INTRODUCTION
Understanding the processes and patterns related to gene flow and local adaptations requires thorough knowledge of how landscape features structure natural populations. The interaction among landscape characteristics and micro-evolutionary processes, such as gene flow, genetic drift and selection, has implications for ecology, evolution and conservation biology (Manel et al. Reference MANEL, SCHWARTZ, LUIKART and TABERLET2003).
Gene flow maintains the connectivity among populations in a landscape and influences several of their aspects, such as the ability to respond to environmental changes (Frankham et al. Reference FRAGOSO, SILVIUS and CORREA2002). Knowledge of gene flow strategies helps understand the genetic structure of populations (Hamrick & Nason Reference HAMRICK, NASON, Young, Boshier and Boyle2000). In plants, gene flow occurs via pollination and seed dispersal. Gene flow via seed dispersal determines the spatial genetic structure (SGS) of populations, which depends on dispersal distance (Vekemans & Hardy Reference WRIGHT2004).
Many landscape features, such as mountains, humidity gradients and rivers, are geographic barriers to gene flow and so promote genetic discontinuity (Funk et al. Reference FUNK, BLOUIN, CORN, MAXELL, PILLIOD, AMISH and ALLENDORF2005, Spear et al. Reference SPEAR, PETERSON, MATOCQ and STORFER2005). Rivers that do not change their course are often effective barriers to gene flow (Mayr Reference MAYR1963). Some researchers consider rivers effective barriers to gene flow (Lamborot et al. Reference LAMBOROT, EATON and CARRASCO2003, Vallinoto et al. Reference VALLINOTO, ARARIPE, REGO, TAGLIARO, SAMPAIO and SCHNEIDER2006), but most studies have focused on animals, whereas only a few have focused on plants (Pinto et al. Reference PINTO, SOUZA and CARVALHO2004, Tero et al. Reference TERO, ASPI, SIIKAMÄKI, JÄKÄLÄNIEMI and TUOMI2003). Rivers are geographic barriers to gene flow for the tropical tree Copaifera langsdorffii (Pinto et al. Reference PINTO, SOUZA and CARVALHO2004), and for the perennial temperate herb Silene tatarica (Tero et al. Reference TERO, ASPI, SIIKAMÄKI, JÄKÄLÄNIEMI and TUOMI2003). However, rivers are not geographic barriers for other plants, such as the temperate herb Primula sieboldii (Kitamoto et al. Reference KITAMOTO, HONJO, UENO, TAKENAKA, TSUMURA, WASHITANI and OHSAWA2005) or the tropical trees Caryocar microcarpum and Caryocar villosum (Collevatti et al. Reference COLLEVATTI, LEOI, LEITE and GRIBEL2009). Hence, the role of rivers as geographic barriers to gene flow in plants with different reproductive systems is still unclear.
In the present study, we describe the genetic structure and gene flow of the tropical tree Handroanthus ochraceus on both banks of the São Francisco River, Brazil, within a seasonally dry tropical forest. We tested the hypothesis that the São Francisco River acts as a geographic barrier to gene flow of this species.
METHODS
Handroanthus ochraceus (Cham.) Mattos (Bignoniaceae) is a common species in the Brazilian savanna, but it is widely distributed in the seasonally dry tropical forests (SDTF) of northern Minas Gerais State, Brazil. Despite the importance and wide distribution of Handroanthus, studies of this genus are restricted to the Amazon, and have focused mainly on distribution, pollination and phenology (Gentry Reference GENTRY1974, Reference GENTRY1990). The few studies with Handroanthus in the Brazilian savanna have focused on pollination (Barros Reference BARROS2001), polyembryony (Bittencourt & Moraes Reference BITTENCOURT and MORAES2010, Mendes-Rodrigues et al. Reference MENDES-RODRIGUES, SAMPAIO, COSTA, CAETANO, RANAL, BITTENCOURT and OLIVEIRA2012), and herbivory (Ribeiro et al. Reference RIBEIRO, PIMENTA and FERNANDES1994, Silva et al. Reference SILVA, ESPÍRITO-SANTO and MELO2012).
Flowering in H. ochraceus is synchronous and its flowers are pollinated by Bombus bumblebees and Centris bees (Barros Reference BARROS2001, Gibbs & Bianchi Reference GIBBS and BIANCHI1993). Handroanthus ochraceus has small, winged seeds that are dispersed by the wind (Lorenzi Reference LORENZI1992, Silva Junior Reference SILVA JUNIOR2005) and may also float on water. On reaching the soil, even after partial submergence, these seeds may retain the potential to germinate as already observed for Tabebuia cassinoides (Bignoniaceae) (Kolb & Joly Reference KOLB and JOLY2010), a phylogenetically close species (Grose & Olmstead Reference GROSE and OLMSTEAD2007).
We carried out our study in Mata Seca State Park (14°56′59″S 44°04′12″W), which covers 15 466.44 ha and is located on the left bank of the São Francisco River, and in Lagoa do Cajueiro State Park (14°92′90″S, 43°92′15″W), which covers 20 500 ha and is located on the right bank of the same river; both sites are located in northern Minas Gerais State, Brazil (Figure 1). The São Francisco is located in eastern Brazil and is 600 m wide at the study site. Despite crossing a semiarid region, the São Francisco is a perennial river and undergoes no period of low flow (Pereira et al. Reference PEREIRA, PRUSKI, SILVA and RAMOS2007).

Figure 1. Location of Handroanthus ochraceus populations from Mata Seca State Park (MSSP 1 and MSSP 2) and Lagoa do Cajueiro State Park (LCSP), within a seasonally dry tropical forest in the São Francisco River Basin.
We sampled three populations from a Brazilian seasonally dry tropical forest. Two populations were located in Mata Seca State Park (MSSP) and were named MSSP 1 and MSSP 2. We collected 112 H. ochraceus individuals in MSSP 1 (56 juveniles and 56 adults), and 30 individuals in MSSP 2 (six juveniles and 24 adults). The third population (LCSP) was located in Lagoa do Cajueiro State Park (LCSP), where we sampled 70 individuals: 12 juveniles and 58 adults (Table 1). We collected expanded leaves from all plants and stored them at −80 °C for DNA extraction. Genomic DNA extraction followed a standard CTAB procedure (Doyle & Doyle, Reference DOYLE and DOYLE1987). Because of a heavy storm, the flower buds of H. ochraceus fell before being pollinated and no fruits were produced during the study period.
Table 1. Characterization of the populations of Handroanthus ochraceus sampled (full population names are presented in the text) based on seven microsatellites. The sample size was 1000 m2 for all populations. The total number of individuals (N) and juveniles (J) differed between populations. The genetic characterization of populations was based on the average number of alleles (A), expected heterozygosity (He), observed heterozygosity (Ho), and fixation index (f) for all loci. All values of f were significant (P < 0.002, Bonferroni adjusted P value for a nominal level of 5%).

We used seven microsatellite loci (Tau07, Tau12, Tau15, Tau17, Tau28, Tau30, Tau31), which were previously developed for Tabebuia aurea by Braga et al. (Reference BRAGA, REIS, LEOI, PEREIRA and COLLEVATTI2007), to genotype 212 individual plants. We performed microsatellite amplifications for genotyping in a 10-μL volume containing 10.0 μm of each primer, 1 unit of Taq DNA polymerase (Phoneutria, MG), 250 μm of each dNTP, 1× reaction buffer (10 mm Tris-HCl, pH 8.3, 50 mm KCl, 1.5 mm MgCl2), 0.25 μg of BSA and 1.0 ng of template DNA.
We performed amplifications using PE9700 thermal controller (Applied Biosystems, CA, USA) under the following conditions: 94 °C for 5 min (one cycle), 94 °C for 1 min, 48 to 62 °C for 1 min (according to each primer), 72 °C for 1 min (35 cycles) and 72 °C for 30 min (one cycle).
We genotyped the PCR products in an ABI Prism 3130xl automated sequencer (Applied Biosystems, CA, USA) and sized them by comparison with a 500-internal lane standard ROX (Applied Biosystems, CA, USA). Fluorescent PCR products were automatically sized using Genescan and Genotyper softwares (Applied Biosystems, CA). We estimated genotyping errors due to stutter bands, allele dropout, and null alleles in the software MICRO-CHECKER (Oosterhout et al. Reference OOSTERHOUT, HUTCHINSON, WILLS and SHIPLEY2004).
We characterized microsatellite loci according to the number of alleles per locus and observed (Ho) and expected heterozygosity under Hardy–Weinberg equilibrium (He) (Nei Reference NEI1978), based on adult individuals. We also estimated the fixation index (f) for each locus and over all loci (Nei Reference NEI1978). We checked the departure from linkage equilibrium for all pairs of loci. We ran analyses and randomization tests with Bonferroni correction in the software FSTAT 2.9.3.2 (Goudet et al. Reference GOUDET, RAYMOND, DE-MEEUS and ROUSSET1996). To test whether the genetic diversity of populations reflects the occurrence of a recent genetic bottleneck we tested whether populations deviate from mutation drift equilibrium (Cornuet & Luikart Reference CORNUET and LUIKART1996). To determine the significant number of loci with heterozygosity excess we used a non-parametric Wilcoxon signed rank test under stepwise mutation model (SMM) and two-phase model (TPM). The variance assumed in the present study was 70% of SMM and 30% TPM. Analyses were performed in the software Bottleneck (Piry et al. Reference PIRY, LUIKART and CORNUET1999).
To check whether all sampled individuals comprised a single gene pool or whether they belonged to different demes or populations, we made a Bayesian analysis of population structure in the software STRUCTURE 2.2. (Pritchard et al. Reference PRITCHARD, STEPHENS and DONNELLY2000). We used a burn-in period of 100 000 generations and 100 000 steps of Markov Chain Monte Carlo simulations to estimate lnPr (X/K), F ST and Q (individual ancestry) for different values of K. The analyses were run for K = 1 to K = 8. In all analyses we considered the admixture model, a reasonable model to deal with the complexity of real populations, and the correlated allele frequencies model, which deals better with inbreeding. For each K-value, we carried out 10 runs to check for the consistency of the results.
We assessed genetic differentiation among populations with Wright's F-statistics (Wright Reference VEKEMANS and HARDY1951) obtained from an analysis of variance of allele frequencies (Cockerham Reference COCKERHAM1969) and with GST (Hedrick Reference HEDRICK2005), a standardized measure of genetic differentiation which deals better with the high mutation rate of microsatellite loci. We ran the analyses in the program FSTAT 2.9.3.2. We made a significance test of differentiation with Bonferroni correction by randomizing genotypes among samples to obtain the log-likelihood G statistics (Goudet et al. Reference GOUDET, RAYMOND, DE-MEEUS and ROUSSET1996).
We estimated gene flow among populations using the indirect form (Wright Reference VEKEMANS and HARDY1951) considering immigrants (Nm) and genetic differentiation (Fst) between populations: Nm = 1/4(1/Fst−1). However, we used GST (Hedrick, Reference HEDRICK2005) for genetic differentiation between populations for gene flow estimation.
We estimated relatedness based on the unbiased regression estimator by Lynch & Ritland (Reference LYNCH and RITLAND1999) in the software MARK (Ritland Reference RITLAND2004). A Monte Carlo simulation provided estimates of relatedness variance and mean relatedness standard error when sample size was adequate. We estimated pairwise relatedness based on the Queller & Goodnight (Reference QUELLER and GOODNIGHT1989) estimator. We ran generalized linear models (Crawley Reference CRAWLEY2002) in R 2.6.1 to test whether individuals from populations of the same bank were more closely related to each other than individuals from populations of different banks.
RESULTS
All seven microsatellite loci were in linkage equilibrium (all P > 0.00238, adjusted nominal 5% level with Bonferroni correction) and had low polymorphism. The observed heterozygosity was lower than the expected under Hardy–Weinberg equilibrium for most loci, with a fixation index significantly different from zero (Table 2). Nevertheless, the combined probability of paternity exclusion (QC) was high and the probability of identity (IC) was very low, ~10−22 (Table 2). The results of the Wilcoxon test showed that all loci were with heterozygosity excess under SMM and TPM. This significant heterozygosity excess (P = 0.019) suggests a deviation of H. ochraceus populations from mutation drift equilibrium, probably due to a recent genetic bottleneck.
Table 2. Characterization of seven microsatellites, based on a sample of 138 adult individuals of Handroanthus ochraceus from Mata Seca State Park and Lagoa do Cajueiro State Park. The microsatellite characterization was based on the average number of alleles (A), expected heterozygosity (He), observed heterozygosity (Ho), fixation index (f), probability of paternity exclusion (Q), combined probability of paternity exclusion (QC), probability of genetic identity (I), and combined probability of genetic identity (IC). Values followed by ns did not differ from zero, for P = 0.005, Bonferroni adjusted P value for a nominal level of 5%.

Allelic richness was very similar among all populations (Table 1). Even the MSSP 2 population exhibited a slightly lower allelic richness than the other populations, but differences were not significant (P > 0.57). The fixation index was high and significant for all populations (Table 1).
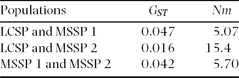
Although genetic differentiation was significantly different among populations (θ = 0.043, P < 0.002 and GST = 0.016, P < 0.002), these values were very low, which suggests low genetic differentiation (Table 3). We found a significant f-value (0.248, P < 0.002), and the F-value was also high and significant (0.280, P < 0.002) (Table 4). The Bayesian analysis showed no population structuring (K = 1, ln P(X/K) = −1984.2). The assignments were roughly symmetric for all populations (~1/K) when K > 1 and no individuals were strongly assigned, which points to a lack of population structure.
Table 3. Population genetic structure of Handroanthus ochraceus based on an analysis of variance of allele frequencies and allele size for each microsatellite. The genetic structure is based on the fixation index (f), total inbreeding coefficient (F), and population differentiation (θ and GST) based on allele size. Values followed by * are significantly different (P < 0.002).

Table 4. Pairwise gene flow based on an analysis of genetic differentiation of Handroanthus ochraceus populations from Mata Seca State Park and Lagoa do Cajueiro State Park.
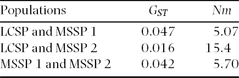
The gene flow estimated based on genetic diversity among populations was high for all pairwise populations (>1), which indicates that gene flow occurs between these populations (Table 4). The genetic relatedness among individuals from populations of the same bank did not differ from the relatedness among individuals from populations of different banks.
DISCUSSION
The populations of H. ochraceus showed low polymorphism and genetic diversity in the seasonally dry tropical forests (SDTF) of northern Minas Gerais State, Brazil. The results also point to the occurrence of a genetic bottleneck in H. ochraceus. A low genetic diversity is characteristic of the founder effect, which happens when a new population begins with only a few members of the original population and, thus, has low genetic diversity (Frankham et al. Reference FRANKHAM, BALLOU and BRISCOE2002). A probable explanation for the low genetic diversity observed in the studied populations of H. ochraceus could be the colonization of the study area by only a few individuals in the Quaternary. The SDTF remnants scattered throughout the Brazilian dry forests and savannas (Werneck 2011) were part of a much larger continuous area of SDTFs that formed the ‘Pleistocenic Arc’ (Prado Reference PRADO2000, Prado & Gibbs Reference PRADO and GIBBS1993). Due to increases in temperature and precipitation, the SDTF got restricted to the extant patches and other Brazilian biomes expanded. Thus, H. ochraceus may have established itself in SDTF areas of northern Minas Gerais State through a few individuals that came from the savanna. Besides, the São Francisco River Basin has a history of disturbance. The basin of this river was occupied in the 17th century, and because of high soil fertility this region has been receiving governmental subsidies for agribusiness and irrigation projects. Thus, these forests have been isolated for many generations (Espírito-Santo et al. Reference ESPÍRITO-SANTO, SEVILHA, ANAYA, BARBOSA, FERNANDES, SANCHEZ-AZOFEIFA, SCARIOT, NORONHA and SAMPAIO2009).
The observed heterozygosity of the studied populations was lower than expected, which resulted in significant values of the fixation index and indicates that mating among individuals of these populations does not occur at random and there is an excess of homozygotes in these populations. Although some loci presented a significant excess of homozygotes, the low value of combined probability of identity (IC) showed that the battery of loci is suitable for kinship analysis. The analysis of raw data in the software MICRO-CHECKER (Oosterhout et al. Reference OOSTERHOUT, HUTCHINSON, WILLS and SHIPLEY2004) showed that the results were not affected by genotyping errors or null alleles (results not shown).
The analysis of genetic structure of H. ochraceus populations showed that there is significant differentiation among all populations studied (θ = 0.043 and GST = 0.016). However, this differentiation is very low by the standards of Frankham et al. (Reference FRAGOSO, SILVIUS and CORREA2002), who assumed that a value above 0.15 is considered an indicator of significant differentiation between populations. Bayesian analyses showed no population structuring (K = 1). These results, reflected in θ and GST, showed that the São Francisco River is not a geographic barrier to gene flow among populations of H. ochraceus and also emphasized the lack of genetic structure, claiming that all individuals belong to the same gene pool. Furthermore, θ and GST were very low and F and f were high, which shows that differentiation due to genetic drift is low, though non-random mating may be important in shaping the genetic structure of these populations (Moreira et al. Reference MOREIRA, FERNANDES and COLLEVATTI2009).
The estimated apparent gene flow has high values among populations. Govindajaru (Reference GOVINDAJARU1989) distinguishes three levels of gene flow: high, when Nm > 1, intermediate, when 0.25 < Nm < 0.99, and low, when Nm < 0.25. According to this definition, the gene flow among the studied populations is high. The results of gene flow corroborate the lack of genetic differentiation among individuals of H. ochraceus. Therefore, there is gene flow among trees from different populations that maintains their homogeneity and prevents differentiation. Kinship analysis revealed that individuals from populations of the same bank of the São Francisco River are not more genetically closely related than individuals from populations of opposite river bank. These results showed once more that the São Francisco River is not a geographic barrier to gene flow.
As the São Francisco is not a geographic barrier to gene flow between populations and there was no genetic differentiation among populations, the gene flow among individuals of H. ochraceus of different populations may be promoted by pollination and seed dispersal. Gene flow between spatially separated populations may occur if pollinators are able to travel long distances (Ghazoul Reference GHAZOUL2005). Handroanthus ochraceus is pollinated by Centris and Bombus bees (Barros Reference BARROS2001), which are large bees that can forage over long distances. Studies on Bombus showed that these bumblebees can fly up to 9.8 km (Goulson & Stout Reference GOULSON and STOUT2001) and 12 km (Hedtke Reference HEDTKE1996). The longest distance ever recorded of a hymenopteran flight was made by Janzen (Reference JANZEN1971), who observed that the pollinating bee of Euglossa imperialis can fly up to 23 km. Nason et al. (Reference NASON, HERRE and HAMRICK1998) found that some wasps (Agaonidae, Chalcidoidea), which pollinate figs (Ficus sp. Moraceae) transferred pollen between trees separated by up to 10 km in a tropical forest. Thus, the pollinating bees of H. ochraceus can move among the studied populations and promote the gene flow among them, despite their separation by the river.
In addition, the seeds of H. ochraceus are dispersed by the wind (Lorenzi Reference LORENZI1992) and have typical morphological adaptations: they are winged, small, lightweight, and produced in large quantity (Lorenzi Reference LORENZI1992, Silva Junior Reference SILVA JUNIOR2005). These factors facilitate seed dispersal by the wind, which may result in long dispersal distances (van der Pijl Reference VAN DER PIJL1982). According to Nathan et al. (Reference NATHAN, KATUL, HORN, THOMAS, OREN, AVISSAR, PACALA and LEVIN2002), seeds dispersed by wind may reach at least a few hundred metres or even tens of kilometres. Müller-Schneider (Reference MÜLLER-SCHNEIDER1955) recorded the distance achieved by some wind-dispersed seeds: 7 km by Abies, 2 km by Pinus sylvestris, 1.6 km by Betula, 4 km by Acer and 0.5 km by Fraxinus excelsior. For Tussilago, the distance reached by its seeds was 14 km, for Populus 30 km, and 200 km for Senecio congestus (van der Pijl Reference VAN DER PIJL1982). Ghazoul (Reference GHAZOUL2005) observed that seeds dispersed by the wind are immune to discontinuities in the landscape. Thus the seeds of H. ochraceus may cross the São Francisco River, colonize the opposite river bank, and maintain the gene flow between individuals of opposite river banks. The São Francisco River changed its hydrological condition in the Quaternary and in the Late Pleistocene its annual average discharge was below its current discharge (3.800 m3 s−1) (Latrubesse et al. Reference LATRUBESSE, STEVAUX, SANTOS, ASSINE, SOUZA, SUGUIO, OLIVEIRA and OLIVEIRA2005), facilitating gene flow between river banks through seeds.
Pinto et al. (Reference PINTO, SOUZA and CARVALHO2004) found out that rivers are geographic barriers to gene flow between populations of the tropical tree Copaifera langsdorffii, showing that populations located on opposite river banks are genetically differentiated. Copaifera langsdorffii is pollinated by small bees, such as Apis mellifera, Scatotrigona depilis and Trigona spinipes (Freitas & Oliveira Reference FREITAS and OLIVEIRA2002), which may not be able to cross the river. Its seeds are dispersed by birds, such as Cyanocorax cristatellus and Turdus rufiventris (Carvalho Reference CARVALHO1994) and some studies revealed that rivers can indeed restrict the movements of some bird species (Capparella Reference CAPPARELLA1988, Reference CAPPARELLA1991; Fernandes et al. Reference FERNANDES, WINK and ALEIXO2012). The same result was observed in the perennial temperate herb Silene tatarica (Tero et al. Reference TERO, ASPI, SIIKAMÄKI, JÄKÄLÄNIEMI and TUOMI2003), which is pollinated by bees and has its seeds dispersed primarily by gravity (Tero Reference TERO2005). Nevertheless, separate river banks are not a geographic barrier to gene flow in the temperate herb Primula sieboldii (Kitamoto et al. Reference KITAMOTO, HONJO, UENO, TAKENAKA, TSUMURA, WASHITANI and OHSAWA2005), a species also pollinated by Bombus bumblebees. Rivers are not barriers to gene flow in two tropical tree species, Caryocar microcarpum and Caryocar villosum (Collevatti et al. Reference COLLEVATTI, LEOI, LEITE and GRIBEL2009), which are pollinated by bats (Gribel & Hay Reference GRIBEL and HAY1993, Martins & Gribel Reference MARTINS and GRIBEL2007) and have their seeds dispersed by a mammal with high swimming ability (Fragoso et al. Reference FRAGOSO, SILVIUS and CORREA2003). In conclusion, our results suggest that the São Francisco River is not a geographic barrier to the gene flow of H. ochraceus and the exchange of genes occurs among populations from left and river banks. The gene flow is responsible for the absence of genetic differentiation among populations of H. ochraceus, pointing out that all individuals belong to the same gene pool.
ACKNOWLEDGEMENTS
The field work was funded by TROPI-DRY [Inter-American Institute for Global Change Research (IAI) CRN II # 021], CNPq (30.9633/2007–9), and Fapemig (CRA 697/06), to whom we are grateful. We also thank José Luis and IEF (Instituto Estadual de Florestas) for their support in Mata Seca State Park, Frederico de Siqueira Neves and Cassia Lima for their help in the field, and Rodrigo O. Pessoa for his help with the map. We thank the Universidade Católica de Brasília for their support with laboratory work, especially Aline Cabral Braga for her help in the laboratory. This study is part of the Master's thesis in Genetics of PAM, and was developed at Universidade Federal de Minas Gerais.