Introduction
Several invertebrates build refuges for themselves through rolling, folding, or tying leaves together with silk, creating a better microclimate, protection from natural enemies, and/or improving the quality of food resources (Fukui Reference Fukui2001, Danks Reference Danks2002, Lill & Marquis Reference Lill, Marquis, Cuddington, Byers, Hastings and Wilson2007, Cornelissen et al. Reference Cornelissen, Cintra and Santos2016, Novais et al. Reference Novais, Aguirre-Jaimes, Quesada and Hernández-Ortiz2020a, Pereira et al. Reference Pereira, Sperandei, Henriques, Silva, Fernandes and Cornelissen2021). After abandonment, these structures might persist on plants and provide microhabitats for other arthropods, thus influencing species diversity through a facilitative interaction process (Henriques et al. Reference Henriques, Cintra, Pereira and Cornelissen2019, Lill & Marquis Reference Lill, Marquis, Cuddington, Byers, Hastings and Wilson2007, Martinsen et al. Reference Martinsen, Floate, Waltz, Wimp and Whitham2000, Novais et al. Reference Novais, DaRocha, Calderón-Cortés and Quesada2017). As such, invertebrates that build leaf shelters are acting as physical ecosystem engineers, organisms that create or modify habitats which, in turn, affect directly or indirectly the availability of resources for other species (Jones et al. Reference Jones, Gutiérrez, Byers, Crooks, Lambrinos and Talley2010).
A large number of studies have demonstrated the role of arthropods (e.g., caterpillars, gall-inducers, mites and wood-boring beetles) acting as ecosystem engineers (Cornelissen et al. Reference Cornelissen, Cintra and Santos2016, Novais et al. Reference Novais, DaRocha, Calderón-Cortés and Quesada2017, Reference Novais, Calderón-Cortés, Sánchez-Montoya and Quesada2018, Reference Novais, Aguirre-Jaimes, Quesada and Hernández-Ortiz2020a, Reference Novais, Cristóbal-Perez, Aguirre-Jaimes and Quesada2021, Vieira & Romero Reference Vieira and Romero2013, Wetzel et al. Reference Wetzel, Screen, Li, McKenzie, Phillips, Cruz, Zhang, Greene, Lee, Singh and Tran2016). However, few studies have shown when and where the facilitation by them will have large or small impacts on associated arthropod communities (but see Novais et al. Reference Novais, Calderón-Cortés, Sánchez-Montoya and Quesada2018, Vieira & Romero Reference Vieira and Romero2013). These physical engineers will have a greater positive effect on the structure of arthropod communities when they increase limited resources that enable the establishment of species that otherwise would be unable to persist in the environment (Crain & Bertness Reference Crain and Bertness2006). These limiting resources are strongly associated with community-structuring processes (e.g., predation, competition and abiotic stress) which may differ among environments or habitats and, therefore, the positive impact of facilitators on the community structure depends on the capacities of the facilitators for alleviating these driving factors (Bruno et al. Reference Bruno, Stachowicz and Bertness2003; Crain & Bertness Reference Crain and Bertness2006).
In stressful environments (e.g., deserts), where the harsh conditions often limit the ability for organisms to inhabit them, physical engineers are likely to have a greater positive impact on the community structure by promoting microclimatic refuges (Crain & Bertness Reference Crain and Bertness2006, Romero et al. Reference Romero, Gonçalves-Souza, Vieira and Koricheva2015). On the other hand, in benign environments such as tropical rainforests, where density-dependent interactions (competition and predation) are major forces structuring natural communities, engineers who create new habitats that alleviate competition and predation pressures, or change the availability of limiting competitive resources, are expected to have major community impacts (Crain & Bertness Reference Crain and Bertness2006, Romero et al. Reference Romero, Gonçalves-Souza, Vieira and Koricheva2015). These hypotheses were supported by a global scale meta-analysis that compared the magnitude of the facilitation by animals (including insects) among geographic regions (Romero et al. Reference Romero, Gonçalves-Souza, Vieira and Koricheva2015). This study reported a stronger facilitation effect in deserts compared to other ecosystems (i.e., forests, grasslands and savannas) and at the tropics compared with temperate biomes at higher latitudes. In this sense, as variations in the physical structure within an ecosystem, especially those resulting from anthropogenic activities, significantly affect arthropod diversity and interactions (Grass et al. Reference Grass, Jauker, Steffan-Dewenter, Tscharntke and Jauker2018, Floren & Linsenmair Reference Floren and Linsenmair2001, Tylianakis et al. Reference Tylianakis, Tscharntke and Lewis2007), it is also expected that the magnitude of the facilitation by shelter-building engineers varies depending on the local environmental context.
Alterations of tropical rainforests are mainly determined by land use change such as deforestation, agricultural and urban expansion (Gatti et al. Reference Gatti, Castaldi, Lindsell, Coomes, Marchetti, Maesano, Di Paola, Paparella and Valentini2015, Parrotta et al. Reference Parrotta, Francis and Knowles2002, Urquiza-Haas et al. Reference Urquiza-Haas, Dolman and Peres2007, Sales et al. Reference Sales, Galetti and Pires2020). In southeast Mexico, the region of Los Tuxtlas represents the northernmost limit of tropical rainforests in the Americas which has suffered extensive habitat loss over the last decades (Dirzo & Garcia Reference Dirzo and Garcia1992, Von Thaden et al. Reference Von Thaden, Laborde, Guevara and Venegas-Barrera2018). Most remaining old-growth forests of Los Tuxtlas are isolated fragments lying within a pasture matrix (Dirzo & Garcia Reference Dirzo and Garcia1992, Guevara et al. Reference Guevara, Laborde, Sánchez-Ríos, Guevara, Laborde and Sánchez-Ríos2004). Overall, forest structure in fragments is greatly affected by altered abiotic conditions at the edges, including higher temperature levels and greater light incidence compared to the interior, which allow an increased diversity and abundance of saplings, herbs and shrubs in the understory of forest edges (Harper et al. Reference Harper, Macdonald, Burton, Chen, Brosofske, Saunders, Euskirchen, Roberts, Jaiteh and Esseen2005). These edge characteristics, in turn, generally result in more diverse and/or abundant vegetation-dwelling arthropod communities when compared to those in the nearby anthropogenic matrices and forest interior (De Araújo and Espírito-Santo Filho Reference De Araújo and do Espírito-Santo Filho2012, Barbosa et al. Reference Barbosa, Leal, Iannuzzi and Almeida-Cortez2005, De Carvalho Guimarães et al. Reference De Carvalho Guimarães, Viana and Cornelissen2014, Jokimäki et al. Reference Jokimäki, Huhta, Itämies and Rahko1998). Another important component of the Los Tuxtlas landscape is the presence of living fences that delimit areas used by cattle, where few tree species are planted very close together and connect some forest fragments (Guevara et al. Reference Guevara, Laborde, Sánchez-Ríos, Guevara, Laborde and Sánchez-Ríos2004). Despite the simple structure, these living fences generate particular environmental conditions that allow some vertebrate (e.g., bats and birds) and invertebrate (e.g., dung beetles) species to move through them among forest fragments (Díaz et al. Reference Díaz, Galante and Favila2010, Estrada et al. Reference Estrada, Cammarano and Coates-Estrada2000, Estrada & Coates-Estrada Reference Estrada and Coates-Estrada2001).
The goal of our study was to evaluate the influence of local environmental context on the colonisation of leaf shelters by arthropod communities. We conducted an experimental study in a Mexican evergreen tropical rainforest comparing the species richness and abundance of arthropods colonising artificially rolled leaves in habitats differing in understory heterogeneity (i.e., forest edge > old-growth forests > living fences). We also verified the consistency of our results evaluating the response of different arthropod guilds (i.e., detritivores, omnivores, predators and herbivores) among these habitats. We hypothesised that habitats with increased habitat heterogeneity favour an increase in arthropod diversity and density-dependent interactions (competition and predation), resulting in a greater occupation of new shelters. We predict that species richness and abundance of arthropods colonising rolled leaves will be greater in forest edges, followed by old-growth forests, and smaller in living fences.
Methods
Study area
This study was carried out in the Los Tuxtlas Biosphere Reserve (LTBR; 18°05′–18°43′N, 94°35′–95°25′W), inside to the Los Tuxtlas Biological Station-UNAM (LTBS) located in southeastern Veracruz, Mexico, which range from 150 to 750 m a.s.l. The vegetation within the reserve consists primarily of evergreen tropical rainforest with a mean annual temperature of 26°C, and a mean annual rainfall of 4,700 mm, with a relatively dry season from March to May (Gutiérrez-García & Ricker Reference Gutiérrez-García and Ricker2011).
Sampling design
During June 2019, we randomly selected 20 plants of non-specific species at least 20 m apart from each other in a transect (up to 500 m) in the understory of 3 distinct habitats: living fences (18°35'21.5"N, 95°04'20.9"W), forest edge (18°35'05.4"N, 95°04'12.2"W) and old-growth forests (18°35'04.4"N, 95°04'34.6"W). In the study area, living fences are generally made with Bursera simaruba (Sapindales: Burseraceae), Erythrina folkersii (Fabales: Fabaceae), or Gliricidia sepium (Fabales: Fabaceae) trees planted very close together, with a width of one to a few metres depending on the tree canopies (Díaz et al. Reference Díaz, Galante and Favila2010). Due to the great diversity of vascular plants in the tropical forest studied (2548 species; Villaseñor et al. Reference Villaseñor, Ortiz and Campos-Villanueva2018) and the natural variation in the occurrence of these species locally, we decided not to use specific plant species in our experiment. Thus, for each habitat, following our experimental design, the first plant individual that met our selection criteria (see below) was selected regardless of the species. In addition, although plant species identity can have a major effect on colonisation of artificial shelters (Wang et al. 2014), not controlling by plant species is advantageous when evaluating this colonisation for the entire plant community on a habitat scale. We only used plant species that did not bear any apparent type of indirect defence (e.g., domatia, extra-floral nectaries), flowers or fruits. For each plant, we selected five young, expanded leaves without apparent damage, ranging from 0.5 to 1.5 m height (N total = 100 leaves per habitat). Prior to the experiment, the leaves were gently cleaned with a soft brush to exclude all arthropods. The leaves were manually completely rolled from the adaxial to the abaxial surface in a manner similar to some engineering by a caterpillar (Figure 1). The leaves were kept rolled with stainless hairpins and stayed in the field during five consecutive days to allow colonisation and establishment of arthropod communities. Artificially rolled leaves from each plant were placed in separate Ziploc® bags (26.8 cm × 27.3 cm) and transported to the laboratory, where they were frozen in order to incapacitate any inhabitants and then carefully unrolled for arthropod collection. Leaf width did not differ among habitats (F = 2.45, P = 0.1). All sampled arthropods were transferred to vials with 70% ethanol for further identification at order level and according to their feeding guilds. Individuals belonging to the orders Coleoptera and Hemiptera were identified at family level and classified in a given guild depending on the predominant feeding habit of their respective family. Species richness (number of morphospecies) and abundance (number of individuals) per plant for all arthropods and for each guild were determined. The sampled arthropods were deposited in the entomological collection of the Laboratory of Evolutionary Ecology and Conservation of Tropical Forests of the National Autonomous University of Mexico.

Figure 1. Artificially rolled leaves simulating the engineering by arthropods. Photo credit: Karla Rodríguez-Hernández.
Data analysis
Generalised linear models were used to test whether richness and abundance of arthropods differed among habitats. Species richness and abundance of all arthropods and per guild were used as response variables, while habitat type (living fences, forest edge and old-growth forests) and average leaf width were used as explanatory variables. We applied a Poisson distribution of errors to the models; overdispersion was adjusted with a negative binomial distribution of errors, while underdispersion was adjusted with a ‘Quasi-Poisson’ when needed. The minimum adequate model was obtained by extracting non-significant terms (P > 0.05) from the full model (Crawley Reference Crawley2013). The package emmeans using Tukey’s method was used for posteriori comparisons (Lenth et al. Reference Lenth, Buerkner, Herve, Love, Riebl and Singmann2021). The explained variance (R2) of significant regressions was calculated using the following formula: Explained deviance = deviance H1/deviance H0. All statistical analyses were conducted with the R software (R Core Team 2020).
Results
In total, 450 arthropods from 125 morphospecies were sampled inside the artificially rolled leaves (Table 1; Supplementary material 1). On average, 7.5 arthropods were found per plant, with 7 arthropods per plant being the most frequent number (8 times), followed by 2 and 3 arthropods (7 times each; Supplementary material 2). Springtails (Collembola) were the most common arthropods found in our studied system (46.2%), followed by Araneae (14%), Blattodea (10.2%), Psocoptera (9.3%), Hymenoptera (5.1%), Thysanoptera (4.2%), Orthoptera (3.6%), Acari (2.2%), Coleoptera (2.2%) and Hemiptera (1.8%). Other orders such as Lepidoptera, Geophilomorpha, Diptera and Thysanura represented less than 1% of all sampled arthropods each. The greater number of morphospecies and individuals were collected at the forest edge habitat (58 morphospecies and 210 individuals), followed by forest interior (53 and 142), and living fences (45 and 98; Table 1). The taxa Collembola, Araneae, Blattodea and Psocoptera together represented 80% of all arthropods sampled and had a greater number of individuals in the rolled leaves at the forest edge, followed by forest interior, and were less abundant in living fences (Table 1).
Table 1. Richness (Rich.) and abundance (Abund.) of arthropod morphospecies sampled in artificially rolled leaves among habitats differing in vegetation structure in Los Tuxtlas tropical rainforest, Veracruz, Mexico. Guilds: F = Fungivores, D = Detritivores, H = Herbivores, Om = Omnivores, P = Predators, Un = Undetermined
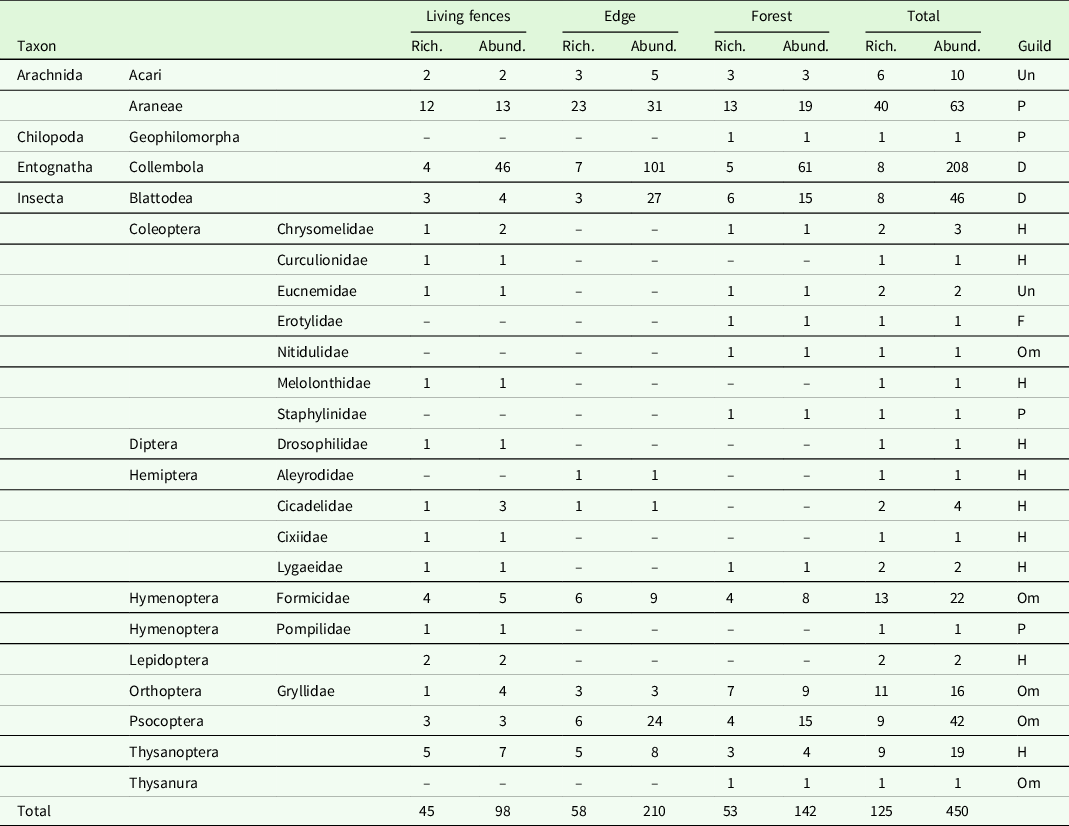
Arthropod species richness did not differ between forest edge and interior habitats, which showed approximately 1.5 times higher species richness compared with the living fence habitat (Table 2; Figure 2A). Detritivores and omnivores showed greater species richness in the forest interior compared to living fences (1.77 and 2.33 times greater, respectively), and there was no difference between edge and interior habitats, neither between edge and living fence habitats (Table 2; Figure 2A). Predators and herbivores did not differ in species richness among habitats (P > 0.05; Table 2; Figure 2A). Predator species richness was positively affected by average leaf width (P = 0.007; Table 2; Figure 3A). Arthropod abundance was 2.16 times greater in forest edge habitat compared to living fence habitat, and there was no difference between edge and interior habitats, neither between interior and living fence habitats (Table 2; Figure 2B). Detritivores and predators showed the same trend (2.6 and 2.21 times greater, respectively), while the abundance of omnivores did not differ between forest edge and interior habitats, which showed approximately 2.9 times higher abundance compared with the living fence habitat (Table 2; Figure 2B). Herbivores did not differ in abundance among habitats (P > 0.05; Table 2; Figure 2B). Predator abundance was positively affected by average leaf width (Table 2; Figure 3B).
Table 2. Results of generalised linear models showing the differences in species richness and abundance of arthropods sampled in artificially rolled leaves among habitats differing in vegetation structure (living fences, forest edge and interior) in Los Tuxtlas tropical rainforest, Veracruz, Mexico. The average width of the leaves per plant was used as a covariate

* Significant differences.

Figure 2. Species richness (a) and abundance of arthropods (b) (mean ± SE) per plant for different guilds sampled in artificially rolled leaves between habitats differing in vegetation structure in Los Tuxtlas tropical rainforest, Veracruz, Mexico. Means followed by the same letters do not differ statistically from each other (P > 0.05).

Figure 3. Relationship between average leaf width and species richness (a) and abundance (b) of predatory arthropods per plant sampled colonising artificially rolled leaves in Los Tuxtlas tropical rainforest, Veracruz, Mexico.
Discussion
Our results demonstrated that local environment context is an important factor that affects the colonisation of arthropods in leaf shelters. In general, the present data support the hypothesis that the colonisation of shelters by arthropod communities in tropical rainforests is stronger in more heterogeneous habitats. The number of individuals of the most representative arthropod taxa (i.e., Collembola, Araneae, Blattodea and Psocoptera) colonising the artificially rolled leaves was greater at the forest edge; a trend that was also observed for average arthropod abundance, and for detritivore and predator guilds. In addition, fewer arthropod species and individuals colonised the artificially rolled leaves in the simplest habitat, the living fences, and this trend was also observed for most arthropod guilds (i.e., detritivores, omnivores and predators).
Although no direct measurements of the arthropod communities in the studied habitats have been carried out, negative effects of simplification of natural ecosystems by anthropogenic activities on arthropod diversity have been reported extensively in the literature (e.g., Beng et al. Reference Beng, Tomlinson, Shen, Surget-Groba, Hughes, Corlett and Slik2016, Floren & Linsenmair Reference Floren and Linsenmair2001, Lichtenberg et al. Reference Lichtenberg, Kennedy, Kremen, Batary, Berendse, Bommarco, Bosque-Pérez, Carvalheiro, Snyder, Williams and Winfree2017, Novais et al. Reference Novais, Macedo-Reis, DaRocha and Neves2016, Schulze et al. Reference Schulze, Waltert, Kessler, Pitopang, Veddeler, Mühlenberg, Gradstein, Leuschner, Steffan-Dewenter and Tscharntke2004). A study conducted in the same study area for dung and carrion beetles found that forest fragments and forest–pasture edges had the highest number of individuals and species compared to living fences (Díaz et al. Reference Díaz, Galante and Favila2010). For arboreal arthropods, the key factors driving this pattern have been associated with more harsh conditions, decreased food resources and suitable microhabitats caused by the reduction in canopy cover, plant abundance and diversity in altered habitats (Floren & Linsenmair Reference Floren and Linsenmair2001, Lichtenberg et al. Reference Lichtenberg, Kennedy, Kremen, Batary, Berendse, Bommarco, Bosque-Pérez, Carvalheiro, Snyder, Williams and Winfree2017, Novais et al. Reference Novais, Macedo-Reis, DaRocha and Neves2016). Although harsh conditions have been suggested as a possible factor that could increase the magnitude of the ecosystem engineering by insects in seasonal tropical forests (Novais et al. Reference Novais, Calderón-Cortés, Sánchez-Montoya and Quesada2018, Vieira & Romero Reference Vieira and Romero2013), the variations in abiotic conditions among habitats in the evergreen tropical rainforest studied are expected to be considerably smaller compared to those between seasons in seasonal forests. In the case studied here, we suggest that the reduced vegetation structure of living fences may have determined a general decrease in arthropod diversity, and consequently, decreasing the probability of a shelter being encountered by a wandering arthropod. In addition, a reduced arthropod diversity in living fences may had led to decreased competition/predation pressures, therefore reducing the importance of the artificially rolled leaves as shelter sites in this habitat.
An opposite mechanism must be determining the highest abundance of arthropods occupying the rolled leaves in the forest edge, which have the most heterogeneous understory vegetation compared with the other habitats. In a study conducted in the Amazon rainforest, Fowler et al. (Reference Fowler, Silva and Venticinque1993) found significantly more individuals of flying insects at the forest edge than in the forest understory, and this pattern was consistent for the majority of insect orders throughout a year. Positive edge effects have also been reported for different arthropod groups belonging to different guilds, such as detritivores (Bogyó et al. Reference Bogyó, Magura, Nagy and Tóthmérész2015, De Smedt et al. Reference De Smedt, Wuyts, Baeten, De Schrijver, Proesmans, De Frenne, Ampoorter, Remy, Gijbels, Hermy and Bonte2016), herbivores (De Araújo & Espírito-Santo Filho Reference De Araújo and do Espírito-Santo Filho2012, Barbosa et al. Reference Barbosa, Leal, Iannuzzi and Almeida-Cortez2005, De Carvalho Guimarães et al. Reference De Carvalho Guimarães, Viana and Cornelissen2014, Wirth et al. Reference Wirth, Meyer, Leal, Tabarelli, Lüttge, Beyschlag and Murata2008) and predators (De Smedt et al. Reference De Smedt, Baeten, Proesmans, Van de Poel, Van Keer, Giffard, Martin, Vanhulle, Brunet, Cousins and Decocq2019). Previous studies have also demonstrated a greater predation pressure by both vertebrate and invertebrate predators in forest edges than in forest understory (Barbaro et al. Reference Barbaro, Brockerhoff, Giffard and van Halder2012, Drozdová et al. Reference Drozdová, Sipos and Drozd2013). Following these patterns, a study conducted by Richards & Coley (Reference Richards and Coley2007) in the lowland moist forest of Barro Colorado Island in Panama demonstrated that the densities of understory plants, young leaves, arthropod herbivores and predators were significantly more abundant in forest gaps than in interior. These authors also evaluated the predation rates on artificial caterpillars and found a higher predation pressure in forest gaps. In our study, as forest edge was also expected to have a greater arthropod diversity, leaf shelters in this habitat should be quickly occupied by an arthropod. Furthermore, as stronger density-dependent interactions are also expected at forest edges compared to the other habitats, a greater limitation of refuges from competitors or predators may have determined the higher colonisation of the artificially rolled leaves in this habitat.
Other studies have reported differences in the magnitude of the facilitation by shelter-building insects within forest ecosystems in time and space (Novais et al. Reference Novais, Calderón-Cortés, Sánchez-Montoya and Quesada2018, Novais et al. Reference Novais, Hernández-Ortiz, Rodríguez-Hernández, Quesada, Valenzuela, Fernandes and Aguirre-Jaimes2020b, Vieira & Romero Reference Vieira and Romero2013). Regarding temporal variations, Vieira & Romero (Reference Vieira and Romero2013) found that vegetation-dwelling arthropods colonising rolled leaves were more abundant in the dry season than in the rainy season in the seasonal Brazilian Atlantic Rainforest. These authors suggested that adverse climatic conditions may have led to an increase in the magnitude of the engineering effect in the dry season. Similarly, Novais et al. (Reference Novais, Calderón-Cortés, Sánchez-Montoya and Quesada2018) found a greater arthropod colonisation in abandoned branch cavities left by wood-boring beetles during the dry season in the understory of a Mexican topical dry forest compared to branches exposed in the rainy season. These authors suggested that this pattern was also influenced by a dramatic reduction in the availability of other shelters in the arboreal vegetation, since most trees shed their leaves as a drought-resistant mechanism in this ecosystem. Unlike our study, which was carried out in an evergreen tropical rainforest and showed a lower colonisation of shelters in the structurally simpler habitat and with harsher conditions (i.e., living fences), the great fluctuation of abiotic factors in seasonal ecosystems (e.g., tropical dry forests) seems to represent a major mechanism in determining variations in the magnitude of facilitation effect by shelter-building engineers. Regarding spatial variations, differences in the magnitude of the facilitation by shelter-building insects have been reported between forest strata. For example, as ant nesting sites represent a limited resource in the arboreal stratum, the importance of the facilitation by wood-boring beetles that create ant nesting cavities is increased in this stratum compared to the ground stratum (Novais et al. Reference Novais, Calderón-Cortés, Sánchez-Montoya and Quesada2018, Reference Novais, Hernández-Ortiz, Rodríguez-Hernández, Quesada, Valenzuela, Fernandes and Aguirre-Jaimes2020b).
The guild of predator arthropods, represented almost exclusively by spiders, was the only arthropod guild that responded significantly to the average leaf width, increasing in species richness and abundance in plants with wider leaves. In general, more complex habitats tend to have higher species richness and abundance of spiders (Diehl et al. Reference Diehl, Mader, Wolters and Birkhofer2013, Jiménez-Valverde & Lobo Reference Jiménez-Valverde and Lobo2007, Langellotto & Denno Reference Langellotto and Denno2004), although the response may differ depending on the spatial scale and foraging strategies (Gonçalves-Souza et al. Reference Gonçalves-Souza, Almeida-Neto and Romero2011, Halaj et al. Reference Halaj, Ross and Moldenke2000). At a small spatial scale, density and species number of hunting spiders (those species lacking webs such as jumping spiders, ambushers and runners) decrease with the simplification of branch structure (Halaj et al. Reference Halaj, Ross and Moldenke2000). A similar result was found for hunting spiders associated with bromeliads, which were more abundant in bromeliad species with more leaves (Gonçalves-Souza et al. Reference Gonçalves-Souza, Almeida-Neto and Romero2011). In our study, spiders may prefer plants with wider leaf shelters because they can favour a more stable microclimate and increased space availability for protection, molting or egg-laying. In addition, as many spiders also curl leaves to build shelters, they may save time and energy occupying preexisting structures (Fukui Reference Fukui2001; Pereira et al. 2020).
Conclusion
This experimental study demonstrated that local environmental context is an important factor affecting the colonisation of leaf shelters by arthropod communities. Our results showed that the colonisation of the shelters was stronger at the forest edge, likely because the new habitats provided through the artificially rolled leaves may have helped to minimise the greater potential of competition and predation pressures on resident arthropod species. Our study also showed a lower colonisation of the shelters in the structurally simplest habitat with harsher conditions, differing from those studies that found a greater magnitude of the facilitation by shelter-building insects in the dry seasons of seasonal forests, when the arboreal stratum is structurally simpler and conditions are very harsh. This difference suggests a greater importance of environmental conditions in determining the magnitude of the facilitation effect in seasonal forest ecosystems than in evergreen rainforests, where density-dependent interactions appear to play a more important role.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467421000523
Acknowledgements
We are grateful to Rosamond Ione Coates, head of the Biological Station Los Tuxtlas-UNAM for her support and provision of facilities to conduct this research. SN thanks CAPES for grant support while GWF thanks CNPq and Fapemig. Noemí Matías Ferrer (INECOL) helped with reviews of previous drafts.
This study was supported by grants from Universidad Nacional Autónoma de México (MQ, PAPIIT # IN212714-3); CONACyT (MQ, # 2009-131008 and # 155016); CONACYT-UNAM-UAGro to LANASE (MQ, 2015-LN250996, 2016-LN271449, 2017-LN280505); and Programa Ibero Americano de Ciencia y Tecnología para el Desarrollo RED CYTED SEPODI (MQ, 417RT0527).
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical statement
Not applicable.







