM, MCIVOR and SMITH1982).
Mangrove forests are often nutrient-limited, with trees showing enhanced growth when nutrient availability is experimentally increased (Feller et al. Reference FELLER, LOVELOCK and PIOU2009, Lovelock et al. Reference LOVELOCK, FELLER, MCKEE, ENGELBRECHT and BALL2004, Naidoo Reference NAIDOO2009). Increasing nutrient availability has profound effects on mangrove forest structure, which include altered species composition and significant changes in wood production, tree size and basal area (Chen & Twilley Reference CHEN and TWILLEY1999). Such changes to forest structure have significant effects on the biodiversity supported by the forest and the flow of nutrients between the mangrove and the surrounding ecosystems (Ewel et al. Reference EWEL, TWILLEY and ONG1998, Field et al. Reference FIELD, OSBORN, HOFFMAN, POLSENBERG, ACKERLY, BERRY, BJORKMAN, HELD, MATSON and MOONEY1998). Increases in soil fertility can also result in increases in the abundance of herbivores (Feller & Chamberlain Reference FELLER and CHAMBERLAIN2007, Onuf et al. Reference ONUF, TEAL and VALIELA1977), as well as increase the capacity of the soil surface to keep up with sea-level rise (McKee et al. Reference MCKEE, CAHOON and FELLER2007).
Because of the importance of nutrients to a range of ecological processes, we investigated whether two common Australian mammals, fruit bats and kangaroos, can transfer terrestrially derived nutrients into the marine environment to the extent that they influence the growth and nutrient relations of mangroves.
METHODS
Study sites
We tested the effects of terrestrial mammals on nutrient availability and mangrove productivity in two separate studies. In one, we assessed the influence of the flying fox Pteropus alecto on the nutrition of mangroves at Watson's Bay on Lizard Island in the Northern GBR (Figure 1). In the second, we assessed the influence of kangaroos on the nutrition of mangroves at Mangrove Bay in Western Australia (Figure 1). The sites were chosen based on their location within oligotrophic marine environments lacking riverine inputs and their large distance from anthropogenic nutrient sources, making the mammalian nutrient subsidy easier to quantify.
Figure 1. A map of Australia showing the two study sites (marked by stars), Lizard Island in the northern Great Barrier Reef lagoon, and Mangrove Bay, located on the upper west coast of North West Cape, Western Australia.
The influence of bats on mangrove nutrition and growth
We assessed the influence of a roosting colony of the black flying fox Pteropus alecto Temminck 1837 (Figure 2a) on the nutrition of mangroves at Watson's Bay on Lizard Island (14°40′00″S, 145°27′07″E, Figure 1) in the Northern Great Barrier Reef Lagoon. Mangrove area on the island is approximately 13 ha that fringes a tidal creek on the seaward edge and freshwater stream on the landward edge (Proske & Haberle Reference PROSKE and HABERLE2012). The forest is dominated by Rhizophora stylosa Griff. but 11 other mangrove species are also present (R. Reef, pers. obs.). A colony of up to several thousand individuals (colony size varies seasonally) of P. alecto roost in trees at the landward edge of the forest in the canopy of tall (3–8 m) R. stylosa, Ceriops tagal (Perr.) C. B. Robinson and Lumnitzera rosea Gaud., making trips to the nearby mainland to forage for fruit and nectar. The bats defecate in their roosts and therefore may provide nutrients derived from the mainland to the mangrove. Lizard Island is a continental island 35 km from the coast whose underlying geology is granite, but supports an extensive fringing coral reef (Rees et al. Reference REES, OPDYKE, WILSON, KEITH FIFIELD and LEVCHENKO2006). Its underlying geology and isolation from the coast result in it being highly oligotrophic. In this setting, we explored the effect of the fruit bat roost on mangrove tree growth and nutrition.

Figure 2. Mammalian herbivores in Australian mangrove forests. Individuals of the black flying fox, Pteropus alecto roosting in Avicennia marina trees in Queensland (a) (photo: R. Reef) and a euro, Macropus robustus in an Avicennia marina forest in Mangrove Bay, Western Australia (b) (photo: R. Kelley).
Tree growth rates
We assessed rates of wood growth of R. stylosa at Lizard Island over a 2-y period using stainless steel dendrometer bands (Hall Reference HALL1944) installed at breast height on eight trees associated with the bat roost and eight that were adjacent in a forest where the bats were not present. Dendrometer bands were installed on 29 June 2008. Radius increments were measured to the nearest 0.01 mm on 8 November 2009 and on 30 July 2010. Only the radial increments recorded during the period between November 2009 and July 2010 were used to calculate basal area increment in order to ensure sufficient time for the band to settle prior to the initiation of measurements (all trees recorded radial increments between June 2008 and November 2009). Differences in increases in basal area between trees associated with the bat roost and trees not associated with the bat roost were tested statistically using a Student's t-test.
Lizard Island nutrient and soil analysis
Foliar nutrient analysis was conducted on fully sun-exposed green and senescent leaves of three mangrove species occurring within and outside the bat roost on 30 July 2010. At each site, leaves were collected from six individual R. stylosa trees, three C. tagal trees and three L. rosea trees. Rhizophora stylosa had no senescent leaves at the time of leaf collection. Three green leaves and senescent leaves were collected from each tree. The difference in nutrient concentrations of green and senescent leaves indicates the plant's resorption efficiency for the tested nutrient with higher resorption efficiencies suggesting the nutrient is limiting growth. Triplicates were later pooled for analysis. Leaves were photographed alongside a ruler, and leaf area was then measured using the image analysis software ImageJ (US National Institutes of Health, Bethesda, Maryland, http://imagej.nih.gov/ij, ver 1.45s). Leaves were dried at 70 °C and were subsequently pulverized using a bead mill. Three samples of relatively fresh bat guano were scraped off leaves from underneath roosting P. alecto. Soil and bat guano samples were dried and ground.
Carbon (C) and nitrogen (N) concentrations (presented as % mass) in dried leaf tissue and bat guano were determined using mass spectrometry (UC Davis Stable Isotope Facility). The total phosphorus (P) concentration (% mass) in the ground material was determined using the methods described in Reef et al. (Reference REEF, BALL, FELLER and LOVELOCK2010a). Briefly, an acidified persulphate autoclave digestion of the organic compounds (Menzel & Corwin Reference MENZEL and CORWIN1965) was followed by quantification of the released orthophosphate in a colorimetric assay with ammonium molybdate and malachite green (van Veldhoven & Mannaerts Reference VAN VELDHOVEN and MANNAERTS1987).
The relative abundance of the stable isotope of 15N increases with trophic level, allowing for the quantification of the amount of nutrition plants derive from higher trophic levels (e.g. from animal sources). We measured relative abundance of the stable isotopes of 15N in leaves and bat guano in order to assess nutrient sources.
Soil porewater salinity was measured at the five locations within the bat roost and five locations in the mangrove outside the roost. Porewater was extracted from 30-cm depth using a suction device (McKee et al. Reference MCKEE, MENDELSSOHN and HESTER1988) and analysed using a handheld refractometer (model 300011, Sper Scientific, Scottsdale AZ, USA). Soil organic matter content was measured in five shallow (0–3 cm deep) soil cores collected from within the bat roost area and from the mangroves outside the roost. Soil organic matter content was measured using the weight-loss-on-ignition method by heating the dried soil sample to 450 °C for 4 h.
Five soil redox potential measurements were made in the bat roost and five in the mangrove forest outside the roost using custom-built platinum probes along with an Ag/AgCl reference probe (IJ14, Ionode Pty Ltd, Brisbane, QLD, Australia). In this system, observed E values were converted to standard reduction potential (EH) by adding 200 mV to each measurement. Probes were pre-tested in freshly prepared saturated quinhydrone solutions of pH 4.01 and pH 7.00, yielding EH values of 269 mV ± 5 mV and 94.1 mV ± 1.52 mV, respectively. Six measuring probes and a reference probe were inserted 5 cm into the soil and allowed to equilibrate for a few minutes before a measurement was made. Each measurement was an average of the readings made from each of the six measuring probes.
The influence of kangaroos on mangrove nutrition
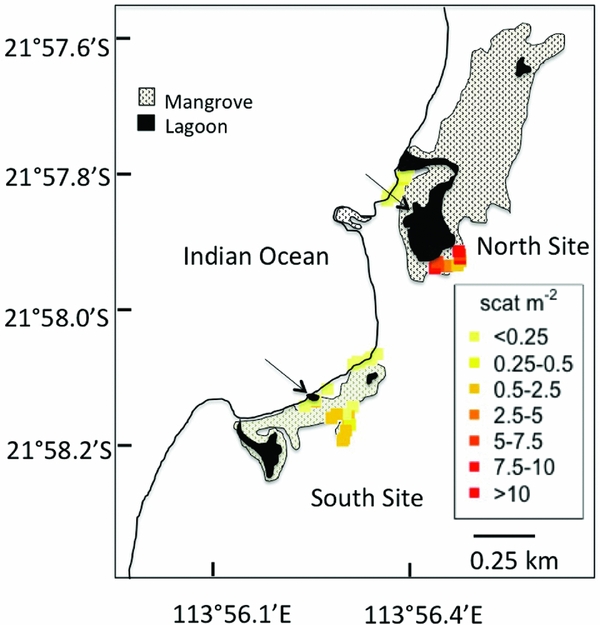
We assessed the influence of kangaroos on the nutrition of mangroves in Mangrove Bay, Western Australia. Mangrove Bay and Ningaloo Reef are on the North West Cape of Western Australia (21°58′S, 113°57′E, Figure 1), which is the most arid coastal region in Australia, where evaporation (3200–4200 mm y−1) greatly exceeds rainfall (<200–700 mm y−1), which occurs mostly between November and May during cyclones (Alongi et al. Reference ALONGI, TIRENDI and CLOUGH2000). Air temperatures range from 14 °C to 40 °C. Here, fringing coral reefs are adjacent to spinifex grasslands growing on coastal dunes. The red kangaroo (Macropus rufus Desmarest 1822) and euro (M. robustus Gould 1841, Figure 2b) are the major mammalian herbivores in this ecosystem. Mangrove vegetation on the Ningaloo coast is restricted to a few patches that occur in association with tidal creeks and lagoons formed in the swales of sand dunes and associated with fossil coral reef limestone. In these settings, they form stands of low trees (<5 m tall) in an essentially tree-less landscape. Although kangaroos are tolerant of high temperatures and low humidity, they use trees for shade, making resting nests under the canopy during the day (Newsome Reference NEWSOME1975). In Mangrove Bay, both M. robustus and M. rufus rest during the heat of the day in the landward regions of the mangrove forest. Additionally, carcasses of kangaroos are found within the forests, which may represent a transfer of nutrients from the terrestrial environment. Mangrove Bay has two distinct mangrove forests composed mainly of the mangrove Avicennia marina (Alongi et al. Reference ALONGI, TIRENDI and GOLDRICK1996). The northern forest is associated with a fossil coral reef formation that gives rise to a shallow lagoon fringed by mangroves while the southern forest occurs within depressions between sand dunes (Figure 3). The relative abundance of kangaroos at the landward and seaward sections of the northern and southern mangrove forests was estimated indirectly by counting faecal pellets. Faecal pellets were counted in ten 20-m2 belt transects laid at each of the four locations.
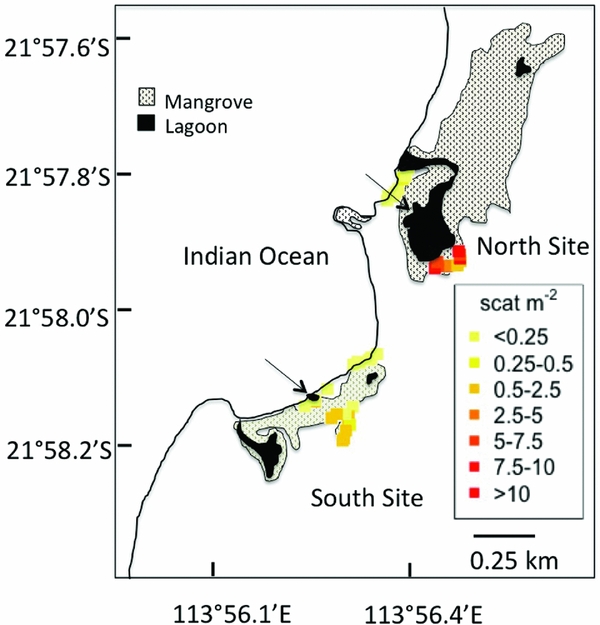
Figure 3. A detailed map of Mangrove Bay shows the two mangrove forests (North Site and South Site) and the arrows point to the North Lagoon (within the North Site) and South Lagoon (within the South Site). Kangaroos rest in the trees on the landward edge of the forests, but less so in those on the seaward edge. Coloured points indicate the density of kangaroo scat at each of the 40 transects.
The relative abundance of 13C is different among plants that utilize different photosynthetic pathways (e.g. C3 or C4 photosynthesis) and thus distinguish between mangroves and grasses on which kangaroos might feed. We used 13C abundance in kangaroo scat in order to establish whether kangaroos fed on terrestrial sources of vegetation (e.g. C4 Spinifex grasses) or rather on C3 mangrove vegetation. The isotopic relative abundances were measured using a PDZ Europa ANCA-GSL elemental analyser interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK) at the UC Davis Stable Isotope Facility. The 13C delta values are presented relative to the international standard V-PDB. 15N delta values are presented relative to air.
Nutrient analysis – Mangrove Bay
We assessed the impact of kangaroos on the nutrition of the mangroves in two forest sites, one around the north lagoon, in the forest frequented by kangaroos and one around the south lagoon, where kangaroo activity was less frequent.
Foliar nutrient analysis was conducted on fully sun-exposed green leaves of A. marina (the most common mangrove species at the site) in September 2009. Three green leaves were collected from each tree for the analysis.
Leaves and fresh kangaroo scat were dried and ground prior to analysis. The three leaves from each tree were pooled together. Carbon and N concentrations (presented as % mass) and stable isotopes of N in leaf tissue and kangaroo scat and stable isotopes of C in kangaroo scat and were analysed using a SerCon elemental analyser coupled with a 20–22 stable isotope ratio mass spectrometer (Sercon, Crewe, UK) at the West Australian Biogeochemistry Centre.
Statistical analysis
Data were analysed using R for Mac OS X ver. 3.0.2. Student's t-tests were used to compare between tree variables within and outside the main mammalian impact area for each species. The distribution of the data was tested for normality using the Shapiro-Wilks normality test. Percentage data were arcsine-transformed prior to further analysis. Rhizophora stylosa δ15N values were logarithmically transformed to conform to normality.
RESULTS
The influence of bats on mangrove nutrition and growth
Rhizophora stylosa trees within the bat roost at Lizard Island had significantly higher growth rates than those outside the colony (t-test, t(7.1) = 2.46, P = 0.04). Stem basal area of eight R. stylosa trees within the bat roost increased on average (±SD) by 1.47 ± 1.54 cm2 y−1, which was nearly six times faster than growth rates for R. stylosa trees growing outside the bat roost (which increased by only 0.25 ± 0.24 cm2 y−1). Trees of all species within the bat roost were taller (3–8 m) than trees outside the roost (where adult tree height was 1–3 m).
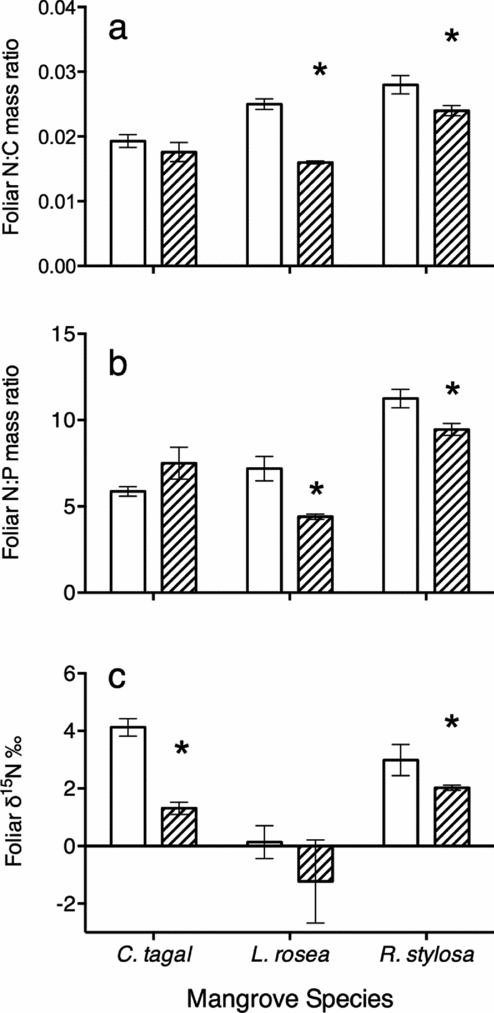
Rhizophora stylosa and L. rosea had significantly higher N:C mass ratios within the roost relative to outside the roost (t-test, t(6.5) = −2.6, P = 0.04 and t(2.6) = −12, P = 0.002 respectively, Figure 4a). The observed increase in foliar N:C within the bat roost was not significant for C. tagal (t(7) = −1, P = 0.33). Rhizophora stylosa and Lumnitzera rosea leaves from trees in the bat colony also had significantly higher N:P mass ratios than leaves from trees outside the roost (t-test, t(7.2) = −3.3, P = 0.01 and t(2.4) = −4.7, P = 0.03 respectively, Figure 4b). N:P mass ratios in green leaves of C. tagal were not significantly affected by the bat roost (t(6) = 1.7, P = 0.15, Figure 4b). Ceriops tagal was the only tree species for which foliar phosphorus concentrations were significantly higher in trees within the bat roost (t(6.5) = −4.2, P = 0.004), increasing from an average (±SD) of 0.11% ± 0.018% of dry mass to 0.15% ± 0.012% within the bat roost.

Figure 4. Mean (±SE) N:C dry mass ratios (a), N:P dry mass ratios (b) and N stable isotope ratios (c) for three mangrove species: Ceriops tagal, Lumnitzera rosea and Rhizophora stylosa within (open bars) and outside (hatched bars) the roosting area of a Pteropus alecto colony. N = 3 for C. tagal and L. rosea and 6 for R. stylosa at each site. Asterisk indicates significant difference between the trees within and outside the roosting area (t-test, α = 0.05).
The observed increase in foliar δ15N ratios in trees within the bat roost than for trees outside the bat roost was statistically significant in C. tagal and R. stylosa trees (t-test, t(4) = 7.6, P = 0.002 and t(5.2) = 0.9, P = 0.049 respectively; Figure 4c).
Phosphorus resorption efficiency was on average 13.2% ± 23.5% and 24.7% ± 11.7% for C. tagal and L. rosea respectively and was not affected by the presence of the bat roost for either species (t-test, t(5.6) = −1.88, P = 0.11 and t(3.8) = 0.27, P = 0.8, respectively). No senescent leaves were present on R. stylosa trees. Nitrogen resorption efficiency was calculated for C. tagal and L. rosea and was not found to be significantly different between trees within and outside the bat colony roosting area (t-test, t(5.3) = −1.5, P = 0.2 and t(2.2) = −0.06, P = 0.95 for C. tagal and L. rosea respectively) averaging (±SD) 64.7% ± 7.4% and 67.2% ± 3.5% for C. tagal and L. rosea respectively.
Guano of P. alecto was composed on average (±SD) of 43.8% ± 1.7% C, 2.7% ± 0.2% N and 0.12% ± 0.05% P as proportions of dry mass. Nitrogen in the bat guano had a δ15N of 2.8‰ ± 0.3‰. Carbon had a δ13C of −27.7‰ ± 0.42‰. Nutrient excretion via urine was not measured in this study.
Some edaphic properties were different between soils collected under the bat roost and soil collected among the trees in the forest outside the bat roost (Table 1). Outside the bat roost, soil porewater was slightly more saline, and there was a significantly larger proportion of organic matter in the soil cores.
Table 1. Soil and porewater properties of soil cores collected within the bat roost and outside the roost (N = 5 for each site). Soil organic matter was calculated using the weight loss on ignition method.

The influence of kangaroos on mangrove nutrition
In our study site in Mangrove Bay, A. marina is the most common mangrove species present. Kangaroo abundance was estimated indirectly by counting faecal pellets along ten 20-m2 belt transects at each site and kangaroos were significantly more abundant in the landward forests (ANOVA, F(1,36) = 41.8, P < 0.001) especially around the northern lagoon (ANOVA, F(1,36) = 26.9, P < 0.001, Figure 3). In the southern lagoon, kangaroo faecal pellets were significantly more abundant on the landward side, 0.84 ± 0.65 m−2 (SD) than on the seaward side (0.08 ± 0.22 m−2). At this site, the lagoon is nestled within the seaward trees. In the northern lagoon, kangaroo faecal pellets were also significantly more abundant on the landward side, 7.03 ± 3.72 m−2 than on the seaward side (0.05 ± 0.12 m−2). At this site, the lagoon is nestled within the landward side trees.
Kangaroo presence did not significantly influence %N in A. marina leaves (Figure 5a) at both the north (t-test, t(2.9) = 0.6, P = 0.6) and south (t(2.9) = −1.6, P = 0.2) sites, but the δ15N isotopic ratio in the leaves of mangroves growing in areas visited by kangaroos were significantly higher than those of leaves from adjacent sites where kangaroos were not present (Figure 5b) at both sites (t-test, t(2.7) = −4.6, P = 0.02 and t(4) = −8.7, P < 0.001 for north and south respectively).
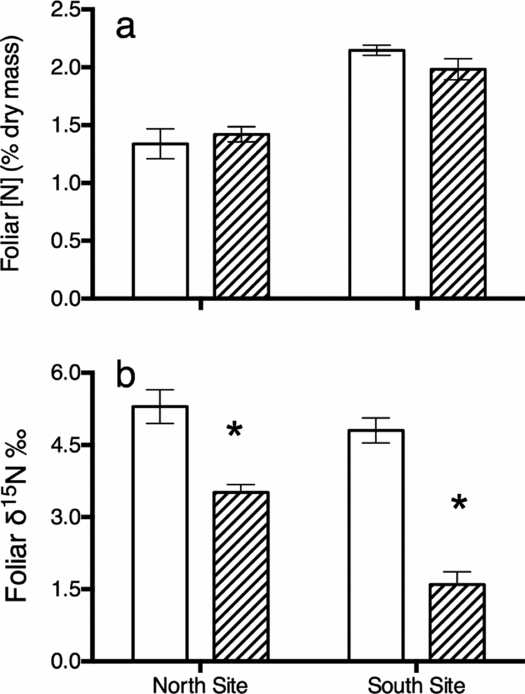
Figure 5. Mean (±SE) foliar N concentration (a) and N stable isotope ratios (b) for Avicennia marina within (open bars) and outside (hatched bars) the areas where kangaroos aggregate at two replicate sites (North and South). N = 3 at each site. Asterisk indicates significant difference between the trees within and outside the areas frequented by kangaroos at each location (α = 0.05).
Fresh kangaroo scat collected from the ground in the mangroves was composed of 34.8% C, 1.3% N and 0.35% P as proportions of dry mass. Nitrogen in the kangaroo scat had a δ15N of 5.02‰ ± 0.4‰ and a δ13C of −14.1‰ ± 0.7‰.
DISCUSSION
Terrestrial mammals frequenting the mangrove forests provided a nutrient subsidy for mangrove trees at both study sites. Due to the high connectivity between mangrove forests and the surrounding marine environment, terrestrial subsidies of nutrients into mangrove forests may in turn influence marine nutrient availability.
The influence of bats on mangrove nutrition and growth
At Lizard Island, the nutrient subsidy by the bat roost led to a significant increase in both the quantity and quality of mangrove vegetation. Rhizophora stylosa trees within the bat colony at Lizard Island had significantly higher growth rates than those outside the colony. Foliar N:C of two of the three dominant mangrove species (R. stylosa and Lumnitzera rosea) increased significantly relative to outside the roost, suggesting a significant N subsidy from the bat roost at this site. Increases in foliar N:C ratios are usually indicative of an increased investment in Rubisco and other components of the chloroplast that leads to higher rates of CO2 assimilation (reviewed in Evans Reference EVANS1989).
Apart from nutrient availability, soil salinity and soil redox potential are both factors that can have a significant negative effect on mangrove productivity (Krauss et al. Reference KRAUSS, LOVELOCK, MCKEE, LÓPEZ-HOFFMAN, EWE and SOUSA2008). Redox potential did not significantly vary between the bat and non-bat sites. Salinity of soil porewater was relatively low at the site (<10 ppt) due to the presence of groundwater. Salinity of porewater was significantly lower outside the roost (1.7 ppt) compared with within the bat roost (9.4 ppt), however previous experimental studies on this species suggest that growth rates of R. stylosa are expected to be similar within the range of salinities measured in this study (Ball & Pidsley Reference BALL, PIDSLEY, Larson, Hanley and Michie1988, Clough Reference CLOUGH1984). Higher-salinity conditions within the bat colony were associated with higher growth rates of trees, which may reflect higher transpiration rates and water use of the canopy (Passioura et al. Reference PASSIOURA, BALL and KNIGHT1992). Given the similarity in salinity and redox potential of soils within and outside the bat roost areas of the forest it is highly likely that the enhanced growth in R. stylosa trees within the bat roost was due to nutrient subsidies from bats and that bats have altered the structure of the forest over time. Pteropus alecto is a widespread bat species in Australia, Papua New Guinea and Indonesia and forms roosts that can exceed 10000 animals, often in mangrove forests (Vardon et al. Reference VARDON, BROCKLEHURST, WOINARSKI, CUNNINGHAM, DONNELLY and TIDEMANN2001). A recent survey along the east coast of Australia (May 2013) has recorded 161000 P. alecto at 108 roosting sites, with the largest roosts in coastal areas (Australian Government, Department of Environment). In two separate surveys from different areas in the Northern Territory, between 20% (Palmer & Woinarski Reference PALMER and WOINARSKI1999) and 33% (Tidemann et al. Reference TIDEMANN, VARDON, LOUGHLAND and BROCKLEHURST1999) of the roosting sites of P. alecto were found in mangrove forests, despite mangrove forests comprising only a small area in the species distribution. Furthermore, other Pteropus species are found in coastal environments, similarly forming large roosting colonies in mangrove forests throughout the Old World tropics. It can thus indirectly be assumed that P. alecto colonies might be contributing significant nutrient subsidies to a large number of mangrove forests throughout their range.
Nutrient limitation in many mangrove ecosystems is primarily due to N limitation (Reef et al. Reference REEF, FELLER and LOVELOCK2010b). Our findings of low resorption efficiency for P in conjunction with the high resorption efficiency for N during senescence at Lizard Island suggests that mangrove growth at this site is limited by N availability, especially for R. stylosa and L. rosea. The high N resorption efficiencies (>65%) for the mangroves at Lizard Island (relative to resorption efficiencies calculated in mangroves elsewhere, Feller et al. Reference FELLER, LOVELOCK and MCKEE2007) suggest N limitation to growth both inside and outside the bat roost. The low (<14, Koerselman & Meuleman Reference KOERSELMAN and MEULEMAN1996) foliar N:P mass ratios measured further support N limitation to growth at this site. Nitrogen limitation to growth in mangroves develops partly due to the low fraction of inorganic N that is useable for plant growth within the total N pool in the soil (Robertson & Phillips Reference ROBERTSON and PHILLIPS1995). Nitrogen provided by bat excretion is in the form of urea and ammonia (Herrera et al. Reference HERRERA, OSORIO and MANCINA2011), which in flooded soils are rapidly hydrolysed by soil micro-organisms to ammonium (Alongi Reference ALONGI1994). Ammonium is the primary form of N used by mangrove trees (Reef et al. Reference REEF, FELLER and LOVELOCK2010b). Thus, bat excretion (also as urine, which was not measured in this study) provides an N subsidy that is in a form that is readily available for mangrove growth.
The organic matter content (and % C) of soils was lower in the bat colony than outside the bat colony (Table 1). Reduction in C content of peat soils was observed when trees were fertilized with N in Belizean mangroves (McKee et al. Reference MCKEE, CAHOON and FELLER2007). This is likely due to reduced allocation to roots by nutrient-enriched trees (Giardina et al. Reference GIARDINA, RYAN, BINKLEY and FOWNES2003, Haynes & Gower Reference HAYNES and GOWER1995) and also possibly due to enhanced decomposition of soil C with N fertilization (McKee et al. Reference MCKEE, CAHOON and FELLER2007).
Ceriops tagal and R. stylosa trees within the bat roost had elevated foliar δ15N isotopic signatures. δ15N is used to measure the trophic structure of communities because of trophic enrichment (a predictable increase in the abundance of 15N from resource to consumer). An elevation in δ15N suggests that the source of foliar [N] could be in part from an N source from a higher trophic level (i.e. from bat guano or bat carcasses from the large roosting colony). The isotopic composition of guano is dependent on the food source and can vary seasonally as different foods become available, especially in a species like P. alecto that exhibits a high level of diet diversity (Palmer et al. Reference PALMER, PRICE and BACH2000). The guano composition presented here represents a single time point and might not represent the average isotopic composition of bat-derived N. The roosting colony can also have indirect effects on the isotopic composition of available N in the soil by enhancing microbial nutrient cycling through fertilization, resulting in 15N enrichment (Natelhoffer & Fry Reference NATELHOFFER and FRY1988).
The influence of kangaroos on mangrove nutrition
In our Western Australian site, kangaroos had similar effects on stable isotope composition as bats had on the mangroves of Lizard Island, but foliar N was not significantly enhanced. Avicennia marina trees where kangaroos rest during the heat of the day had significantly higher δ15N ratios than A. marina trees in areas less frequented by kangaroos. Although kangaroos have been reported to feed on mangrove seedlings (Smith Reference SMITH1987), the high (less negative) δ13C value in kangaroo scat (−14.1‰) indicates C4 grasses were likely a main component of kangaroo diets (Iles et al. Reference ILES, KELLEWAY, KOBAYASHI, MAZUMDER, KNOWLES, PRIDDEL and SAINTILAN2010) and it is unlikely that mangroves contributed significantly to the diet of kangaroos at this site (mangrove δ13C values ranged between −26‰ and −29.3‰). Thus, the contribution of N to the mangrove forest is of terrestrial origin, offering an avenue for new, allochthonous N to enter the system.
In conclusion, mangrove habitats provide important structure for resting sites for birds, bats and other mammals, including kangaroos. Here, we provide evidence that these terrestrial organisms provide a nutrient subsidy that is taken up by mangrove trees, enhancing growth and productivity and may be exported further into adjacent marine environments. The importance of mangroves in nutrient cycling in the coastal zone is well recognized, but our study indicates that through their importance to terrestrial fauna, they may be sites of localized nutrient enrichment, which may enhance productivity and diversity.
ACKNOWLEDGEMENTS
This study was funded by ARC awards DP0774491, DP1096749 and DE120101706. We thank H. Penrose for assistance in the field and N. Santini and F. Adame for helpful comments on the manuscript. We thank R. Kelley for the photo of the euro.