INTRODUCTION
In comparison with water and soil, light is the most important resource that influences the abundance, growth and survival of plants in tropical forest (Chazdon et al. Reference CHAZDON, PEARCY, LEE, FETCHER, Mulkey, Chazdon and Smith1993, Larcher Reference LARCHER2003, Valladares & Niinemets Reference VALLADARES and NIINEMETS2008). Based on light requirements, tropical tree species have been generally classified as pioneer species (light-demanding or gap-dependent species) that can establish and grow in tree-fall gaps where light levels are high and non-pioneer species (shade-tolerant species) that can establish and persist in the shade (Denslow Reference DENSLOW1980, Poorter & Arets Reference POORTER and ARETS2003). Non-pioneer species may be the largest group of the total set of tropical forest species and this group has been subdivided into truly shade-tolerant species and non-pioneer light-demanding species with intermediate light requirements (Montgomery Reference MONTGOMERY2004, Poorter & Arets Reference POORTER and ARETS2003).
However, the assignment of the species to ecological groups may be arbitrary because species responses to shade can be complex and few quantitative data are available on the variation in physiology and growth in response to light levels, particularly within shade-tolerant species (Montgomery Reference MONTGOMERY2004, Valladares & Niinemets Reference VALLADARES and NIINEMETS2008).
Canopy disturbance occasioned by falling trees and forest edges causes high light heterogeneity in tropical forest and plants growing in the understorey may be able to change their physiology and growth in response to spatial and temporal changes in light availability (Chazdon et al. Reference CHAZDON, PEARCY, LEE, FETCHER, Mulkey, Chazdon and Smith1993). In comparison with leaves produced under high light levels, leaves grown in low-light environments have high leaf area, specific leaf area and stomatal density, and usually show low light compensation points, carboxylation efficiency and leaf nitrogen content per unit area, that result in reduced dark respiration rate, low net CO2 assimilation and electron transport rates at light saturation and low light saturation points (Larcher Reference LARCHER2003, Valladares & Niinemets Reference VALLADARES and NIINEMETS2008). Leaf trait variation in sun versus shade environments can be different among species and the levels of phenotypic plasticity can be important to explain the coexistence of species in tropical forest (Laurans et al. Reference LAURANS, MARTIN, NICOLINI and VINCENT2012).
This paper examines ecophysiological correlations between three tree fern species of three different genera and the understorey environment in which the taxa normally are found in a Mexican cloud forest. Alsophila firma inhabits riparian habitats and performs like a species of mature forest because it exhibits slow growth, Cyathea divergens is widely distributed in the forest and Lophosoria quadripinnata grows best on the edges of the forest (Arens & Sanchez-Baracaldo Reference ARENS and SANCHEZ-BARACALDO1998, Bernabe et al. Reference BERNABE, WILLIAMS-LINERA and PALACIOS-RIOS1999, Mehltreter & García-Franco Reference MEHLTRETER and GARCÍA-FRANCO2008). Despite their different preferences in terms of microhabitat, the three fern species can grow together in open and closed understorey environments in forest. If the response of species to canopy openness is a significant source of niche differentiation in tropical forest (Bazzaz Reference BAZZAZ1979, Denslow Reference DENSLOW1980, Engelbrecht et al. Reference ENGELBRECHT, KURSAR and TYREE2005, Poorter & Arets Reference POORTER and ARETS2003), then it could be expected that the distribution of the three coexisting tree fern species in the forest was associated with canopy openness and leaf physiological characteristics. We tested the hypothesis that the species with preferred distribution towards shady places (Alsophila firma) would show leaf traits that were intended to deal with the lower end of the understorey light gradient (i.e. lower maximum electron transport, carbon assimilation, dark respiration, saturation light intensity and greater specific leaf area), while the broadly distributed species (Cyathea divergens, Lophosoria quadripinnata) would show leaf features associated with major tolerance of sunny environments (i.e. thicker leaves, high predawn water potential, higher density and smaller stomata), and the species with preferred distribution towards forest edges (Lophosoria quadripinnata) would have the lowest tolerance of low light.
METHODS
Study site and species
This study was conducted in a 32-ha cloud-forest fragment in the private El Riscal reserve (19°28′55″N, 96°59′47″W, 1600 m asl), near the city of Xalapa, Veracruz. The site is located on the eastern slopes of the Sierra Madre Oriental, an extensive massif that occupies the eastern region of Mexico. During the fieldwork (June 2009–May 2010) on a weather station located 4 km away from the study site, the precipitation was 2105 mm, with rainfall mainly between June and October. The average maximum and minimum air temperature in the coldest month (January) was 16.8 °C and 8.7 °C, while these values were 26.8 °C and 14.6 °C in the hottest month (May). The most abundant trees on the site are Carpinus caroliniana Walter, Clethra mexicana DC., Liquidambar styraciflua L., Ostrya virginiana (Mill) K. Koch, Platanus mexicana Moric and Quercus spp. Alsophila firma (Baker) D.S. Conant and Cyathea divergens Kunze var. tuerckheimii (Maxon) R.M. Tryon (Cyatheaceae) tree ferns grow up to 12 m tall and are distributed in the neotropics, mainly in cloud forests or rain forests. Lophosoria quadripinnata (J.F. Gmel.) C. Chr. (Dicksoniaceae) grows 1–2 m tall and is distributed in cloud and temperate forests from Mexico to Argentina (Mickel & Smith Reference MICKEL and SMITH2004).
Tree fern distribution and field light gradient
On a slope with a north-east exposure, five perpendicular transects were established from the crest to the stream. They were 4 m wide and 70, 70, 80, 110 and 120 m long. The lengths of the transects were chosen in response to the distance between the stream and the crest of the slope. Each transect was divided into quadrats 10 m long. Canopy openness was estimated from 10h00 to 15h00 on 26 November 2009 as the proportion of the photosynthetic photon flux density (PPFD) at a totally exposed site (Machado & Reich Reference MACHADO and REICH1999). Two LI-190SA light sensors (Li-Cor Inc. Lincoln, Nebraska, USA) were plugged into a datalogger that was programmed to take a measurement every minute at 1 m in height in each one of the 45 quadrats, as well as in a site that was totally exposed outside the forest. The number and height of the sporophytes > 0.2 m of each species were recorded in each quadrat. The morphological similarities among plants < 0.2 m in the two species of Cyatheaceae did not allow us to differentiate between them unequivocally.
Physiological leaf response to canopy openness
Five individuals from each species that were growing in two sites with contrasting light availabilities were selected. One site was under closed canopy, and the other was under open canopy. Individuals from the three species under closed canopy were located on the same place near the stream; those individuals growing under open canopy were located in three populations on the top of the slope. Special care was taken to select only individuals with a simple trunk. The height of these individuals ranged between 0.5 and 1.0 m in A. firma and C. divergens and between 0.2 and 0.5 m in L. quadripinnata. PPFD, air temperature and relative humidity were recorded at a height of 1 m via an LI-190SA and an LI-1000–16 (Li-Cor, Lincoln, Nebraska, USA), as well as HMP45A (Vaisala Inc., Finland) sensors that were connected to an LI-1000 datalogger at the closed-canopy site during the entire study. Under open-canopy sites, climatic data were recorded in various periods; however, the light received by the populations growing in the open-canopy sites was not significantly different (ANOVA, P > 0.05).
Gas exchange
The maximum CO2 assimilation rate at light saturation (Amax), photosynthetic flux density to 90% of Amax (ALSP) and dark respiration rate (Rd) were estimated during the morning. This was done with a portable infrared gas analyser with a leaf chamber and LED red light source (LI-6400, Li-Cor, Lincoln, Nebraska, USA). Red light is a suitable light source for photosynthesis in leptosporangiate ferns, the group to which the selected tree ferns belong, due to the fact that intact stomata of leaves open in response to red light but not in response to blue light (Doi et al. Reference DOI, WADA and SHIMAZAKI2006). Using the light curve program of the LI-6400, the PPFD was increased in nine steps, starting with 0 and ending with 1500 μmol m−2 s−1. The relative humidity and temperature of the leaf were kept close to environmental conditions, the flow rate ranged from 100 to 200 μmol s−1, and the CO2 was kept at slightly above ambient (450 μmol m−2 s−1).
Chlorophyll a fluorescence
The apparent maximum electron transport rate (ETRmax) and the photosynthetic flux density to saturation when ETR reached 90% of ETRmax (ETRLSP) were estimated during the morning by using the light-curve program of a pulse-modulated fluorometer coupled to the 2030-B leaf clip holder (MiniPAM, Walz, Effeltrich, Germany) (Rascher et al. Reference RASCHER, LIEBIG and LÜTTGE2000). A segment of a leaf was darkened for 20 min and then placed inside the leaf clip holder. There, actinic light was increased in nine steps, starting with 0. The maximum quantum yield of the leaf (Fv/Fm) was recorded at predawn (5h00–6h00), and the light-adjusted quantum yield of the leaf (Fv′/Fm′), apparent rate of electron transport (ETR) through photosystem II, PPFDi and leaf temperature measurements were made throughout the day by using the MiniPAM (Maxwell & Johnson Reference MAXWELL and JOHNSON2000).
Water status, leaf and stomatal dimensions
Measurements of predawn (5h00–6h00) and midday (12h00–14h00) leaf water potential (Ψ) were performed in five individuals under closed and open canopies by using a pressure chamber (PMS Corvallis, Oregon, USA). Predawn and midday soil Ψ measurements were done at 15 cm from the stalk of each individual and 10 cm deep in the soil by using C-52 sample chambers connected to an HR-33T dew point microvoltmeter (Wescor, Logan, Utah, USA). The relative water content (RWC); thickness from the middle of a mature leaf; and specific leaf area (SLA), calculated as the quotient between the area and the dry weight of the sample and density and length of stomata in the abaxial surface of the leaf were determined.
Leaf water loss and quantum yield
Apical pinnae from plants growing under closed and open canopies were cut at the base and placed in water overnight. The next day, after 30 min in darkness, the pinnae were placed on a laboratory bench, and Fv′/Fm′ and fresh weight were recorded while the pinnae were losing water. After the last measurement, the pinnae were dried at 80 °C, and RWC was calculated. During the experiment the average leaf temperature was 26 °C (range = 23 °C–28 °C), and relative humidity fluctuated between 60% and 90%.
Data analysis
To analyse the variation in the abundance of ferns with canopy openness in the quadrats of the transects, we used a contingency table with three species and five canopy-openness classes, as well as a residuals adjusted test for the difference between the observed and expected values (Everitt Reference EVERITT1977). The variation of Amax, ALSP, Rd, ETRmax, ETRLSP, stomatal length and density were subjected to factorial ANOVA. Fv/Fm, predawn Ψ, midday Ψ, SLA and leaf thickness were recorded in more than one period of the year in the same individuals, and therefore, their variation was subjected to an analysis of variance with repeated measures (ANOVAR) (Winer et al. Reference WINER, BROWN and MICHELS1991). Non-parametric ANOVAR was used to analyse the variation in RWC and soil Ψ (Brunner et al. Reference BRUNNER, DOMHOF and LANGER2002). Amax, ALSP, ETRmax and ETRLSP values were estimated when adjusting the exponential function described in Rascher et al. (Reference RASCHER, LIEBIG and LÜTTGE2000). Though this model appeared to describe the data well, the adjusted Rd was always higher than that observed and very close to zero. Then, the exponential function was not used to estimate Rd. The relationships between Fv′/Fm′ and leaf temperature, PPFDi and ETR, and Fv′/Fm′ and PPFDi were analysed with linear and quadratic regression models. The data normality was analysed with a Shapiro–Wilk W test and standardized residual plots. ALSP, Rd, predawn Ψ, midday Ψ and leaf thickness data were transformed into logarithms. All statistical tests used Statistica 7 (StatSoft, USA), but the non-parametric ANOVAR used the nparLD 2.0 package in R 2.14.0 (Free Software Foundation, Inc., USA).
RESULTS
Tree fern distribution and field light gradient
The comparison of the number of observed and expected ferns along the slope showed that A. firma was significantly more abundant in small gullies and at the edge of the stream, where the canopy was more closed, while L. quadripinnata was more abundant near the crest of the slope or at open sites. The fern C. divergens was more abundant in sites with intermediate canopy openness values (χ2 = 136, df = 8, P < 0.0001, Table 1). Small individuals (≤ 1 m) of A. firma and C. divergens were distributed from the ridge to the creek, but the larger individuals of A. firma were found in the sites with the most closed canopy. C. divergens was found in the most open canopy sites (Figure 1a, b).
Table 1. Observed (Obs.) and expected (Exp.) number of tree ferns, depending on canopy openness, estimated as proportion of photosynthetic photon flux density (PPFD) in relation to a completely exposed site in a cloud forest of El Riscal, Veracruz, Mexico. Adjusted residual values are shown; absolute values > 1.96 are significant at 5% of the normal distribution.


Figure 1. Distribution of three tree fern species across a light gradient (canopy opening) in a cloud forest of El Riscal, Veracruz, Mexico. Number of plants ≤ 100 cm high (a) and > 100 cm high (b) of Alsophila firma (black bars), Cyathea divergens (diagonal lines) and Lophosoria quadripinnata (empty bars) related to the degree of canopy openness, estimated as proportion of photosynthetic photon flux density (PPFD) at 100 cm high, compared with a completely exposed site outside cloud forest. The sampled area was 1800 m2.
Leaf response to canopy openness
The mean daily PPFD was 7.7 ± 2.41 μmol m−2 s−1 (mean ± SE) and 97.1 ± 24.1 μmol m−2 s−1 under closed and open canopies, respectively (Figure 2a). The difference between the two canopy environments in terms of relative humidity, air temperature and vapour pressure deficit was relatively high during the hottest month of the year (May) (Figure 2b–d).

Figure 2. Microclimatic data for the El Riscal reserve, Veracruz, Mexico. Photosynthetic photon flux density (PPFD) (a), air temperature (b), relative humidity (RH) (c) and vapour pressure deficit (DPV) (d) at 100 cm high under closed canopy (full circles) and open space (empty circles) in a cloud forest. Each point is the daily mean recorded during the sunlit period at the beginning of the rainiest period (June–July 2009), the end of the rainiest period (September–October 2009) and the end of the driest period (May 2010).
At the end of the wettest period of the year, Amax was not affected by canopy openness, and A. firma showed the lowest Amax value, although the difference among species was not significant (FAmax (2,20) = 2.70, P = 0.09, Tables 2 and 3). ALSP and Rd were similar between species, but were significantly lower under closed canopy (FA-LSP (1,20) = 8.51, P = 0.008; FRd (1,15) = 8.11, P = 0.01). At the end of the wettest period of the year C. divergens had significantly higher values of ETRmax and ETRLSP, and A. firma presented the lowest values (FETRmax (2,20) = 7.36, P = 0.004; FETR-LSP (2,20) = 5.18, P = 0.015, Table 2). ETRmax was not affected by the opening of the canopy, but ETRLSP was significantly higher at the open site (FETR-LSP (1,20) = 4.89, P = 0.039). The highest values of ETR and PPFDi occurred more frequently in the morning at the closed site in all three species, while at the open site, they occurred during the afternoon (Figure 3). The increase in the ETR with PPFDi followed a quadratic function in all species at both sites (R2 > 0.99, P < 0.01). Only in C. divergens was ETR with increasing PPFDi significantly more pronounced in the open site as compared with closed site (tb1-b2 = 5.13, P < 0.01). All species showed the highest ETR values at the open site in May 2010, reaching values up to 70 μmol m−2 s−1, however, ETR saturation values were not observed in any of the recording periods, probably due to the low PPFDi values that prevail in the understorey under both closed and open canopies. The average value of Fv/Fm during the study period was significantly higher in C. divergens (0.84 ± 0.004) and L. quadripinnata (0.85 ± 0.004) as compared with A. firma (0.82 ± 0.004) (F(2,20) = 8.2, P < 0.001, Table 4). The three species showed significantly lower values of Fv/Fm under closed canopy (average of three species = 0.83 ± 0.003) as compared with open canopy (0.85 ± 0.003) (F(1,20) = 14.8, P < 0.001). In all species, Fv′/Fm′ was relatively high throughout the year and decreased during the morning and sometimes in the afternoon. Lower values of Fv′/Fm′ were observed in the driest period of the year (May), but they were never < 0.5 (Figure 3). Under open canopy, Fv′/Fm′ decreased significantly in all three species with increasing PPFDi. This followed a linear function in A. firma, but it followed a quadratic function in the other species (R2 = 0.21–0.67, P < 0.05), while under closed canopy, this decrease followed a quadratic function and was significant only for C. divergens (R2 = 0.4, P < 0.05). The increased leaf temperature in the daytime decreased the Fv′/Fm′ linearly and significantly in all species, both under closed and open canopies (R2 = 0.1–0.36, P < 0.01).
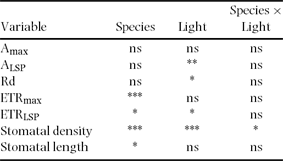
Table 2. Effect of understorey light environment on the photosynthetic traits and stomatal dimensions of three tree fern species in a cloud forest of El Riscal, Veracruz, Mexico. CO2 assimilation rate at light saturation (Amax), photosynthetic flux density to 90% of Amax (ALSP), dark respiration rate (Rd), apparent maximum electron transport rate (ETRmax), photosynthetic flux density to 90% of ETRmax (ETRLSP), stomatal density and stomatal length of ferns growing under closed (7.7 μmol m−2 s− mean daily PPFD) and open (97.1 μmol m−2 s− mean daily PPFD) canopies. Mean ± 1 SE are shown. n = 4–5, but n = 2 in stomatal density and length of Alsophila firma in the closed site. Data were recorded during the rainiest period. Different letters indicate significant differences between light environments. All variables labelled with an asterisk indicate significant differences among species. nd = no data.

Table 3. ANOVA of the leaf traits of three tree fern species (Alsophila firma, Cyathea divergens and Lophosoria quadripinnata) and two understorey light environments (closed and open canopies) in a cloud forest of El Riscal, Veracruz, Mexico. See Table 2 for abbreviations. * = P < 0.05; ** = P < 0.01; *** = P < 0.0005; ns = not significant.
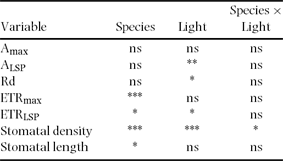
Table 4. ANOVA with repeated measures of leaf traits of three tree fern species (Alsophila firma, Cyathea divergens and Lophosoria quadripinnata), two understorey light environments (closed and open canopies) and time of year in a cloud forest of El Riscal, Veracruz, Mexico. Fv/Fm, along with predawn and midday leaf Ψ, were recorded during three periods of the year (the beginning and end of the rainiest period and the end of the driest period). Predawn and midday soil Ψ, RWC, SLA and leaf thickness were recorded during two periods of the year (the end of the rainiest period and end of the driest period). See Figures 2 and 3 for abbreviations. * = P < 0.05; ** = P < 0.01; *** = P < 0.0005; ns = not significant.


Figure 3. Effect of the daily and seasonal variation on instant photon flux (PPFDi), chlorophyll fluorescence variables and temperature in the leaves of three tree fern species in a cloud forest of El Riscal, Veracruz, Mexico. PPFDi, apparent electron transport rate (ETR), maximum (Fv/Fm) and light-adjusted quantum yield (Fv′/Fm′) and the leaf temperature of Alsophila firma (a–d), Cyathea divergens (e–h) and Lophosoria quadripinnata (i–l) growing under closed (full symbols) and open (empty symbols) canopies in the cloud forest. Each point is the mean ± 1 SE (n = 5) of the values recorded during various hours of days 157 and 158 at the beginning of the rainiest period (June 2009), days 291 and 292 at end of the rainiest period (October 2009) and day 131 at end of the driest period (May 2010).
In the leaves of the three species, the predawn Ψ was significantly less negative at the beginning of the rainiest period (F(2,26) = 75.1, P < 0.001; Figure 4, Table 4). The predawn Ψ of L. quadripinnata was significantly less negative as compared with those of the other two species (F(2,13) = 8.37, P < 0.001), while the midday Ψ was similar among species. When they grew under open canopy early in the wettest period (June), the three fern species showed midday Ψ that was significantly more negative, but at the end of the rainiest period (October), midday Ψ was more negative when ferns grew under closed canopy (F(2,32) = 8.15, P < 0.005). The latter was also observed in the A. firma predawn Ψ because its values were more negative under closed canopy at the end of the rainiest period (October) and driest period (May) of the year, although the differences were not significant. Even though the predawn and midday soil Ψ were significantly (P < 0.001) more negative in the driest period of the year, their values were close to zero throughout the study (Table 4). The predawn soil Ψ were −0.01 ± 0.0228 MPa and −0.12 ± 0.004 MPa at the end of the rainy season and in the driest period of the year, respectively, while the soil Ψ at midday were −0.01 ± 0.026 MPa and −0.16 ± 0.0019 MPa for the same periods. The midday soil Ψ at the open site was more negative than that under closed canopy, although the difference was not significant (P = 0.06). The greatest decrease in midday soil Ψ (−0.26 ± 0.0674 MPa) occurred under C. divergens in open canopy during the driest period of the year. The leaf RWC remained high (96.6 ± 0.61) and was significantly similar among species, sites and periods (Table 4).

Figure 4. Effect of seasonal variation in leaf water potential (Ψ) of three tree fern species in a cloud forest of El Riscal, Veracruz, Mexico. Predawn (a) and midday (b) leaf Ψ of Alsophila firma (Af), Cyathea divergens (Cd) and Lophosoria quadripinnata (Lq) growing under closed (full bars) and open (empty bars) canopies at the beginning of the rainiest period, in June; at end of the rainiest period, in October 2009, and at end of the driest period, in May 2010. Mean ± 1 SE (n = 4–5, n = 3–2 in seven cases).
The leaves of A. firma had significantly higher SLA and were thinner, while the leaves of L. quadripinnata had lower SLA and greater thickness (FSLA(2,20) = 15.2, P < 0.01; FThickness(2,19) = 22.7, P < 0.001; Figure 5, Table 4). All species had higher SLA values and leaf thicknesses at the start of the wettest period (FSLA(1,20) = 11.9, P < 0.005; FThickness(1,19) = 652, P < 0.001). Under closed canopy, the leaves of the three species were significantly thinner (FThickness(1,19) = 18.7, P < 0.001) and had higher SLA values as compared with the open site, but differences in the SLA values between sites were not significant. Cyathea divergens and L. quadripinnata plants that grew in the open site had a higher density of stomata than those that grew under closed canopy, and L. quadripinnata showed a greater stomatal density as compared with C. divergens (F(1,14) = 4.91, P < 0.04, Table 3). The stomatal length of L. quadripinnata was significantly lower compared with that of C. divergens (F(1,6) = 7.92, P < 0.04). The difference in C. divergens or L. quadripinnata stomatal length between sites was not significant (Table 3). Although the density and length data for A. firma stomata were scarce, their values were similar to those of C. divergens.

Figure 5. Effect of seasonal variation in the leaf traits of three tree fern species in a cloud forest of El Riscal, Veracruz, Mexico. Specific leaf area (SLA) (a, c) and leaf thickness (b, d) of Alsophila firma (Af), Cyathea divergens (Cd) and Lophosoria quadripinnata (Lq) growing under closed (black bars) and open (empty bars) canopies during the beginning of the rainiest period, in June 2009, and at end of the driest period, in May 2010. Mean ± 1 SE, n = 5.
Leaf water loss and quantum yield
The curves of water loss and the decrease in the light-adjusted quantum yield with time in the leaves from the open and closed sites were similar within each species. Water loss and the decreasing quantum yield during the desiccation of the leaves on the laboratory bench were faster in A. firma (Figure 6). Ten hours after the start of the drying, the leaf water loss of L. quadripinnata and C. divergens were 50% and 66%, respectively, and the quantum yield decreased from 0.80 to 0.72 and from 0.79 to 0.67, respectively, while water loss of A. firma during the same period was 96%, and the quantum yield decreased from 0.77 to 0.09 (χ2(RWC, 10h) = 19.8, df = 2, P < 0.005). Notwithstanding the above, all species retained high quantum yield values (0.75–0.72) when they lost 50% of their water.

Figure 6. Effect of leaf water loss on quantum yield in leaves of three tree fern species in a cloud forest of El Riscal, Veracruz, Mexico. Relative water content (RWC) (a) and light-adjusted quantum yield (Fv′/Fm′) (b) of Alsophila firma (filled triangles), Cyathea divergens (empty circles) and Lophosoria quadripinnata (filled squares) during the desiccation of the leaves of plants that were growing under closed and open canopies. Mean ± 1 SE, n = 5.
DISCUSSION
The physiological performance associated with photosynthesis indicates that the three tree ferns studied are shade species. The highest CO2 assimilation rates in the tree ferns were similar to or lower than the values widely reported in terrestrial and epiphytic vascular plants in shade habitats (Chazdon et al. Reference CHAZDON, PEARCY, LEE, FETCHER, Mulkey, Chazdon and Smith1993, Durand & Goldstein Reference DURAND and GOLDSTEIN2001, Hietz & Briones Reference HIETZ and BRIONES2001, Larcher Reference LARCHER2003, Ludlow & Wolf Reference LUDLOW and WOLF1975, Mooney et al. Reference MOONEY, FIELD, VÁZQUEZ-YANEZ, Medina, Mooney and Vázquez-Yanez1984, Saldaña et al. Reference SALDAÑA, GIANOLLI and LUSK2005, Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010). The ETRmax for tree fern species growing in the cloud forest was relatively low compared with those of species growing with higher light availabilities: three legume tree species (Caesalpinia echinata, C. ferrea, Machaerium obovatum) in a semi-deciduous tropical rain forest (Geβler et al. Reference GEβLER, DUARTE, FRANCO, LÜTTGE, DE MATTOS, NAHM, RODRIGUES, SCARANO and RENNENBERG2005), two shrub species (Andira legalis, Clusia hilariana) and an hemicryptophyte (Allagoptera arenaria) in a sandy coastal plant community (Gessler et al. Reference GESSLER, NITSCHKE, DE MATTOS, ZALUAR, SCARANO, RENNENBERG and LÜTTGE2008), a deciduous broad-leaved tree species (Quercus mongolica var. crispula) (Kitao et al. Reference KITAO, KITAOKA, KOMATSU, UTSUGI, TOBITA, KOIKE and MARUYAMA2012), a broad-leaved pioneer tree species (Alnus formosana) (Wong et al. Reference WONG, CHEN, HUANG and WENG2012), a warm temperate evergreen shrub species (Buxus sempervirens) (Letts et al. Reference LETTS, RODRÍGUEZ-CALCERRADA, ROLO and RAMBAL2012) and a sun fern species (Pyrrosia lingus) (Wong et al. Reference WONG, CHEN, HUANG and WENG2012). However, the ETRmax in the studied species showed similar values to the higher ETR or ETRmax values in plants living under limiting light levels: five tree fern species (Cyathea cunninghamii, C. dealbata, C. medularis, C. smithii, Dicksonia squarrosa) associated with shade environments in a rain temperate forest in New Zealand (Bystriakova et al. Reference BYSTRIAKOVA, BADER and COOMES2010), three fern species (Blechnum magellanicum, B. mochaenum, B. penna-marina) growing in the shade in an evergreen temperate forest (Saldaña et al. Reference SALDAÑA, HERNANDEZ, COOPMAN, BRAVO and CORCUERA2010), two herbaceous shade fern species (Archangiopteris somai, Diplazium donianum) (Wong et al. Reference WONG, CHEN, HUANG and WENG2012) and four tree species (Alnus crispa var. sinuata, Rubus spectabilis, Vaccinum alaskaense, V. ovalifolium) growing in a high-latitude coastal temperate rainforest (Quigg Reference QUIGG2012). ETRLSP values in the studied tree ferns were lower in comparison with those of the three legume tree species but similar to the three fern species of the genus Blechnum mentioned above (Saldaña et al. Reference SALDAÑA, HERNANDEZ, COOPMAN, BRAVO and CORCUERA2010).
Although Amax and ETRmax were not sensitive to the light environment in the studied ferns species, the correlation of Amax and ETRmax with sun and shade has been documented in many plants, including epiphyte and tree fern species (Chazdon et al. Reference CHAZDON, PEARCY, LEE, FETCHER, Mulkey, Chazdon and Smith1993, Durand & Goldstein Reference DURAND and GOLDSTEIN2001, Hietz & Briones Reference HIETZ and BRIONES2001, Kitao et al. Reference KITAO, KITAOKA, KOMATSU, UTSUGI, TOBITA, KOIKE and MARUYAMA2012, Larcher Reference LARCHER2003, Letts et al. Reference LETTS, RODRÍGUEZ-CALCERRADA, ROLO and RAMBAL2012, Ludlow & Wolf Reference LUDLOW and WOLF1975, Quigg Reference QUIGG2012, Saldaña et al. Reference SALDAÑA, GIANOLLI and LUSK2005, Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010). Compared with open spaces, the fact that the lowest ALSP values were found under closed canopy may indicate that the studied species are efficient in absorbing the little light available in order to assimilate CO2 under canopy shade (Durand & Goldstein Reference DURAND and GOLDSTEIN2001, Hietz & Briones Reference HIETZ and BRIONES2001).
The maximum quantum yield in the studied tree ferns varied around 0.83, which is the optimal value for most unstressed plants (Maxwell & Johnson Reference MAXWELL and JOHNSON2000), including shade-tolerant and light-demanding tree ferns (Durand & Goldstein Reference DURAND and GOLDSTEIN2001, Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010). It has been mentioned that facing temperature decreases between 10 °C and 20 °C, plants from the tropics show low quantum yield values due to decreasing chlorophyll concentration, poor chloroplast development, lower electron transport, the photo-degradation of photosystem II components, the night retention of depoxidized xanthophylls of photo-protective efficiency regulation for photosystem II, and a drop in Calvin cycle enzyme activity (Lambers et al. Reference LAMBERS, CHAPIN and PONS1998, Lin et al. Reference LIN, HWANG and LO2007, Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010). The minimum air temperature (average 12 °C) during the colder months in the study site could have diminished the photosynthesis in the three fern species because the lowest maximum quantum yield values were recorded under closed canopy during this period. Cold winter mornings in Australian forests, despite a temperature of > 10 °C, caused the maximum quantum efficiency to be lower in winter than in summer in Dicksonia antarctica and Cyathea australis (Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010). Increasing air temperature caused light-adjusted quantum efficiency to decrease in the three fern species, indicating that the photosynthesis of the studied ferns is a heat-sensitive process, just as it is in many plants (Berry & Björkman Reference BERRY and BJÖRKMAN1980).
Although tropical forests receive substantial rainfall, plants can be exposed to insufficient water supply during the dry season, and drought stress can be a major factor in seedling mortality (Engelbrecht et al. Reference ENGELBRECHT, KURSAR and TYREE2005). Foliar Ψ decreases in the three studied species during the driest season might be caused by increased transpiration, lower water flow in conducting tissues and, to a lesser extent, lower soil water availability. It has been documented that fern stipes shows lower water-specific conductivity than seed plants (Watkins et al. Reference WATKINS, HOLBROOK and ZWIENIECKI2010). On the other hand, it is likely that the higher drop in fern foliar Ψ values during the coldest season under closed canopy as compared with open canopy was a consequence of a protective mechanism against low temperatures. In this study, the lowest Fv/Fm values were recorded during October under closed canopy, when the air temperature was < 17 °C and the leaf temperature was ≤ 15 °C. In Zea mays, it has been reported that the decrease in relative water content works as a form of photosystem II protection against decreasing temperatures (Aroca et al. Reference AROCA, IRIGOYEN and SANCHEZ-DIAZ2003).
The reduction in quantum yield during the drought reflects a decline in the efficiency of light use for electron transport by PSII (Poulos et al. Reference POULOS, GOODALE and BERLYN2007). During leaf desiccation, the lower ability to contain water made quantum yield decrease much faster in A. firma, which grows best in the darkest areas of the forest, than the other tree ferns. Even so, quantum yield decrease due to leaf water loss was similar in the three studied tree ferns. Epiphytic ferns growing in the same cloud forest in which this study was carried out showed differences in the decrease in the quantum yield due to water loss. When RWC dropped to 30%, the humidity-loving fern Trichomanes bucinatum, which grows on the humid and shady bases of tree trunks, was affected first, while Polypodium puberulum and Asplenium cuspidatum, which grow in the outer canopy and mid-canopy, respectively, were affected to a lesser extent (Hietz & Briones Reference HIETZ and BRIONES2001).
Thin leaves are associated with lower levels of solar radiation because the relatively large proportion of spongy mesophyll and specialized anatomical structures permit the scarce flashing light in the understorey to be used for photosynthesis (Lambers et al. Reference LAMBERS, CHAPIN and PONS1998). The studied ferns produced thinner leaves when they grew under closed canopy, and the species with the thinnest leaves (A. firma) was the one that showed a preference for growing in the shadiest sites in the forest. Individuals of Cyathea caracasana in sunny environments produced up to 50% thicker leaves than individuals in shady environments (Arens Reference ARENS1997), and foliage thickness was positively correlated with distribution inside the crown of eight epiphyte ferns in a cloud forest (Hietz & Briones Reference HIETZ and BRIONES1998). SLA variation in the studied ferns is consistent with light-gathering maximization (Lambers et al. Reference LAMBERS, CHAPIN and PONS1998, Larcher Reference LARCHER2003, Poorter Reference POORTER2009). The maximum SLA values under closed canopy during the humid season show that the studied ferns had greater leaf area to absorb light per unit invested in dry matter when grown in shade micro-environments (Larcher Reference LARCHER2003). SLA values in three coexisting Blechnum fern species decreased as the canopy opened in a Chilean evergreen temperate forest (Saldaña et al. Reference SALDAÑA, GIANOLLI and LUSK2005). Both in summer and winter, Dicksonia antarctica and Cyathea australis showed an inverse relationship between SLA and irradiance, although SLA values were lower in winter, when light intensity was lower and water availability was higher (Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010). Sun leaves of angiosperms and ferns have higher stomatal density than shade leaves (Lambers et al. Reference LAMBERS, CHAPIN and PONS1998, Larcher Reference LARCHER2003). In the same way, stomatal density in C. divergens and L. quadripinnata was related to the light environment in which their leaves were developed. Three terrestrial fern species from shady habitats had lower stomatal densities than three species from sunny environments, and epiphytic fern species growing close to the bases of trunks had lower stomatal densities than species found in the exposed crowns of trees in a cloud forest (Hietz & Briones Reference HIETZ and BRIONES1998, Ludlow & Wolf Reference LUDLOW and WOLF1975). However, in Dicksonia antarctica, Cyathea australis and C. caracasana tree ferns, the stomatal density was not related to the light environment (Arens Reference ARENS1997, Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010).
Studies carried out in humid forests in South America, New Zealand and Australia have shown that habitat specialization is a common feature in tree ferns (Arens & Sanchez-Baracaldo Reference ARENS and SANCHEZ-BARACALDO1998, Bystriakova et al. Reference BYSTRIAKOVA, BADER and COOMES2010, Jones et al. Reference JONES, OLIVAS ROJAS, TUOMISTO and CLARK2007, Volkova et al. Reference VOLKOVA, BENNETT and TAUSZ2010). Differences observed in the microhabitats of the tree ferns studied coincide with observations carried out on the same species in other regions. In Costa Rica, A. firma was found to be associated with streams and glens in sites with large-basal-area trees (Jones et al. Reference JONES, OLIVAS ROJAS, TUOMISTO and CLARK2007), while L. quadripinnata was restricted to open, sunny spaces in the cloud forest in the Colombian Andes (Arens & Sanchez-Baracaldo Reference ARENS and SANCHEZ-BARACALDO1998). In central Mexico, Cyathea divergens lives in several micro-sites in the cloud forest and has shown higher survival in sunny environments than A. firma (Eleutério & Pérez-Salicrup Reference ELEUTÉRIO and PÉREZ-SALICRUP2009, Mehltreter & García-Franco Reference MEHLTRETER and GARCÍA-FRANCO2008). In a site near the one studied, L. quadripinnata was observed to be associated with the cloud-forest edges and sunnier places (Bernabe et al. Reference BERNABE, WILLIAMS-LINERA and PALACIOS-RIOS1999).
Spatial and temporal variation in growth conditions may explain the differences in the distributions of the small and large individuals across the light gradient. The role of spore germination and gametophyte plasticity in habitat selection may also be important. A correspondence between spore germination and habitat preference was observed in a study including two of the three selected tree fern species. The germination of spores of Lophosoria quadripinnata and Alsophila firma had the widest (11 °C–35 °C) and lowest (15 °C–28 °C) tolerance of extreme temperatures, respectively (Pérez-García & Riba Reference PÉREZ-GARCÍA and RIBA1982). It has been shown that gametophytes can establish themselves in a broad range of environments, while sporophytes may be restricted to more stable habitats (Farrar et al. Reference FARRAR, DASSLER, WATKINS, CHANDA, Ranker and Haufler2008). Studies on resource and environmental requirements during various stages of the two-generation life cycle will improve the understanding of the niche differentiation and coexistence of tree ferns.
In conclusion, the spatial distribution patterns of A. firma, C. divergens and L. quadripinnata in the cloud forest were associated with the degree of openness of the canopy, and this suggests that they differ in terms of habitat specialization. The seasonality of the cloud forest affected leaf physiology, and the studied species showed physiological acclimatization to the light environment in which they grew. The magnitude of the physiological parameters associated with photosynthesis and leaf water state indicates that the three tree fern species are shade species and suggests that A. firma has a higher shade tolerance than the other two species. The evidence found in this study supports the hypothesis that tree fern distribution and leaf physiological characteristics are associated with the degree of openness of the canopy in the cloud forest.
ACKNOWLEDGEMENTS
The authors appreciate Yareni Perroni's and Rogelio Macías's hospitality in Private Reserve El Riscal, and Mario Márquez's field help. We also express thanks to two thoughtful reviewers for useful comments on the manuscript. The authors thanks the scholarship and grant by Conacyt (276041, CB-2011-168682).