INTRODUCTION
Leaf-cutting ants (Atta spp. and Acromyrmex spp., Formicidae, Hymenoptera) are prevalent herbivores and dominant invertebrates of tropical and subtropical America, as they are able to harvest up to 15% y−1 of the standing leaf crop in their foraging areas (Urbas et al. Reference URBAS, ARAÚJO, LEAL and WIRTH2007, Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003). In tropical forests, however, the overall effects of leaf-cutting ants (especially Atta spp.) may go far beyond occasional simple foliage removal. Much of their influence can be attributed to the construction and maintenance of their nests, which often reach more than 100 m2 in surface area (Cherrett Reference CHERRETT, Lieth and Werger1989). These prodigious structures has been argued to (1) enhance soil nutrient availability, (Farji-Brener & Silva Reference FARJI-BRENER and SILVA1995, Moutinho et al. Reference MOUTINHO, NEPSTAD and DAVIDSON2003, Sternberg et al. Reference STERNBERG, PINZON, MOREIRA, MOUTINHO, ROJAS and HERRE2007); (2) improve soil penetrability relative to non-nest soils (Moutinho et al. Reference MOUTINHO, NEPSTAD and DAVIDSON2003); (3) promote active removal of leaf litter (Weber Reference WEBER1972); and (4) create ‘nest clearings’ or vegetation gaps above and immediately around Atta nest sites (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Farji-Brener & Illes Reference FARJI-BRENER and ILLES2000). Because of their diverse effects on the vegetation, leaf-cutting ants have been denoted as keystone species (Fowler et al. Reference FOWLER, PAGANI, SILVA, FORTI and SAES1989) and ecosystem engineers (Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003).
Ecosystem engineering is a scale-dependent process generating shifts in the resource availability to other organisms, which, in the case of leaf-cutting ants (LCAs), has been well documented at the spatial scale of ant nests, particularly in terms of plant recruitment while colonies are active (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011a) or after nest abandonment (Bieber et al. Reference BIEBER, OLIVEIRA, WIRTH, TABARELLI and LEAL2011). In contrast, the broader spatial scale of the foraging area has been neglected. Through a well-formed system of foraging trails (e.g. covering up to 9050 m2; Silva et al. Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009), these ants harvest large quantities of plant material for cultivating their fungus garden (Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003). By creating small foliage gaps through the removal of leaves in the canopy, LCA herbivory causes a patchy increase in light transmittance and thus an increased frequency/variability of light availability on the forest floor (Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003). Such increased heterogeneity in the light regime has been proposed to affect plant recruitment and species coexistence via niche partitioning (Svenning Reference SVENNING2000). This perspective is consistent with the fact that light availability is one of the most important ecological factors affecting the growth, survival and establishment of seedlings and saplings (Augspurger Reference AUGSPURGER1984, Nicotra et al. Reference NICOTRA, CHAZDON and IRIARTE1999).
In addition to herbivory in the forest canopy, LCA activities in their foraging areas frequently include both massive and occasional seed harvesting (Dalling & Wirth Reference DALLING and WIRTH1998, Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007), and seedling/sapling defoliation (Rao et al. Reference RAO, TERBORGH and NUNEZ2001, Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007, Vasconcelos & Cherrett Reference VASCONCELOS and CHERRETT1997), particularly close to foraging trails (Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007). In synthesis, many mechanisms allow us to expect that LCA activities impose effects at the scale of foraging areas, particularly in terms of plant recruitment. Such effects are likely to be more evident across regenerating forest patches, in which LCAs may attain increased density and reduced foraging range (Silva et al. Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009). Here we address seedling-assemblage attributes, seed germination and seedling survival across foraging sites of Atta cephalotes colonies inhabiting second-growth patches (25–47 y old) of Atlantic forest in order to identify potential effects from LCA activities on plant recruitment. In particular, we examine the hypothesis that seedling assemblages in foraging areas are impoverished and taxonomically distinct from those inhabiting forest areas free of ant activity. We also expected reduced seed germination and seedling survival in foraging areas.
METHODS
Study area
The study was carried out at Usina Serra Grande – a 667-km2 privately held sugarcane plantation in the state of Alagoas, north-eastern Brazil (8°30′S, 35°50′W). Soils include yellow-red latosols and podzols. Annual rainfall is approximately 2000 mm, and the dry season (< 60 mm mo−1 rainfall) extends from November to February (Santos et al. Reference SANTOS, PERES, GRILLO, ALVES-COSTA and TABARELLI2008). The Serra Grande landscape still retains nearly 9000 ha of evergreen and semi-deciduous lowland forests (< 400 m asl), including the 3500-ha Coimbra Forest – the largest and best-preserved private forest patch in north-east Brazil (Santos et al. Reference SANTOS, PERES, GRILLO, ALVES-COSTA and TABARELLI2008). Fabaceae, Lauraceae, Sapotaceae, Euphorbiaceae, Chrysobalanaceae and Lecythidaceae are the most species-rich families (Grillo et al. Reference GRILLO, OLIVEIRA, TABARELLI, Porto, Almeida-Cortez and Tabarelli2006).
Seedling assemblages
We assessed Atta cephalotes colonies across 15 second-growth patches (ranging in size from 0.53 to 7.4 ha, with a total area of 43.3 ha), which consisted of formerly clear-cut sites within flat, core areas of the Coimbra Forest, our study spot (Figure 1). Although embedded in the same matrix of mature forest, these second-growth patches were apart from each other (i.e. spatially independent patches) and represented well-known 25–47-y-old chronosequence created by the abandonment of slash-and-burn gaps following 5–10 y of subsistence agriculture (maize, cassava, beans) as described elsewhere (Santos et al. Reference SANTOS, PERES, GRILLO, ALVES-COSTA and TABARELLI2008, Silva et al. Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009). Within each patch we selected a single A. cephalotes colony, resulting in a set of 15 similar-sized colonies (20.5 ± 2.7 m2; mean ± SE). The average distance between colonies was 1477 ± 94 m (range = 34–3400 m; Figure 1). Colony foraging areas were mapped via a single, monthly observation during the peak phase of daily harvesting activity by measuring compass bearings and lengths of all quasi-linear trail segments. This procedure was repeated throughout 1 y (2005), resulting in 12 estimates per colony, which were further collapsed into a single annual measure of foraging area per colony as described by Silva et al. (Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009) and Kost et al. (Reference KOST, GAMA, OLIVEIRA, KNOCH and WIRTH2005). The resulting annual foraging area was 5870 ± 618 m2 (mean ± SE) and tripled across the regeneration chronosequence ranging from 3010 m2 in a 25-y-old to 9050 m2 in a 47-y-old patch (Silva et al. Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009).

Figure 1. Map of the Usina Serra Grande landscape in north-east Brazil showing the location of 15 second-growth patches sampled to evaluate seedling assemblage attributes, seed germination and seedling survival across foraging sites of Atta cephalotes colonies. Within each patch we selected a single Atta cephalotes colony. The hatched polygon represents the Coimbra forest with the sampled second-growth patches (filled circles); white area indicates the matrix of sugar-cane monoculture.
During a 1-y period (2006), seedlings were recorded at monthly intervals in 1-m2 plots disposed within foraging zones and control areas, i.e. forest sites in the vicinity of, but clearly outside a given foraging zone (c. 30–50 m away). Control areas were pre-assigned based on the previous assessments of annual foraging areas (Silva et al. Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009) and monitored monthly to ensure that they remained LCA-free throughout the study period. Plot location in both habitats varied monthly. Foraging-zone plots were located at random distances from the ant nest, 1 m away from major active ant trails to avoid foraging sectors without ant activity (Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003). Nest-to-plot distances averaged 46.0 ± 56.2 (mean ± SD) and mean inter-plot distance was 36 ± 55 m. Similarly, control plots were randomly placed into the corresponding control areas with a minimum distance of 20 m to each other. This design resulted in 12 plots per colony per treatment (foraging zone and control area), yielding a total sampling effort of 180 m2 per treatment. Seedlings were defined as individuals up to 50 cm tall, including shrub, tree, liana and palm species. Individuals with evidence of resprouting or subterranean stems (stolons) were disregarded. Seedlings were assigned to species with the help of a local parataxonomist and through comparisons with specimens deposited in the Federal University of Pernambuco (Brazil), UFP Herbarium (voucher numbers 34,445–51,604 for the study site) and in the Serra Grande seedling and fruit/seed collection stored at the Plant Ecology Laboratory of the Federal University of Pernambuco as previously adopted by Santos et al. (Reference SANTOS, PERES, GRILLO, ALVES-COSTA and TABARELLI2008).
Seed germination and seedling mortality
During the 2007 rainy season, we collected 3675 fresh seeds from three tree species in the Coimbra Forest: Tapirira guianensis Aubl. (Anacardiaceae, 1350 seeds), Pouteria sp. (Sapotaceae, 1200 seeds) and Simarouba amara Aubl. (Simaroubaceae, 1125 seeds). Seeds were buried individually (1 cm deep) across five distances from the nest edge (3, 6, 9, 12 and 15 m) of our 15 focal colonies. Seedling germination and seedling mortality were monitored during a 17-mo period based on weekly censuses. Dead seedlings were assigned to six sources of mortality: dried out, herbivory by LCAs, other herbivores, physical damage, failed radicle attachment and unknown reason.
Data analysis
Between-habitat differences of seedling density and species richness were examined using the following approach. We first ran analyses of covariance (ANCOVA; Sokal & Rohlf Reference SOKAL and ROHLF1995), considering habitat as a factor and age of second-growth stands as covariate. As stand age proved to be not significant (P > 0.05), habitats were further compared via paired t-tests. We also estimated species richness of seedlings per habitat (i.e. foraging-zone plots vs. control plots) using mean species–area accumulation curves and Chao's non-parametric estimators: Sobs, Chao-1 and Chao-2, with 95% confidence intervals (Chazdon et al. Reference CHAZDON, COLWELL, DENSLOW, GUARIGUATA, Dallmeier and Comiskey1998). These estimators are based on the incidence of species and have been described in the literature as the best estimators of species richness in tropical forests (Chazdon et al. Reference CHAZDON, COLWELL, DENSLOW, GUARIGUATA, Dallmeier and Comiskey1998). Here we particularly adopted them to verify the consistency of between-habitat differences relative to species richness (foraging areas vs. control plots). Species accumulation curves were obtained by randomizing seedling records from 180 1-m2 plots, which were obtained by sampling, once monthly over 1 y, 15 foraging zones and their respective control areas. We performed 1000 randomizations of the total dataset using the software EstimateS 8.2. We also conducted rarefaction analysis of total species richness considering a standard sample of 597 seedlings (i.e. the maximum number of seedlings recorded in one of the second-growth stands) in order to compare habitats based on the same number of individuals.
To examine species similarity among plots, we performed a non-metric multidimensional scaling (NMDS) ordination (via PRIMER software) of all 30 plots (collapsing all monthly records) using their Bray–Curtis similarity matrix of species composition (Krebs Reference KREBS1999). Species abundance data were square-root-transformed and standardized in order to avoid any bias resulting from highly abundant species and differences in sample sizes (i.e. seedling density per plot). To examine the relationships between habitat type and patterns of species similarity between plots, habitat was considered as a factor in the ANOSIM procedure. Additionally, we performed an indicator species analysis (Dufrêne & Legendre Reference DUFRÊNE and LEGENDRE1997) based on two groups of seedling plots identified by both NMDS ordination and ANOSIM test: one consisting exclusively of foraging zone plots and another of control plots. The influence of indicator species on patterns of taxonomic similarity between habitats was investigated via a SIMPER analysis. Finally, differences in seed germination and seedling mortality relative to nest distance and age of second-growth stands were examined via Survival Analyses adopting Cox's proportional hazard models, while between-species differences in terms of seedling-mortality causes were examined via ANOVAs (one ANOVA per each source of mortality). All percentages were arcsine-transformed prior to analyses (Sokal & Rohlf Reference SOKAL and ROHLF1995). NMDS, ANOSIM and SIMPER procedures were performed with PRIMER 5, ANCOVAs, ANOVAs and Survival Analyses via ESTATISTICA 8.0.
RESULTS
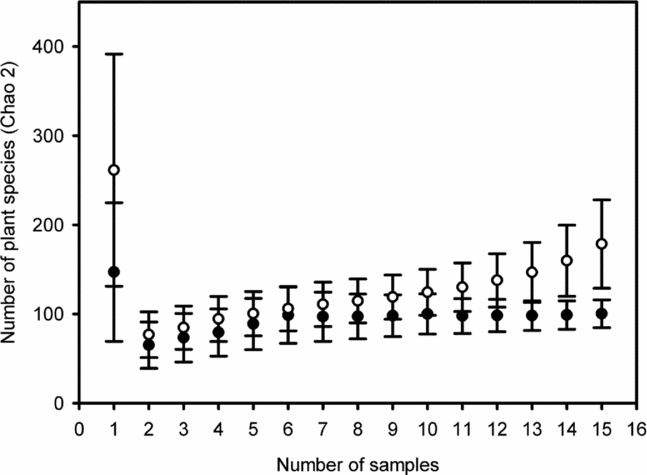
A total of 1862 seedlings from 108 plant species were recorded across both habitats – 597 (32%) seedlings and 74 (69%) species in foraging-zone plots and 1265 (68%) seedlings and 94 (87%) species in control plots. Seedling assemblage attributes (density and richness) were not affected by the age of second-growth stands (F = 0.94, df = 1, P = 0.333 and F = 2.79, df = 1, P = 0.095, respectively). However, average seedling density in foraging-zone plots (3.31 ± 0.23 m−2; mean ± SE) was half that in control plots (7.02 ± 0.44 m−2; t = 8.03, df = 17, P < 0.0001), and average seedling richness also differed: 2.09 ± 0.10 species in foraging zones vs. 3.22 ± 0.13 species in control plots (t = 8.37, df = 17, P < 0.0001). At the community level, species–area accumulation curves revealed significant differences in terms of total species richness regardless of the estimator adopted (Figure 2). In foraging zones, total expected richness estimates varied from 74 (Sobs) to 139 (Chao-2); whereas it achieved 80.6 (Sobs) and 299 (Chao-2) in control sites; Chao-1 offered intermediate scores of species richness. However, the rarefaction method for a standard set of 597 seedlings indicated a similar pattern of tree species in the two habitats.
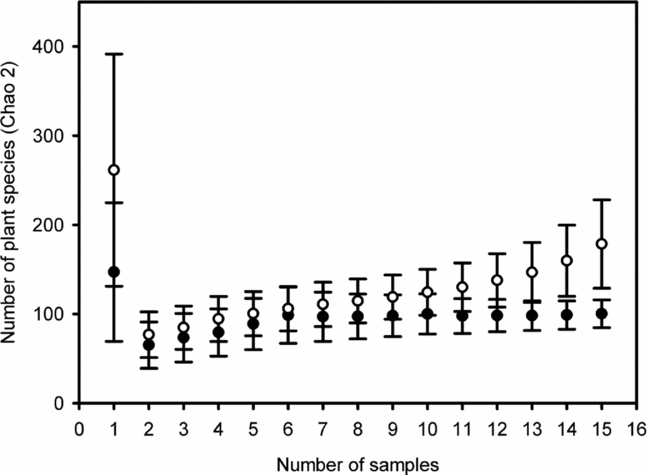
Figure 2. Species–area accumulation curves (Chao-2) in foraging zone plots of 15 Atta cephalotes colonies (filled circles) and in control plots (open circles) recorded at 15 second-growth stands of Atlantic forest in Serra Grande, north-east Brazil. 12 plots (1 m2) were surveyed per colony and per treatment (foraging and control plots), yielding a total sampling effort of 180 m2 for each group. Bars indicate 95% confidence intervals.
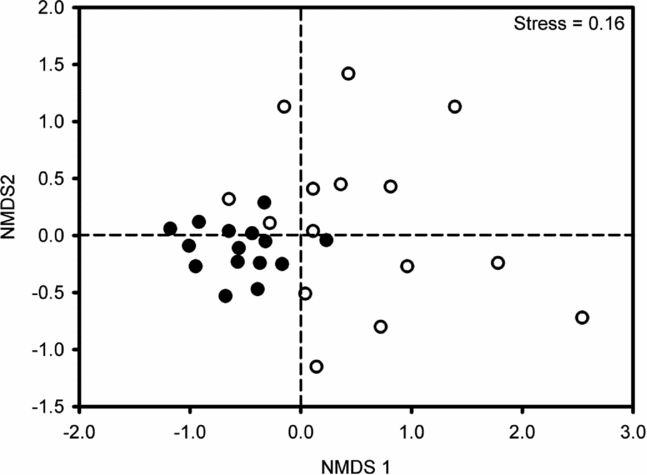
NMDS ordination of seedling plots based on species similarity and abundance resulted in two consistent and clearly segregated clusters: one formed by the 15 foraging-zone plots and another formed by control plots (Figure 3). Plot ordination was well supported by low stress levels of 0.16 and the ANOSIM detected a significant effect of habitat type (R = 0.372, P = 0.001). Moreover, between-plot species similarity was much higher among those located in the foraging zones (87.3 ± 0.21) than in control plots (81.6 ± 0.31; F = 227, df = 1, P < 0.0001). The indicator species analysis generated a higher indicator value (IV) for three species in the control plots: Thyrsodium spruceanum Benth. (Anacardiaceae), Myrcia sylvatica (G.Mey.) A. DC. (Myrtaceae) and Erythroxylum mucronatum Benth. (Erythroxylaceae), while it failed to detect any indicator species across foraging-zone plots. These three species were among the ten best plot-discriminating species, which accounted for a cumulative contribution of 28.6% on average between-plot species similarity (Table 1, SIMPER analysis of Bray–Curtis dissimilarity).
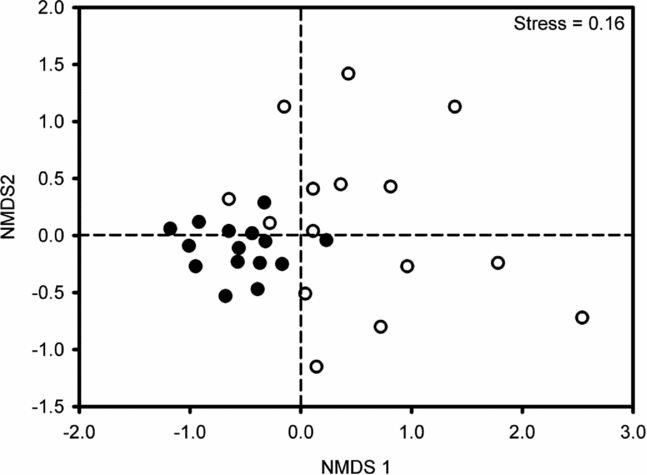
Figure 3. Non-metric multidimensional scaling ordination of seedling assemblages across 30 plots (360 m2) based on Bray–Curtis similarity coefficients: 15 foraging-zone plots (filled circles) and 15 control plots (open circles) located in second-growth stands of Atlantic forest in Serra Grande, north-east Brazil.
Table 1. Scores of a SIMPER analysis indicating the contribution of tree species to the average Bray–Curtis dissimilarity between foraging-zone plots (FZP) and control plots (CP) in Atlantic forests patches at Serra Grande, north-east Brazil. The following values are given: average abundance observed in foraging-zone and control plots, average dissimilarity ± SD for each species and proportional contribution of each species to the dissimilarity index between FZP and CP. The list of species contains only the 10 tree species that contributed most to the dissimilarity between FZP and CP, accounting for 28.6% of the overall dissimilarity.

After a 1-y period a total of 552 seeds germinated but total germination was highly variable among tree species, ranging from 15.3% in Simarouba amara to 43.2% in Pouteria sp. For these two tree species, seed germination did not correlate significantly to either distance from ant nests or age of second-growth stands, while in the case of Tapirira guianensis total germination correlated positively (Beta = 0.19, Wald statistic = 13.4, P = 0.0002) with distance from ant nests (χ2 = 14.1, df = 2, P < 0.001 for the whole model). After 1 y, total seedling mortality achieved c. 93% in S. amara, but lower scores were recorded in T. guianensis (57%) and Pouteria sp. (54%). Similar to germination, seedling mortality was not correlated to distance from nests or age of second-growth stands in the case of S. amara and Pouteria sp. However, mortality among T. guianensis seedlings correlated positively with distance from ant nests (Beta = 0.23, Wald statistic = 9.87, P = 0.016), but negatively with the age of second-growth stands (χ2 = 15.5, df = 2, P = 0.0001 for the whole model). Finally, tree species exhibited differential susceptibility to mortality factors in all studied species. In T. guianensis seedlings experienced high mortality caused by herbivores, probably gall-inducing arthropods, while most S. amara and Pouteria sp. seedlings died from desiccation (Figure 4). Moreover, LCAs killed a total of 76 seedlings through defoliation and were thus responsible for a proportional mortality of 3.1% in T. guianensis and 13.6% in S. amara.

Figure 4. Average seedling mortality within six categories of death causes in the species Tapirira guianensis, Simarouba amara and Pouteria sp. across second-growth stands of Atlantic forest at Serra Grande, north-east Brazil. Letters above bars (± SE) denote significant differences at the 5% level (ANOVAs, LSD post hoc tests).
DISCUSSION
Our results suggest that foraging zones of A. cephalotes ants in the Atlantic forest support less dense and impoverished seedling assemblages at local and habitat scale. With a reduction of one third of species richness, such assemblages are taxonomically distinct and lack species that are characteristic of ant-free forest zones (e.g. Thyrsodium spruceanum, Myrcia sylvatica and Erythroxylum mucronatum). In fact, seedling assemblages in ant foraging zones were floristically much more similar to each other than in ant-free zones, and formed a discrete species cluster; i.e. a clear signal of floristic convergence. Finally, shifts in seedling assemblages across foraging zones may partially result from (1) seedling defoliation by ants, and (2) reduced seed germination close to ant nests as observed in Tapirira guianensis. We addressed seedling assemblages through a whole year by shifting plot location every month within each of the 15 foraging zones located in differently aged second-growth stands (25–47 y old) immersed in a single large forest remnant and exposed to the same pool of plant species. Thus, the patterns uncovered here, cannot be considered ephemeral or related to microhabitat selection by colony founding ant queens (Vieira-Neto & Vasconcelos Reference VIEIRA-NETO and VASCONCELOS2010). In other words, our results suggest that leaf-cutting ants act as an important selective filter for seedling establishment, affecting seedling density, species richness and taxonomic composition.
LCAs are expected to interfere directly and indirectly on the nature of seedling assemblages via a large set of activities and processes: reduced seed production due to intense herbivory on adult trees (Rockwood Reference ROCKWOOD1973), seed harvesting (Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007, Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003), seedling defoliation related to nest-clearing activities (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011b) or collection of plant material to feed the symbiotic fungus (Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007, Vasconcelos & Cherrett Reference VASCONCELOS and CHERRETT1997). Leaf-cutting ants may directly cut the leaves and/or epicotyls of seedlings during foraging, particularly close to nests and in the vicinity of trunk trails (Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007, Vasconcelos & Cherrett Reference VASCONCELOS and CHERRETT1997). In most Atta species this behaviour seems less common than foraging on large canopy trees (Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003), but we observed seedling defoliation by A. cephalotes apparently in the context of foraging trail clearing activities at our study site. Likewise, A. sexdens has been considered the most important herbivore operating in abandoned pastures of the Amazon region by promoting defoliation with detectable impacts on seedling survivorship and growth (Nepstad et al. Reference NEPSTAD, UHL, PEREIRA and SILVA1996), while Rao et al. (Reference RAO, TERBORGH and NUNEZ2001) attributed the reduced plant recruitment on small forest islands (0.3–3.0 ha) to high levels of Atta herbivory in Lago Guri, Venezuela. Massive defoliation of seedlings by LCA has also been reported from the Atlantic forest (Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007), where it was primarily concentrated on seedlings recruited close to nest entrances. Again, it is important to note that the ants do not necessarily use seedlings as fungus garden substrate but rather remove them while clearing newly established foraging trails (Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003).
Although seedling defoliation imposed by LCAs partially explains the differences among seedling assemblages documented in this study, we would like to speculate about additional mechanisms that act complementarily. LCAs are largely associated with the construction and maintenance of their nests, which can reach 100 m2 or more in surface area (Cherrett Reference CHERRETT, Lieth and Werger1989). These prodigious structures have been argued to promote the active removal of leaf litter, resulting in bare ground on and in the vicinity of nests, thus creating nest clearings or vegetation gaps above and immediately around Atta nest sites, where understorey vegetation growing on or overhanging the immediate nest surface is constantly cleared (Farji-Brener & Illes Reference FARJI-BRENER and ILLES2000). These gaps increase the amount of light passing through the foliage and reaching the understorey, and affect microclimatic conditions such as soil temperature, moisture (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011a) and soil penetrability (Bieber et al. Reference BIEBER, OLIVEIRA, WIRTH, TABARELLI and LEAL2011). Such intense physical alterations above the nests and their vicinities (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011a, Reference MEYER, LEAL, TABARELLI and WIRTH2011b) may potentially operate as a filter for seed germination and seedling recruitment; i.e. they represent an inhospitable environment for: (1) plant species bearing ant-preferred seedlings; (2) small-seeded tree species without resprouting abilities, (3) light-sensitive, shade-tolerant species; and (4) plant species whose seeds require undisturbed habitats for better germination and establishment (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011b, Silva et al. Reference SILVA, LEAL, WIRTH and TABARELLI2007).
Whatever the consequences of leaf-cutting ant activities on the nature of seedling assemblages, their intensity and spatial extent depends on both nest density and size of foraging zones. It has already been documented that several LCA species tend to proliferate in human-disturbed habitats such as second-growth stands or edge and road-affected habitats (Dohm et al. Reference DOHM, LEAL, TABARELLI, MEYER and WIRTH2011, Meyer et al. Reference MEYER, LEAL and WIRTH2009, Silva et al. Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009, Vasconcelos & Cherrett Reference VASCONCELOS and CHERRETT1995, Vasconcelos et al. Reference VASCONCELOS, VIEIRA-NETO, MUNDIM and BRUNA2006, Wirth et al. Reference WIRTH, MEYER, ALMEIDA, ARAUJO, BARBOSA and LEAL2007). But in addition to increased colony densities in disturbed habitats, the patterns of ant activities are expected to differ from those documented across undisturbed habitats due to a higher availability of palatable pioneer plants (Farji-Brener Reference FARJI-BRENER2001, Peñaloza & Farji-Brener Reference PEÑALOZA and FARJI-BRENER2003, Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003), and lower pressure imposed by natural enemies (Almeida et al. Reference ALMEIDA, WIRTH and LEAL2008, Rao Reference RAO2000). For example, Wirth et al. (Reference WIRTH, MEYER, ALMEIDA, ARAUJO, BARBOSA and LEAL2007) and Urbas et al. (Reference URBAS, ARAÚJO, LEAL and WIRTH2007) observed that A. cephalotes colonies are able to multiply their nest density, scale down their foraging ranges by a factor of 2.5 (0.9 vs. 2.3 ha per colony y−1), and more than double their herbivory pressure in Atlantic forest edge zones (14.3% vs. 7.8% of standing leaf crop). Furthermore, Silva et al. (Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009) estimated that the ants accessed up to 100% of the area covered by early secondary patches (25 y old), whereas they explored only a small portion of the available forest (16–23%) in mid-successional patches (42–47 y old). It is thus not surprising considering the second-growth scenario of the present study that we documented shifts in seedling abundance, species richness and composition on those sites exposed to LCAs activities.
While manifold impacts of LCAs on plant assemblages have previously been documented in tropical forests, particularly on nest sites and their close vicinity (Bieber et al. Reference BIEBER, OLIVEIRA, WIRTH, TABARELLI and LEAL2011, Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Garrettson et al. Reference GARRETTSON, STETZEL, HALPERN, HEARN, LUCEY and MCKONE1998, Hull-Sanders & Howard Reference HULL-SANDERS and HOWARD2003, Meyer et al. 2011a), this study is the first to document tangible shifts at the spatial scale of ant foraging areas as initially proposed by Wirth et al. (Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003). In fact, our seedling plots were located between 2 and 414 m from nest mounds (46 m on average). Impoverished and floristically convergent seedling assemblages at such spatial scales reinforce the notion that LCAs can operate as ecosystem engineers at spatial scales far beyond ant nests (Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011a, Wirth et al. Reference WIRTH, BEYSCHLAG, RYEL, HERZ and HÖLLDOBLER2003). In addition to seedling defoliation (Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011b), it is reasonable to propose that environmental shifts resulting from nest-activities and foliage removal from the canopy alter resource availability (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011a) and may act as potential environmental filters for particular plant guilds (i.e. reduce the recruitment of sensitive tree and shrub species). Such a cascade has already been documented across nest vicinities in edge-affected habitats (Corrêa et al. Reference CORRÊA, SILVA, WIRTH, TABARELLI and LEAL2010, Meyer et al. Reference MEYER, LEAL, TABARELLI and WIRTH2011b). In synthesis, our results suggest that LCAs modify seedling assemblages at broader spatial scale than previously reported. The consequences of such a disturbance regime still deserve further investigation as it may alter all attributes of plant assemblages attending to forest dynamic; i.e. impoverished and convergent assemblages. This is particularly relevant in the case of human-modified landscapes, where LCAs attain increased colony densities and their activities are able to cover the whole area of forest patches (Silva et al. Reference SILVA, BIEBER, LEAL, WIRTH and TABARELLI2009).
ACKNOWLEDGEMENTS
We thank ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (CNPQ), ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior’ (CAPES-PNPD), ‘Deutscher Akademischer Austausch Dienst’ (DAAD) for funding this study. IRL and MT also thank CNPq for research grants. During manuscript preparation, PSDS was supported by a post-doctorate fellowship from ‘Fundação de Amparo à Pesquisa do Estado da Bahia’ (FAPESB). We thank A. G. Bieber for helpful comments on the manuscript.