INTRODUCTION
Tropical forests are extremely diverse environments (Morellato & Haddad Reference MORELLATO and HADDAD2000, Putz et al. Reference PUTZ, BLATE, REDFORD and FIMBEL2001, ter Steege et al. Reference TER STEEGE, PITMAN, SABATIER, BARALOTO, SALOMÃO, GUEVARA, PHILLIPS, CASTILHO, MAGNUSSON, MOLINO, MONTEAGUDO, NÚÑEZ VARGAS, MONTERO, FELDPAUSCH, CORONADO, KILLEEN, MOSTACEDO, VASQUEZ, ASSIS and TERBORGH2013) and the identification of functional groups facilitates understanding of more complex ecological processes (Powers & Tiffin Reference POWERS and TIFFIN2010). Leaf δ13C can be used to organize plant functional groups because it reflects conditions during photosynthesis, with increasing discrimination against 13C as intercellular CO2 availability increases (Farquhar et al. Reference FARQUHAR, O'LEARY and BERRY1982), leading to lower values of δ13C. Leaf δ13C can also vary with leaf habit, morphology, genetics and irradiance (Dawson et al. Reference DAWSON, MAMBELLI, PLAMBOEK, TEMPLER and TU2002, Franco et al. Reference FRANCO, BUSTAMANTE, CALDAS, GOLDSTEIN, MEINZER, KOZOVITS, RUNDEL and CORADIN2005, Rossatto et al. Reference ROSSATTO, HOFFMANN, DE CARVALHO RAMOS SILVA, HARIDASAN, STERNBERG and FRANCO2013, Sobrado & Ehleringer Reference SOBRADO and EHLERINGER1997, Vitoria et al. Reference VITORIA, VIEIRA, DE, CAMARGO and SANTIAGO2016), which may reflect differences in photosynthetic water use efficiency (WUE).
In sites with high mean annual precipitation (MAP), lower δ13C values are observed (Cornwell et al. Reference CORNWELL, WRIGHT, TURNER, MAIRE, CERNUSAK, DAWSON, ELLSWORTH, FARQUHAR, GRI, KEITEL, KNOHL, REICH, WILLIAMS, BHASKAR, CORNELISSEN, RICHARDS and SCHMIDT2018, Leffler & Enquist Reference LEFFLER and ENQUIST2002, Ma et al. Reference MA, SUN, LIU and CHEN2012). Cornwell et al. (Reference CORNWELL, WRIGHT, TURNER, MAIRE, CERNUSAK, DAWSON, ELLSWORTH, FARQUHAR, GRI, KEITEL, KNOHL, REICH, WILLIAMS, BHASKAR, CORNELISSEN, RICHARDS and SCHMIDT2018) showed that woody evergreen species have lower leaf δ13C than woody deciduous species when MAP is higher than 1000 mm, and an inverse pattern when MAP is lower than 1000 mm. In temperate zones, deciduous species and species with short leaf lifespans generally exhibit lower δ13C and presumably lower WUE than evergreen species and species with long leaf lifespans (Ehleringer & Cooper Reference EHLERINGER and COOPER1988, Marshall & Zhang Reference MARSHALL and ZHANG1994). However, the relationship between leaf δ13C and deciduousness has been somewhat controversial in the tropics, with reports of lower discrimination against 13C in deciduous compared with evergreen species in Venezuela (Sobrado & Ehleringer Reference SOBRADO and EHLERINGER1997), greater discrimination against 13C in deciduous compared with evergreen species in Brazil, and no difference in other studies (Franco et al. Reference FRANCO, BUSTAMANTE, CALDAS, GOLDSTEIN, MEINZER, KOZOVITS, RUNDEL and CORADIN2005, Leffler & Enquist Reference LEFFLER and ENQUIST2002, Powers & Tiffin Reference POWERS and TIFFIN2010). Variation of δ15N in plants and soil can reflect temporal and spatial variation in N sources, soil N availability and N acquisition from alternative sources such as biological N2-fixation, mycorrhizal associations and atmospheric deposition (Bai et al. Reference BAI, BOUTTON, LIU, WU, ARCHER and HALLMARK2009, Bustamante et al. Reference BUSTAMANTE, MARTINELLI, SILVA, CAMARGO, KLINK, DOMINGUES and SANTOS2004, Dawson et al. Reference DAWSON, MAMBELLI, PLAMBOEK, TEMPLER and TU2002, Ometto et al. Reference OMETTO, EHLERINGER, DOMINGUES, BERRY, ISHIDA, MAZZI, HIGUCHI, FLANAGAN, NARDOTO and MARTINELLI2006, Powers & Tiffin Reference POWERS and TIFFIN2010). Values of δ15N in soil and plants systematically decrease with the increasing of MAP due to variation in the openness of the N cycle until the point of soil waterlogging (Amundson et al. Reference AMUNDSON, AUSTIN, SCHUUR, YOO, MATZEK, KENDALL, UEBERSAX, BRENNER and BAISDEN2003, Austin & Vitousek Reference AUSTIN and VITOUSEK1998, Handley et al. Reference HANDLEY, AUSTIN, ROBINSON, SCRIMGEOUR, RAVEN, HEATON, SCHMIDT and STEWART1999, Nardoto et al. Reference NARDOTO, PIERRE, BALBAUD, EHLERINGER, HIGUCHI, MARIA and MARTINELLI2008, Santiago et al. Reference SANTIAGO, KITAJIMA, WRIGHT and MULKEY2004, Schuur & Matson Reference SCHUUR and MATSON2001). Differences in the frequency of leaf N re-translocation with leaf lifespan have been suggested to also influence δ15N values of tropical trees, where shorter leaf lifespans re-translocate leaf N more frequently than evergreen species (Santiago et al. Reference SANTIAGO, KITAJIMA, WRIGHT and MULKEY2004) and δ15N becomes enriched during re-assimilation of nitrate and leaf N re-metabolism (Evans Reference EVANS2001).
We determined leaf δ13C and δ15N in evergreen and deciduous species in the understorey from two tropical Atlantic forests in Brazil that differ in MAP: 1200 mm and 1900 mm in order to determine the influence of leaf habit and site on leaf δ13C and δ15N. We hypothesized that in understorey leaves: (1) deciduous species have traits associated with maximizing C gain with higher leaf N and lower δ13C than evergreen species; (2) evergreen species show lower δ15N than deciduous species; and (3) values for δ13C and δ15N are greater at the drier site.
METHODS
Study sites and species
This study was carried out in two evergreen tropical Atlantic forests in Brazil (IBGE 2012): one in Bahia state that receives 1200 mm of annual precipitation and one in Rio de Janeiro state that receives 1900 mm y−1 (Figure 1). The 1200-mm site is located within a semi-arid region of Brazil in Chapada Diamantina National Park (CDNP), north-eastern Brazil (12°28′S, 41°23′W). However, due to moist air masses, altitude, orography and climatic conditions, it shows floristic similarities to the humid forest along the Brazilian coast (Funch et al. Reference FUNCH, RODAL, FUNCH, Thomas and Britton2008). The vegetation of the 1200-mm site is classified as evergreen seasonal forest (Richards Reference RICHARDS1998) and the soil is yellowish-red latosol with sandy clay loam texture (Funch et al., Reference FUNCH, RODAL, FUNCH, Thomas and Britton2008). The topography is undulating and the altitude ranges between 400 and 600 m asl, which confers a mesothermal climate to this site (Cwb) (Kottek et al. Reference KOTTEK, GRIESER, BECK, RUDOLF and RUBEL2006). Mean temperature oscillates around 18°C in April–September and exceeds 22°C in the hotter months of October–February (Funch et al. Reference FUNCH, FUNCH and BARROSO2002). The 1200-mm site has clouds for much of the year, and the rainy season occurs between December–April, with a peak between March–April, whereas the dry season varies between 5–6 mo occurring between May–October.

Figure 1. Map of the studied areas. Geographic map indicating the two tropical forests in Brazil that differ in mean annual precipitation: the 1200-mm site is in Bahia state, located in Chapada Diamantina National Park, and the 1900-mm site is in Rio de Janeiro state, located in the União Biological Reserve.
The 1900-mm site is located in south-eastern Brazil, in the União Biological Reserve (22°27′S, 42°02′W). This site has a gently undulating topography ranging from sea level to 370 m asl. The vegetation of the 1900-mm site is classified as lowland wet forest (Braga et al. Reference BRAGA, VITÓRIA, SOUZA, BARROS and FREITAS2016). The soil is dystrophic red-yellow podzolic with sandy clay texture (Lima et al. Reference LIMA, VILLELA, FILHO and PÉREZ2011). Climate is tropical humid (Aw) (Kottek et al. Reference KOTTEK, GRIESER, BECK, RUDOLF and RUBEL2006), with an average annual temperature of 25°C, and about 85% of precipitation occurring between October–April. Although precipitation does occur throughout the year, there are 3–4 mo of dry season.
Thirty-eight species of 18 families were sampled: 20 at the 1200-mm site and 18 at the 1900-mm site. Species were chosen according to previous information about leaf habit. The classification scheme for leaf habit of the 38 species is based on Frankie et al. (Reference FRANKIE, BAKER and OPLER1974) and included evergreen, evergreen with discontinuous production, deciduous and semi-deciduous species. For data presentation and analyses, evergreen species and species evergreen with discontinuous production were grouped under the category evergreen, whereas deciduous and semi-deciduous species were considered deciduous. For each species, five adult individuals 10–15 m tall with diameter at breast height of 10–30 cm had leaves at heights of 2.5–5.0 m sampled, except for the following species at the 1900-mm site: Ficus gomelleira (n = 4), Cupania racemosa (n = 4), Brosimum glazioui (n = 4), Virola gardneri (n = 2), Micropholis guianensis (n = 2), Ocotea diospyrifolia (n = 2). Photosynthetically active leaves of the second or third pair, under shade conditions (< 40 µmol m−2 s−1 in both sites) were collected. We opted to collect samples in the shade conditions due to the differences in the cloud cover between forests, and consequently irradiance differences in the canopy leaves, since irradiance has an influence on the δ13C (Vitoria et al. Reference VITORIA, VIEIRA, DE, CAMARGO and SANTIAGO2016). Our leaf isotopic composition data can therefore be considered as conservative in terms of emphasizing differences in climate between the two sites. Leaves were collected between 2011–2013 at the 1200-mm site and in 2015 at the 1900-mm site. No substantial variation in MAP in these areas was observed in these years.
C and N analyses
Leaves were dried at 60°C for at least 2 d and ground to a fine powder before analysis. Samples of around 1 mg were combusted on a continuous flow elemental analyser (Flash 2000 Organic Elemental Analyser), which measured elemental concentrations of C and N, coupled to a stable isotope ratio mass spectrometer (IRMS Delta V Advantage, Thermo Scientific, Germany), which measured isotopic composition of C and N. Pee Dee Belemnite (PDB) and atmospheric N were used as standard values for C and N analyses, respectively. The analytical precision was ± 0.1‰ for δ13C and ± 0.2‰ for δ15N and the accuracy for elemental and isotopic compositions were determined by certified standard (Protein OAS/Isotope Cert 114859; Elemental Microanalysis).
Data analyses
Comparisons of parameters between evergreen and deciduous leaf habit and the 1200-mm and 1900-mm sites were performed using factorial ANOVA (Statistica 6.0) followed by Tukey's test (P ≤ 0.05). Regression and correlation coefficients were calculated with the software package Sigma Plot 11.0 (SPSS; Chicago, IL, USA).
Non-metric multidimensional scaling (nMDS) was used to explore the spatial distribution of species characterized by leaf habit and site and potential grouping with leaf C, leaf N, leaf δ13C, leaf δ15N and leaf C/N ratio for the 38 study species. Traits were first normalized and then used to compute pairwise Euclidean distances among plant species. Significant differences (P ≤ 0.05) between leaf habit or site were assessed with analysis of similarity (ANOSIM, Clarke & Gorley Reference CLARKE and GORLEY2006). Analysis of similar percentages (SIMPER) was used to identify which leaf traits contributed most to the differentiation among defined groups. All multivariate analyses were performed using Primer v6.0 (Clarke & Gorley Reference CLARKE and GORLEY2006).
In order to detect phylogenetic effects, phylogenetic independent contrasts (PIC) were performed using the ‘Ape’ package in R. Analysis for phylogenetic signal of each leaf trait was performed with the ‘Picante’ package in R (Kembel et al. Reference KEMBEL, ACKERLY, BLOMBERG, CORNWELL, COWAN, HELMUS, MORLON and WEBB2010). PICs were calculated for each leaf trait and correlations on these contrasts were performed and compared to Pearson's correlation coefficients on the raw data. To build a trait-based phenogram, a distance matrix based on the values of leaf C concentration, δ13C, N concentration, δ15N and C/N ratio was computed among species. The leaf traits were first normalized and then used to compute pairwise Euclidean distances among plant species with Primer v6.0 (Clarke & Gorley Reference CLARKE and GORLEY2006). The matrix file was edited and imported into PAUP*4.0a146 (Swofford Reference SWOFFORD2002) to build a trait phenogram using the Neighbour joining method. The trait-based phenogram was refined using Adobe Illustrator v. 16.0.4. Finally, the trait-based phenogram was contrasted against the molecular-based phylogeny of the same plant species. The phylogenetic tree was obtained using Phylomatic v3 (http://www.phylodiversity.net/phylomatic) and the stored megatree by Zanne et al. (Reference ZANNE, TANK, CORNWELL, EASTMAN, SMITH, FITZJOHN, MCGLINN, O'MEARA, MOLES, REICH, ROYER, SOLTIS, STEVENS, WESTOBY, WRIGHT, AARSSEN, BERTIN, CALAMINUS, GOVAERTS and HEMMINGS2014).
RESULTS
No significant differences for mean values of δ13C and δ15N between deciduous and evergreen species were observed (Table 1). Leaf δ13C ranged from −28.4‰ in Copaifera langsdorffii, a deciduous species from the 1200-mm site, to −33.8‰ in Geissospermum laeve, an evergreen species from the 1900-mm site (Figure 2). Geissospermum laeve also presented the highest leaf δ15N (+6.1‰), whereas the lowest leaf δ15N was −3.3‰ in Myrcia obovata, a deciduous species from the 1200-mm site. Significant differences for leaf C concentration between deciduous and evergreen species were observed only at the 1900-mm site, with higher values in evergreen than in deciduous species (Table 1 and 2). Higher values of leaf C, leaf C/N ratio and leaf δ13C were found at the 1200-mm site, whereas higher leaf δ15N and N were found at the 1900-mm site, regardless of the leaf habit (Tables 1 and 2). There were no significant differences in δ15N between potentially N2-fixing leguminous species (Apuleia leiocarpa +1.8‰, Copaifera langsdorffii −1.6‰ and Pseudopiptadenia contorta +1.6‰) and species from other families. Rather, leaf δ15N values were strongly influenced by site (Figure 2). No statistical differences in N concentration between leguminous species and species from other families were found.
Table 1. Mean (± SD) of C and N concentrations (%), C/N ratio, δ13C (‰), and δ15N (‰) of evergreen and deciduous species from two tropical forests in Brazil that differ in mean annual precipitation (MAP), 1200-mm in Bahia state and 1900-mm in Rio de Janeiro state. Capital letters compare between sites (same leaf habit), and lowercase letters compare between leaf habit (same site). Factorial ANOVA. P ≤ 0.05. Mean = mean of all species (evergreen + deciduous) in the same site.


Figure 2. Leaf C and N isotopic composition of 38 tree species from two tropical forests in Brazil that differ in mean annual precipitation: 1200-mm site (black bars) and 1900-mm site (white bars). Leaf C (δ13C) (a) and leaf N (δ15N) (b) isotopic composition. Evergreen (unhatched) and deciduous (hatched) species are differentiated within each site.
Table 2. Species, plant family, leaf habit (EG: evergreen, EG-DP: evergreen with discontinuous production, DC: deciduous, SM-DC: semi-deciduous), mean annual precipitation (MAP) and concentration of leaf C (C; %), N (N; %) and C/N ratio from two tropical forests in Brazil that differ in MAP: 1200-mm in Bahia state, and 1900-mm in Rio de Janeiro state.

The nMDS analysis identified two major clusters structured by site with the 1200-mm site differing significantly from the 1900-mm site (P = 0.001). No grouping based on leaf habit was observed (Figure 3, Table 3). The two sites were mostly differentiated by leaf δ15N (25.3%), although the contribution of leaf C/N ratio (19.9%), leaf N concentration (18.5%), leaf δ13C (18.3%) and leaf C concentration (18.0%) were also important.
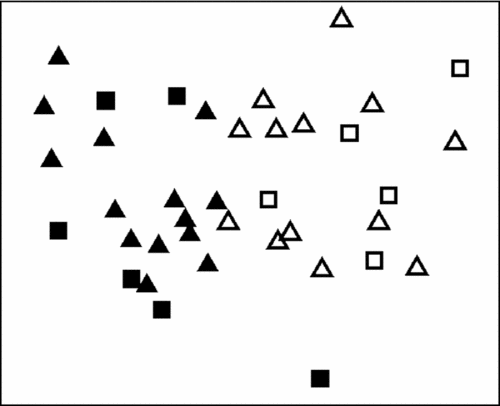
Figure 3. Non-metric multidimensional scaling (nMDS) analysis considering mean of all leaf traits, C and N concentrations, C and N isotopic composition, and C/N ratio (2D Stress: 0.13), from two tropical forests in Brazil that differ in mean annual precipitation. The 1200-mm site and the 1900-mm site are represented by closed and open symbols, respectively. Leaf habits (evergreen: triangles, deciduous: squares) are also given for each species.
Table 3. R values and significance levels (P) from the analysis of similarity (ANOSIM) for the different comparison tests assessing differences in leaf traits, C and N concentrations, C and N isotopic composition, and C/N ratio between two tropical forests in Brazil that differ in mean annual precipitation (1200 mm and 1900 mm). * Significance: P ≤ 0.05.

A bi-plot between leaf δ13C and δ15N showed negative correlation (P = 0.0003, Figure 4) with 1900-mm samples distributed in a broader range of δ15N values. However, no correlation was observed between leaf δ13C and δ15N from the 1200-mm site, whereas leaf δ13C and δ15N from 1900-mm site showed significant correlation (r = −0.59, P = 0.0099).

Figure 4. Correlation between leaf δ13C and δ15N for two tropical forests in Brazil that differ in mean annual precipitation. The 1200-mm site and the 1900-mm site are represented by closed and open symbols, respectively. Leaf habits (evergreen: triangles, deciduous: squares) are also given for mean of each species.
Correlations between leaf traits revealed that in the 1200-mm site, species with lower C/N ratio also had higher δ13C and N concentration, and species with lower C concentration also had lower δ15N (Table 4). Correlations between leaf traits in the 1900-mm site species showed: (1) weak, but significant differences between deciduous and evergreen for C/N ratio; (2) species with higher δ13C also had lower δ15N; and (3) species with lower C/N ratio had higher N concentration as also shown by the species in the 1200-mm site (Table 4). In general, these correlations were robust compared with PIC analysis, except for the relationship between δ13C and C/N ratio in the species from the 1200-mm site, which became non-significant in PIC analysis, and the relationships between δ15N and N concentration and between δ15N and C/N ratio, which became significant in species from the 1900-mm site (Table 4). However, when the more conservative method Bonferroni was applied, only the negative correlation between C/N ratio and N in the 1900-mm site remained (for Pearson's correlation (r) and PIC). The trait-based phenogram, suggested no phylogenetic effect on the grouping pattern according to the data, and clearly reinforced the influence of site on leaf traits (Appendix 1).
Table 4. Correlation coefficients (r) for leaf traits of tropical tree species from two tropical forests in Brazil that differ in mean annual precipitation (MAP), 1200-mm in Bahia state and 1900-mm in Rio de Janeiro state. Cross-species r in the upper right section of the matrix (n = 20 for 1200-mm site; n = 18 for 1900-mm site). Phylogenetic independent contrast in lower left section of the matrix (n = 19 for 1200 mm site; n = 17 for 1900-mm site). Significance: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.

DISCUSSION
Our data show that in shaded leaves, there were no consistent differences between species based on leaf habit. Within sites, only leaf C varied with leaf habit at the 1900-mm site. Across all traits, nMDS showed two major clusters, highly structured by site. We also anticipated large differences between sites based on differences in mean annual precipitation, with the wetter site expected to show relatively low δ13C and δ15N. Our data supported the pattern that leaf δ13C values were significantly lower at the wetter site, but did not support the pattern of lower δ15N at the wetter site, suggesting that factors besides openness of the N cycle were important in differentiating N cycling processes between these two forests (Evans Reference EVANS2001). Overall, site differences were the main factor distinguishing traits among species, suggesting strong functional convergence to local climate and soils among shade leaves within each site. In addition, we suggest that higher leaf N concentration, and associated higher leaf δ15N, in the 1900-mm site than in the 1200-mm site allows trees to increase photosynthesis, leading to lower δ13C in the 1900-mm site (Figure 4).
One of the most notable findings of this study was the muted difference in leaf isotopic composition and elemental concentrations between evergreen and deciduous species. The lack of differences in δ13C values between coexisting deciduous and evergreen species in both forests indicates that over the long term, these species maintained similar ranges of internal CO2 concentration during photosynthesis (Farquhar & Richards Reference FARQUHAR and RICHARDS1984, Farquhar et al. Reference FARQUHAR, O'LEARY and BERRY1982, Reference FARQUHAR, EHLERINGER and HUBICK1989). This finding runs counter to a number of studies showing that although dry-season-deciduous tropical tree species do not gain C through leaf photosynthesis during the unfavourable dry season, they often have greater maximum rates of photosynthesis than evergreen species during the growing season (Chabot & Hicks Reference CHABOT and HICKS1982, Eamus & Prior Reference EAMUS and PRIOR2001, Santiago et al. Reference SANTIAGO, KITAJIMA, WRIGHT and MULKEY2004), potentially leading to differences in leaf δ13C. Deciduous species are generally considered less conservative in the use of water than evergreen species (Lloyd & Farquhar Reference LLOYD and FARQUHAR1994), as they appear to maximize photosynthetic C gain during a shorter season than evergreen species. Our study on shaded leaves represents a conservative assay and does not exclude the possibility that differences in evergreen and deciduous leaf function are manifested under high light conditions. For example, in dry tropical ecosystems, greater δ13C in deciduous than evergreen species has been shown and related to maximizing C gain in deciduous species through higher concentrations of leaf N and P and higher photosynthetic rates than in evergreen species (Franco et al. Reference FRANCO, BUSTAMANTE, CALDAS, GOLDSTEIN, MEINZER, KOZOVITS, RUNDEL and CORADIN2005, Hasselquist et al. Reference HASSELQUIST, ALLEN and SANTIAGO2010a, Sobrado & Ehleringer Reference SOBRADO and EHLERINGER1997). For a given stomatal aperture, greater rates of photosynthesis results in lower leaf internal CO2 concentration, leading to relatively more incorporation of 13CO2 (Farquhar & Richards Reference FARQUHAR and RICHARDS1984). Yet, Leffler & Enquist (Reference LEFFLER and ENQUIST2002) showed no significant differences in δ13C between evergreen and deciduous tropical tree species in Guanacaste, Costa Rica, similar to our findings. Consistent with our lack of difference in δ13C between deciduous and evergreen species, we also found no differences in leaf N concentration between evergreen and deciduous species, suggesting similar photosynthetic capacity across these groups.
However, it is important to highlight that our results were obtained from shaded leaves in the understorey due to constant cloudiness in the 1200-mm site, whereas most of the literature data is from sun leaves. It is widely known that δ13C varies between sun and shade leaves, with shade leaves being 13C-depleted (Leffler & Enquist Reference LEFFLER and ENQUIST2002, Medina et al. Reference MEDINA, STERNBERG and CUEVAS1991, Xiao et al. Reference XIAO, YANG, SUN, LI and GUO2013). Thus, it is expected that canopy leaves are 13C-enriched in comparison with understorey leaves and the possibility that differences between deciduous and evergreen species could still be evident in canopy leaves can not be ruled out, although no differences due to leaf habit were described in δ13C for canopy leaves in wet forest (Leffler & Enquist Reference LEFFLER and ENQUIST2002).
In general, deciduous species compensate for the shorter leaf payback period with an increase in the potential payback capacity or maximum photosynthetic rate (Kikuzawa Reference KIKUZAWA1991, Pringle et al. Reference PRINGLE, ADAMS, BROADBENT, BUSBY, DONATTI, KURTEN, RENTON and DIRZO2011). However, no significant correlation between δ13C and N were found for deciduous species in either site. It is likely that direct measurements of photosynthetic gas exchange or RUBISCO activity are needed to fully evaluate these relationships. It is also necessary to consider that understorey leaves are not at maximum photosynthetic capacity due to irradiance limitations and that RUBISCO activity may not be sufficient to assimilate all the C available in the sub-stomatal cavity, as occurs in the sun leaves of deciduous species, which leads to lower 13C discrimination.
When analysing all leaf traits together, no significant differences between deciduous and evergreen were found, even in the 1200-mm site, suggesting that differences in water scarcity between the two sites is not felt in understorey leaves to the same degree as canopy leaves in other studies (Franco et al. Reference FRANCO, BUSTAMANTE, CALDAS, GOLDSTEIN, MEINZER, KOZOVITS, RUNDEL and CORADIN2005, Hasselquist et al. Reference HASSELQUIST, ALLEN and SANTIAGO2010a, Sobrado & Ehleringer Reference SOBRADO and EHLERINGER1997). Despite the lower MAP, the presence of clouds could also modulate water loss from the 1200-mm site, leading to more similar water deficits than expected based on MAP alone.
Deciduousness is more evident in dry than wet tropical forests (Borchert Reference BORCHERT1998). In addition, more variability in growth and photosynthetic gas exchange has been described in trees in dry sites than in wet sites (Ruzicka et al. Reference RUZICKA, PUETTMANN and BROOKS2017). Thus, both sites showed a greater degree of functional convergence than expected, which has been described under low-disturbance conditions (ter Steege & Hammond Reference TER STEEGE and HAMMOND2001), as well as a function of limited resources including light (Valladares et al. Reference VALLADARES, SKILLMAN and PEARCY2002) and phosphorus (Fonseca et al. Reference FONSECA, OVERTON, COLLINS and WESTOBY2000). However, when water is the limited resource, physiological trade-offs become extreme, resulting in greater divergence between strategies that on one hand favour withstanding the long dry season as evergreen trees, or on the other hand, reducing costs due to transpiration and respiratory C losses as deciduous trees (Santiago et al. Reference SANTIAGO, KITAJIMA, WRIGHT and MULKEY2004). The lack of difference between evergreen and deciduous species in the understorey of these sites reinforces the idea that water is not the only limiting factor for these forests. Although the question of variable functional convergence along precipitation gradients has not been addressed much in the literature, there is some evidence based on the fact that differences in leaf traits of lianas and trees are greatest in seasonally-dry tropical forests and converge above around 2500 mm (Asner & Martin Reference ASNER and MARTIN2012), suggesting that reduced seasonal variability of water availability leads to a loss of an advantage for some drought resistance strategies and promotes functional convergence as annual precipitation increases.
A difference in leaf δ15N of approximately 4.4‰ was found between the 1200-mm and 1900-mm sites, showing a strong influence of the environment on this leaf trait. Plants are integrators of δ15N from different sources, and their δ15N values reflect source δ15N and internal N cycling (Dawson et al. Reference DAWSON, MAMBELLI, PLAMBOEK, TEMPLER and TU2002). At ecosystem and landscape scales δ15N is thought to be controlled by pathways of N loss, in which losses favour the light isotope and lead to enrichment of δ15N, proportional to the amount of loss (Amundson et al. Reference AMUNDSON, AUSTIN, SCHUUR, YOO, MATZEK, KENDALL, UEBERSAX, BRENNER and BAISDEN2003). Ecosystems rich in N can become progressively enriched in 15N relative to ecosystems with relatively little N (Amundson et al. Reference AMUNDSON, AUSTIN, SCHUUR, YOO, MATZEK, KENDALL, UEBERSAX, BRENNER and BAISDEN2003, Hasselquist et al. Reference HASSELQUIST, SANTIAGO and ALLEN2010b, Martinelli et al. Reference MARTINELLI, PICCOLO, TOWNSEND, CUEVAS, ROBERTSON and TRESEDER1999). At the global scale, decreases of leaf δ15N occur with increasing water availability (Amundson et al. Reference AMUNDSON, AUSTIN, SCHUUR, YOO, MATZEK, KENDALL, UEBERSAX, BRENNER and BAISDEN2003). However, at local scales, where differences in climate may be more subtle, other factors may play a larger role (Nardoto et al. Reference NARDOTO, PIERRE, BALBAUD, EHLERINGER, HIGUCHI, MARIA and MARTINELLI2008). Lower leaf δ15N values have also been interpreted as an indication of relatively low N availability in the ecosystem (Bai et al. Reference BAI, BOUTTON, LIU, WU, ARCHER and HALLMARK2009, Högberg Reference HÖGBERG1997, Martinelli et al. Reference MARTINELLI, PICCOLO, TOWNSEND, CUEVAS, ROBERTSON and TRESEDER1999). The drier conditions of the 1200-mm site compared to the 1900-mm site could limit aqueous N dissolution and plant uptake, consequently limiting N availability, consistent with lower leaf N values at the 1200-mm site. Other factors including temperature, N re-translocation in plants, N fractionation after plant uptake, atmospheric deposition and plant leaf habit are known to influence leaf δ15N (Amundson et al. Reference AMUNDSON, AUSTIN, SCHUUR, YOO, MATZEK, KENDALL, UEBERSAX, BRENNER and BAISDEN2003, Dawson et al. Reference DAWSON, MAMBELLI, PLAMBOEK, TEMPLER and TU2002, Högberg Reference HÖGBERG1997, Martinelli et al. Reference MARTINELLI, OMETTO, FERRAZ, VICTORIA, CAMARGO, MOREIRA, Martinelli, Ometto, Ferraz, Victoria, Camargo and Moreira2009, Ometto et al. Reference OMETTO, EHLERINGER, DOMINGUES, BERRY, ISHIDA, MAZZI, HIGUCHI, FLANAGAN, NARDOTO and MARTINELLI2006, Santiago et al. Reference SANTIAGO, SILVERA, ANDRADE and DAWSON2005, Reference SANTIAGO, SILVEIRA, ANDRADE and DAWSON2017). Alternative N sources, such as biological N2- fixation and mycorrhizal associations also affect δ15N (Evans Reference EVANS2001). Fabaceae, the legume plant family with the potential for biological N2-fixation, is the richest family in both of our sites with similar contribution to the community composition: 11.9% at the 1200-mm site (Couto et al. Reference COUTO, FUNCH and CONCEIÇÃO2011), and 14.3% at the 1900-mm site (Carvalho et al. Reference CARVALHO, NASCIMENTO and OLIVEIRA FILHO2008). However, no clear pattern was observed between potentially N2-fixing and non-legume plant species. Thus it is likely that differences in water availability, soil type, or sources of atmospheric deposition are the main factors influencing mean site δ15N (Nardoto et al. Reference NARDOTO, PIERRE, BALBAUD, EHLERINGER, HIGUCHI, MARIA and MARTINELLI2008, Schulze et al. Reference SCHULZE, WILLIAMS, FARQUHAR, SCHULZE, LANGRIDGE, MILLER and WALKER1998).
Our results indicate that deciduous and evergreen species in the understorey from two sites in Brazilian Atlantic forest that differ in annual precipitation show similar leaf C and N concentration and isotopic composition. This indicates that leaf habit is not a strong factor in distinguishing C and N resource acquisition in shaded leaves, as in sun leaves of other tropical forest regions. Overall, our data showed that leaf isotopic composition and elemental concentration data tended to group species according to site and not according to broad categorical functional groups, such as evergreen and deciduous species. Such functional information as provided by leaf isotopic and elemental analysis can aid in interpreting responses of vegetation to environmental changes. If trends in decreasing precipitation in the tropics continue (Joetzjer et al. Reference JOETZJER, DELIRE, DOUVILLE, CIAIS, DECHARME, FISHER, CHRISTOFFERSEN, CALVET, DA COSTA, FERREIRA and MEIR2014, Pan et al. Reference PAN, BIRDSEY, FANG, HOUGHTON, KAUPPI, KURZ, PHILLIPS, SHVIDENKO, LEWIS, CANADELL, CIAIS, JACKSON, PACALA, MCGUIRE, PIAO, RAUTIAINEN, SITCH and HAYES2011), patterns of resource acquisition revealed by isotopic analysis may serve as indicators of ecological change.
ACKNOWLEDGEMENTS
The authors thank Marcelo Gomes Almeida for helping with isotopic analyses, Thiago Rangel for preparing Figure 1, and Prof. Rafael Silva Oliveira for suggestions. A.P.V., L.F. and C.E.R. thank the Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) from Brazil for the fellowship. L.S.S. thanks the University of California, Riverside Department of Botany and Plant Sciences and the USDA National Institute of Food and Agriculture for support. This research was supported by the State of Rio de Janeiro Research Foundation (FAPERJ E-26/010.002.261/2014.)
Appendix 1.

A comparison between a trait-phenogram and a molecular phylogeny including the 38 plant species (18 families) studied in two tropical forests in Brazil (1200 mm and 1900 mm) using five leaf traits = leaf C concentration, δ13C, N concentration, δ15N and C/N ratio. Crossing lines indicate inconsistencies between evolutionary relationships and trait relationships among species. 1200-mm site and 1900-mm sites are represented by closed circles and open squares, respectively. Species names in black represent evergreen species, whereas deciduous species are represented in red.










