INTRODUCTION
Understanding community assembly mechanisms is a central goal in community ecology. Many studies have focused on the two ecological processes: environmental filtering that leads to a convergence in ecological strategy (Cornwell et al. Reference CORNWELL, SCHWILK and ACKERLY2006, Siefert et al. Reference SIEFERT, RAVENSCROFT, WEISER and SWENSON2013), and competitive interactions that result in a divergence in ecological strategies (Chalmandrier et al. Reference CHALMANDRIER, MUNKEMULLER, GALLIEN, DE BELLO, MAZEL, LAVERGNE and THUILLER2013, Purschke et al. Reference PURSCHKE, SCHMID, SYKES, POSCHLOD, MICHALSKI, DURKA, KUHN, WINTER and PRENTICE2013). Functional diversity has the potential to disengage community assembly (Conti & Diaz Reference CONTI and DIAZ2013, Roscher et al. Reference ROSCHER, SCHUMACHER, LIPOWSKY, GUBSCH, WEIGELT, POMPE, KOLLE, BUCHMANN, SCHMID and SCHULZE2013). Species tend to functional convergence under strong environmental filtering, but tend to functional divergence under strong competitive interactions (Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). However, the outcome of competitive interactions may depend on the relative strength of niche differences and fitness differences. Competitive interactions can also drive functional convergence if fitness differences are stronger (Mayfield & Levine Reference MAYFIELD and LEVINE2010).
The strength of environmental filtering and competitive interactions may depend on environmental conditions (Katabuchi et al. Reference KATABUCHI, KUROKAWA, DAVIES, TAN and NAKASHIZUKA2012). Studies have found that functional diversity is often significantly related to the variation in soil and climate at larger scales (Fortunel et al. Reference FORTUNEL, PAINE, FINE, KRAFT and BARALOTO2014, Siefert et al. Reference SIEFERT, RAVENSCROFT, WEISER and SWENSON2013), or changes with successional stage and land-use (Janecek et al. Reference JANECEK, DE BELLO, HORNIK, BARTOS, CERNY, DOLEZAL, DVORSKY, FAJMON, JANECKOVA, JIRASKA, MUDRAK and KLIMESOVA2013, Purschke et al. Reference PURSCHKE, SCHMID, SYKES, POSCHLOD, MICHALSKI, DURKA, KUHN, WINTER and PRENTICE2013). There was evidence that multivariate functional diversity is low under strong abiotic stress, but elevated in the highly productive and competitive environments (Weiher & Keddy Reference WEIHER and KEDDY1995). However, a study also found that multivariate functional diversity was not significantly related to environmental gradients (Cornwell et al. Reference CORNWELL, SCHWILK and ACKERLY2006). Different traits may be associated with different niche axes (i.e. tree height and leaf size are linked to plant stature, but specific leaf area, leaf dry matter content and chlorophyll concentration are related to leaf traits and resource acquisition), and multivariate functional diversity may mask community assembly processes when traits are associated with opposing niche axes (Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). Therefore, investigating how single traits are associated with different niche axes may contribute to understanding community assembly (Katabuchi et al. Reference KATABUCHI, KUROKAWA, DAVIES, TAN and NAKASHIZUKA2012, Swenson & Enquist Reference SWENSON and ENQUIST2009).
In this study, we evaluated the relationships between functional diversity and environmental conditions in a 20-ha Dinghushan (DHS) Forest Dynamics Plot in Southern China, to infer community assembly processes across environmental gradients, and to assess the relative importance of environmental factors in controlling functional diversity. We addressed the following two hypotheses: (1) Functional dominance significantly correlates with environmental gradients at the small scale under the environmental filtering effects; functional diversity changes with environmental gradients, co-occurring species tend to be functionally convergent in relative abiotic stressful environments due to environmental filtering effects, but tend to be functionally divergent in environments with high resource availability, where competitive interactions and resource partitioning are dominant. (2) Environmental factors related to key limiting resources in subtropical forests, such as light and soil fertility, have the most important effects on functional diversity.
METHODS
Study site
The study area is located in the 20-ha DHS Forest Dynamics Plot (subtropical evergreen broad-leaved forest) in the Dinghushan Natural Reserve (1155 ha, established in 1956), Southern China (23˚09′21″–23˚11′30″N, 112˚30′39″–112˚33′41″E). The mean annual temperature and precipitation are 20.9°C and 1927 mm, respectively, and mean relative humidity is 85%. The altitude of the DHS plot ranges from 230 to 470 m asl. The soil is composed mainly of lateritic red and mountain yellow brown soil. The 20-ha DHS plot was established in the core of the DHS Natural Reserve in 2005 (Bin et al. Reference BIN, YE, MULLER-LANDAU, WU, LIAN and CAO2012). All stems with a diameter at breast height (dbh) ≥1 cm were measured, mapped and tagged in the initial census (Shen et al. Reference SHEN, SANTIAGO, MA, LIN, LIAN, CAO and YE2013). A total of 178 species and 61,125 individuals were recorded in the second census in 2010. The canopy height of DHS plot is more than 15 m, and Castanopsis chinensis, Schima superba, Engelhardtia roxburghiana, Cryptocarya chinensis and Cryptocarya concinna are the dominant species in this community (Bin et al. Reference BIN, LIAN, WANG, YE and CAO2011).
Functional traits
Six key functional traits were measured on 6–12 individuals randomly for each of the 92 species in the DHS plot (Appendices 1 and 2), using the standardized methods of Cornelissen et al. (Reference CORNELISSEN, LAVOREL, GARNIER, DIAZ, BUCHMANN, GURVICH, REICH, TER STEEGE, MORGAN, VAN DER HEIJDEN, PAUSAS and POORTER2003), and selected individuals should be without obvious symptoms of pathogens or herbivore attack and without substantial cover of epiphylls. The 92 species make up 95.5% of the cumulative community basal area in the plot; these species are the most important species in determining community assembly and ecosystem function. We used a 10-m telescopic fibreglass measuring pole, and assisted by climbing to collect canopy leaves for tall trees. Four relatively young, fully expanded and healthy leaves were measured for each individual.
Leaf chlorophyll concentration (g m−2) was evaluated as the average of three points on each leaf by a portable chlorophyll meter (SPAD 502, Plus Chlorophyll Meter, Konica Minolta, USA) (Loh et al. Reference LOH, GRABOSKY and BASSUK2002). Leaf size (cm2) was determined using a scanner (CanoScan LiDE 700F), and image processing software (ImageJ version 1.43u, National Institute of Mental Health, Bethesda, Maryland, USA). Leaf lamina thickness (mm) was measured twice on each side of the main vein at the widest part of each leaf using a micrometer and avoiding large secondary veins. Leaves were placed in an oven at 60°C for at least 72 h after weighing for fresh mass, and then re-weighed to determine dry mass. Specific leaf area (SLA; cm2 g−1) was determined by dividing leaf size by oven-dried mass. Leaf dry matter content (LDMC; g g−1) was then expressed as the ratio of leaf dry mass to leaf fresh mass.
Wood samples were collected from six randomly chosen individuals of each of the 92 species outside the plot. An increment borer was used to extract a tree core at approximately 1.3 m height on the main stem for trees larger than 6 cm dbh, but for smaller trees and shrubs, we collected stem segments (10 cm length and 1 cm diameter) from terminal branches. We quantified wood sample volume, and dry mass was determined after at least 96 h at 60°C (Cornelissen et al. Reference CORNELISSEN, LAVOREL, GARNIER, DIAZ, BUCHMANN, GURVICH, REICH, TER STEEGE, MORGAN, VAN DER HEIJDEN, PAUSAS and POORTER2003). Wood density (g cm−3) was calculated as the ratio of dry mass to fresh volume. All functional traits of individuals were averaged, resulting in one mean value of each trait for each species, which was used to represent the characters of species.
Traits selected in this study have been proposed to be good predictors of plant performance. Chlorophyll is linked to leaf nitrogen content and hence to photosynthetic rates (Chaturvedi et al. Reference CHATURVEDI, RAGHUBANSHI and SINGH2011). Larger leaves tend to have less frequent branching and to bear larger fruits, and have a thicker boundary layer which overheats more easily, leading to higher respiration and transpiration costs (Wright et al. Reference WRIGHT, ACKERLY, BONGERS, HARMS, IBARRA-MANRIQUEZ, MARTINEZ-RAMOS, MAZER, MULLER-LANDAU, PAZ, PITMAN, POORTER, SILMAN, VRIESENDORP, WEBB, WESTOBY and WRIGHT2007). Thinner leaves tend on average to higher nitrogen concentration, fuelling onward assimilation and growth rates (Poorter & Bongers Reference POORTER and BONGERS2006). SLA measures light-intercepting leaf area per dry mass invested, leaves of higher SLA tend to have higher nitrogen content per unit leaf area, consequently, higher metabolic and growth rates (Wright et al. Reference WRIGHT, REICH, WESTOBY, ACKERLY, BARUCH, BONGERS, CAVENDER-BARES, CHAPIN, CORNELISSEN, DIEMER, FLEXAS, GARNIER, GROOM, GULIAS, HIKOSAKA, LAMONT, LEE, LEE, LUSK, MIDGLEY, NAVAS, NIINEMETS, OLEKSYN, OSADA, POORTER, POOT, PRIOR, PYANKOV, ROUMET, THOMAS, TJOELKER, VENEKLAAS and VILLAR2004). LDMC reflects the amount of assimilatory tissue versus structural compound in a leaf. A lower LDMC corresponds to a higher proportion of assimilatory tissue and lower structural component, leads to higher photosynthetic and growth rates of species (Kazakou et al. Reference KAZAKOU, VILE, SHIPLEY, GALLET and GARNIER2006). Lower wood density represents less biomass invested per unit volume, thus results in higher growth rates of species (King et al. Reference KING, DAVIES, SUPARDI and TAN2005, Poorter et al. Reference POORTER, WRIGHT, PAZ, ACKERLY, CONDIT, IBARRA-MANRIQUES, HARMS, LICONA, MARTINEZ-RAMOS, MAZER, MULLER-LANDAU, PENA-CLAROS, WEBB and WRIGHT2008).
Environmental conditions
Topographical indices of habitat were measured as quadrat altitude (m), convexity (m) and slope angle (°) (Appendix 2). We measured the altitude of each 20 × 20-m quadrat within the DHS 20-ha plot using an electronic total station, averaging values from the four corners of each quadrat (Legendre et al. Reference LEGENDRE, MI, REN, MA, YU, SUN and HE2009). Convexity of each 20 × 20-m quadrat was calculated as the altitude of the focal plot minus the mean altitude of the eight 20 × 20-m quadrats around each focal plot. For the edge quadrat, convexity was the altitude of the centre point minus the mean of its four corners. Slope was defined as the mean angular deviation from horizontal of each of the four triangular planes formed by connecting three of its corners.
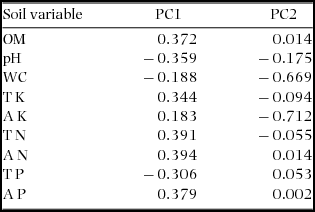
Soil-based indices of habitat were determined by sampling soils at 30-m grid points within the plot for a total of 710 sampling points (Appendix 3). At each point, we collected 500 g of topsoil (0–10 cm depth) and analysed nine soil properties: organic matter (OM; mg g−1), soil pH, water content (WC, %), total potassium (TK; mg g−1), available potassium (AK; mg g−1), total nitrogen (TN; mg g−1), available nitrogen (AN; mg g−1), total phosphorus (TP; mg g−1) and available phosphorus (AP; mg g−1) (Lin et al. Reference LIN, STRALBERG, GONG, HUANG, YE and WU2013). Soil values for each 20 × 20-m quadrat were calculated using kriging methods. Variables of soil fertility were strongly correlated with each other, thus we computed principal components from the nine soil variables and used only the first two components (PC1 and PC2), which explained 77.9% of the total variance in soil variables (Table 1). PC1 was associated with soil fertility, and PC2 was associated with concentration of available K and water content (Table 1).
Table 1. Factor loadings of the first two components of the Principal Component Analysis (PCA) on soil variables in the Dinghushan (DHS) 20-ha plot. OM, organic matter; pH, soil pH; WC: water content of soil; TK, total K; AK, available K; TN, total N; AN, available N; TP, total P; AP, available P.
Data analysis
Functional diversity was represented by functional dispersion (FDis) (Appendix 4), which quantifies the mean distance of individual species to the centroid of all species in the community (Laliberte & Legendre Reference LALIBERTE and LEGENDRE2010). The weighted centroid was calculated as:
where c is the weighted centroid in the i-dimensional spaces, aj is the abundance of species j, aij is the attribute of species j for traits i. The FDis was calculated as:
where zj is the distance of species j to the weighted centroid c. The relative abundances of species can be taken into account in the calculation of FDis, which can eliminate the effects of different abundances of species across different quadrats, and can be used for single traits and multiple traits (Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). Furthermore, functional dispersion is independent from species richness, which allows comparison of quadrats and size classes with different species richness without bias (Laliberte & Legendre Reference LALIBERTE and LEGENDRE2010, Villeger et al. Reference VILLEGER, MASON and MOUILLOT2008).
Community weighted mean (CWM) trait values were also estimated to represent functional dominance in this study, which were calculated as the mean trait value weighted by species abundance in the community (Garnier et al. Reference GARNIER, CORTEZ, BILLES, NAVAS, ROUMET, DEBUSSCHE, LAURENT, BLANCHARD, AUBRY, BELLMANN, NEILL and TOUSSAINT2004). Functional diversity and functional dominance were calculated for each quadrat.
In order to eliminate the possible spurious effect of spatial autocorrelation, we used a partial Mantel test to estimate the relationships between CWM trait values and environmental gradients (Cielo et al. Reference CIELO, GNERI and MARTINS2007, Houle et al. Reference HOULE, MCKENNA and LAPOINTE2001, Legendre & Fortin Reference LEGENDRE and FORTIN1989), and then another partial Mantel test was also used to test associations between environmental gradients and functional diversity. In the partial Mantel test, we calculated three dissimilarity matrices, CWM trait values dissimilarity or functional diversity dissimilarity, environmental dissimilarity and the matrix of geographic distances among quadrats for controlling space effects. These univariate partial Mantel tests can identify statistically significant relationships between CWM and diversity and environments, and permit a first comparison of the direction (Poorter et al. Reference POORTER, WRIGHT, PAZ, ACKERLY, CONDIT, IBARRA-MANRIQUES, HARMS, LICONA, MARTINEZ-RAMOS, MAZER, MULLER-LANDAU, PENA-CLAROS, WEBB and WRIGHT2008). Multiple stepwise regression models were used to examine the relationships between functional diversity and combinations of environmental variables, which can eliminate unimportant variables, and evaluate the relative importance (R2 and P value) of environmental variables in regulating functional diversity patterns. Seven models including single traits and multiple traits were established. Finally, in order to determine the relative importance of each environmental factor in the multiple-trait regression model, we decomposed R2 using hierarchical partitioning method (Gromping Reference GROMPING2006, Mac Nally Reference MAC NALLY2002).
Data analyses were conducted in R (3.0.1, 2013), functional dispersion was calculated by the FD R language package (Laliberte & Legendre Reference LALIBERTE and LEGENDRE2010), the partial Mantel tests were performed in the vegan package, and the hierarchical partitioning was analysed in the relaimpo package (Gromping Reference GROMPING2006).
RESULTS
The partial Mantel test between environmental gradients and CWM trait values indicated that environmental conditions had strong effects on the distributions of trait values (Table 2), especially convexity and soil PC1. For instance, LDMC, leaf size, SLA and wood density significantly increased with increasing convexity and soil PC1. However, no significant relationships between altitude, slope, PC2 and CWM trait values were observed in this study.
Table 2. Partial Mantel correlation coefficients between environmental conditions and community-weighted mean (CWM) trait values for all individuals in the Dinghushan (DHS) 20-ha plot. LDMC, leaf dry matter content; SLA, specific leaf area; PC1, the first principal component of soil variables; PC2, the second principal component of soil variables.* P < 0.05; ** P < 0.01; *** P < 0.001.

Functional diversity was largely affected by convexity and soil properties (Table 3). For example, diversity of LDMC, leaf size, SLA and thickness tended to be higher in the high-convexity quadrats. In the high-fertility quadrats, diversity of LDMC, leaf size, SLA also tended to be greater. Leaf thickness was more diverse in the steep-slope quadrats. However, functional diversity of chlorophyll concentrations of trees in quadrats increased with decreasing concentration of available K and water content (PC2) (Table 3). For multivariate functional diversity, species were functionally divergent in the quadrats of high convexity and soil fertility (PC1).
Table 3. Partial Mantel correlation coefficients between environmental conditions and functional dispersion (FDis) for single traits and multiple traits in the Dinghushan (DHS) 20-ha plot. See Table 2 for abbreviations in the text.* P < 0.05; ** P < 0.01; *** P < 0.001.

Stepwise multiple regression models showed that different environmental conditions had different importance in affecting functional diversity patterns (Table 4). For example, all regressors except for PC2 were significant in the model of leaf size, and explained 41% of the variation in the functional dispersion of leaf size. However, only altitude and convexity were significant in the model of wood density, and only 10% of the variation was explained. For the model of multiple traits, all regressors were significant, and convexity and soil properties (PC1 and PC2) had more important effects on functional diversity patterns (P values were smaller). In addition, 30% of the variation in functional dispersion can be explained (Table 4).
Table 4. Stepwise multiple regression coefficients and P values for the relationships between environments and functional diversity (FDis), including single traits and multiple traits. R2 of models were showed. See Table 2 for abbreviations in the text.* P < 0.05; ** P < 0.01; *** P < 0.001; NS, not significant.

When we decomposed the R2 of the multiple-trait model to find the best predictors of functional diversity, they were convexity and soil PC1 (Figure 1), which contributed 30.5% and 29.0% of total R2 to this model.

Figure 1. Relative importance of each environmental factor in the multiple traits regression model for 92 species in the Dinghushan (DHS) 20-ha plot. See Table 2 for abbreviations in the text.
DISCUSSION
Environmental filtering and competitive interactions are important ecological processes in community assembly. Consequently, functional diversity patterns have the potential to detect the two opposing effects (Mason et al. Reference MASON, DE BELLO, MOUILLOT, PAVOINE and DRAY2013, Mouchet et al. Reference MOUCHET, VILLEGER, MASON and MOUILLOT2010, Siefert et al. Reference SIEFERT, RAVENSCROFT, WEISER and SWENSON2013). In our study, co-occurring species were functionally divergent in area with high terrain convexity and soil fertility (Table 3),which suggests that functional diversity is high in regions of high resource availability and strong competitive interactions, and decreased in regions of relative strong abiotic stress (Cornwell & Ackerly Reference CORNWELL and ACKERLY2009, Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). These results are consistent with our first hypothesis. However, functional convergence may also derive from competitive interactions if differences in competitive ability overcome niche differences (Mayfield & Levine Reference MAYFIELD and LEVINE2010). For instance, competitors may differ in a specific trait, i.e. tree height, so competitive ability difference will occur when light is a limiting resource. In this situation, competitive interactions will exclude all but the tallest species, and then drive clustering. This may occur in specific regions of a homogeneous habitat where niche differences are not significant, but may not occur in a community with complicated habitats such as the DHS plot (Shen et al. Reference SHEN, SANTIAGO, MA, LIN, LIAN, CAO and YE2013, Wang et al. Reference WANG, YE, CAO, HUANG, LIAN, LI, WEI and SUN2009).
Our results showed that CWM trait values changed in response to environmental gradients (Table 2), especially terrain convexity and soil fertility (PC1), which indicates that environmental filtering is occurring in this forest (Spasojevic & Suding Reference SPASOJEVIC and SUDING2012), which is also consistent with the first hypothesis. Convexity reflects the contrast in altitude between a focal quadrat and surrounding quadrats, where very high convexity may indicate a hilltop and very low convexity may indicate bottomlands or a local hollow (Detto et al. Reference DETTO, MULLER-LANDAU, MASCARO and ASNER2013). Convexity is often negatively associated with soil moisture and nutrient availability (Lan et al. Reference LAN, HU, CAO and ZHU2011, McEwan et al. Reference MCEWAN, LIN, SUN, HSIEH, SU, CHANG, SONG, WANG, HWONG, LIN, YANG and CHIANG2011, Noguchi et al. Reference NOGUCHI, ITOH, MIZUNO, SRI-NGERNYUANG, KANZAKI, TEEJUNTUK, SUNGPALEE, HARA, OHKUBO, SAHUNALU, DHANMMANONDA and YAMAKURA2007), and potentially has effects on plant performance, such as species composition and richness (Legendre et al. Reference LEGENDRE, MI, REN, MA, YU, SUN and HE2009), species distribution patterns (Lan et al. Reference LAN, HU, CAO and ZHU2011, Wang et al. Reference WANG, YE, CAO, HUANG, LIAN, LI, WEI and SUN2009) and above-ground biomass (McEwan et al. Reference MCEWAN, LIN, SUN, HSIEH, SU, CHANG, SONG, WANG, HWONG, LIN, YANG and CHIANG2011). However, high convexity can be associated with high light availability (Enoki & Abe Reference ENOKI and ABE2004, Tsujino et al. Reference TSUJINO, TAKAFUMI, AGETSUMA and YUMOTO2006), which is one of the most limiting resources for the understorey in tropical forests (King et al. Reference KING, DAVIES, SUPARDI and TAN2005, Poorter et al. Reference POORTER, BONGERS, STERCK and WOLL2005, Ruger et al. Reference RUGER, HUTH, HUBBELL and CONDIT2009). High convexity (associated with high light) and high soil fertility (PC1) can also promote species with large leaf size, high specific leaf area and chlorophyll concentration (Table 2), and can result in high light-capturing and photosynthetic rates, as well as high growth rates and carbon gain in high-light environments (Carreno-Rocabado et al. Reference CARRENO-ROCABADO, PENA-CLAROS, BONGERS, ALARCON, LICONA and POORTER2012, Poorter et al. Reference POORTER, WRIGHT, PAZ, ACKERLY, CONDIT, IBARRA-MANRIQUES, HARMS, LICONA, MARTINEZ-RAMOS, MAZER, MULLER-LANDAU, PENA-CLAROS, WEBB and WRIGHT2008, Sterck et al. Reference STERCK, POORTER and SCHIEVING2006). The positive relationships between wood density, convexity and PC1 may be due to the fact that wood density increased with declining soil moisture (Cornwell & Ackerly Reference CORNWELL and ACKERLY2009), and trees exposed to stronger winds in high-convexity quadrats may have higher wood density to resist natural stresses. However, LDMC was positively associated with convexity and PC1, which was not consistent with the findings of Carreno-Rocabado et al. (Reference CARRENO-ROCABADO, PENA-CLAROS, BONGERS, ALARCON, LICONA and POORTER2012).
In previous studies, environmental conditions were associated with functional diversity patterns at larger scales (Cornwell & Ackerly Reference CORNWELL and ACKERLY2009, Fortunel et al. Reference FORTUNEL, PAINE, FINE, KRAFT and BARALOTO2014, Siefert et al. Reference SIEFERT, RAVENSCROFT, WEISER and SWENSON2013, Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). However, our results showed that variation in environments at smaller scales also had strong effects on the patterns of functional diversity (Table 3). Terrain convexity and soil fertility (PC1) were the most important factors that affected the functional diversity patterns in this study (Figure 1), which is consistent with the second hypothesis. Trees in tropical forests not only compete for soil fertility and moisture, but also for light. Trees located in the quadrats of high convexity might intercept more light (Enoki & Abe Reference ENOKI and ABE2004, Tsujino et al. Reference TSUJINO, TAKAFUMI, AGETSUMA and YUMOTO2006). There is evidence that relative growth rates of large trees are positively related to convexity in this study site (Shen et al. Reference SHEN, SANTIAGO, SHEN, MA, LIAN, CAO, LU and YE2014). We suggest that, due to higher light availability, trees in the high-convexity quadrats have stronger competitive interactions and resource partitioning, resulting in functional divergence. Conversely, traits may respond to light limitation, resulting in higher similarity of strategy (environmental filtering) in the low-convexity quadrats.
Some studies have reported that functional diversity is related to soil properties. For example, soil moisture was the strong predictor of trait distribution patterns in coastal California (Cornwell & Ackerly Reference CORNWELL and ACKERLY2009), and soil fertility characteristics (i.e. pH, K and P) have the potential to affect functional diversity (Mason et al. Reference MASON, RICHARDSON, PELTZER, DE BELLO, WARDLE and ALLEN2012) and functional beta diversity (Siefert et al. Reference SIEFERT, RAVENSCROFT, WEISER and SWENSON2013). Furthermore, functional diversity in single traits such as height, SLA and chlorophyll concentration can be strongly associated with soil moisture and N (Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). In our study, PC1 was associated with high OM, K, N and available P (Table 1), and represented the gradients of soil fertility. PC1 had positive effects on functional diversity (Table 3), which was consistent with the fact that species differed in resource strategy in high-fertility environments, but were increasingly convergent in strategy as soil fertility declined (Mason et al. Reference MASON, RICHARDSON, PELTZER, DE BELLO, WARDLE and ALLEN2012, Sutton-Grier et al. Reference SUTTON-GRIER, WRIGHT, MCGILL and RICHARDSON2011). Total P was negatively correlated with available P (r = −0.59; P < 0.001), suggesting that the negative effects of Total P on functional diversity are the result of secondary correlation with available P. Study in this site also found that TP was not significantly correlated with AP, but N addition significantly increased AP (Liu et al. Reference LIU, MENG, LIANG, LI, XU, HUANG and YAN2014). However, other studies have shown that variation in altitude, slope and soil moisture (PC2) can potentially affect plant performance (Comita & Engelbrecht Reference COMITA and ENGELBRECHT2009, Harms et al. Reference HARMS, CONDIT, HUBBELL and FOSTER2001, Legendre et al. Reference LEGENDRE, MI, REN, MA, YU, SUN and HE2009), but was not observed in our result (Tables 2, 3), reflecting a fact that trees mainly tended to partition light and soil fertility in this forest.
Species in the quadrats of high convexity and soil fertility were more diverse in leaf traits of LDMC, leaf size, SLA and thickness (Table 3), and these traits reflect the relationships among light-capture rates, leaf biomass invested and defensive strategy. Species with low LDMC, thinner leaves, large leaf size and SLA tend to have high photosynthetic rates and carbon gain, but have lower leaf biomass invested (Kazakou et al. Reference KAZAKOU, VILE, SHIPLEY, GALLET and GARNIER2006, Martínez-Vilalta et al. Reference MARTÍNEZ-VILALTA, MENCUCCINI, VAYREDA and RETANA2010, Poorter & Bongers Reference POORTER and BONGERS2006, Poorter et al. Reference POORTER, WRIGHT, PAZ, ACKERLY, CONDIT, IBARRA-MANRIQUES, HARMS, LICONA, MARTINEZ-RAMOS, MAZER, MULLER-LANDAU, PENA-CLAROS, WEBB and WRIGHT2008). This suggests that species partition light (Carreno-Rocabado et al. Reference CARRENO-ROCABADO, PENA-CLAROS, BONGERS, ALARCON, LICONA and POORTER2012, Mason et al. Reference MASON, DE BELLO, DOLEZAL and LEPS2011), and that below-ground resources (soil fertility) (Mason et al. Reference MASON, RICHARDSON, PELTZER, DE BELLO, WARDLE and ALLEN2012, Siefert et al. Reference SIEFERT, RAVENSCROFT, WEISER and SWENSON2013) may increase in these quadrats, responding to the high light availability and high soil fertility.
Our finding was consistent with the hypothesis that species are functionally convergent in relative abiotic stressful environments because of environmental filtering, and divergent in more benign environments as competitive interactions become more important. However, studies also found evidence that functional diversity increased with abiotic stress for some traits, which indicated that different traits might be related to different niche axes, and associated with different ecological processes (Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). Our results supported this conclusion. The stepwise multiple regressions analysis showed that environmental factors had distinguishing effects on diversity of different traits (Table 4), more variation in the diversity of LDMC, leaf size, SLA and multiple traits could be explained, but this was not the case for chlorophyll, thickness and wood density since R2 values were much lower. This result implied that traits that are related to different niche axes, result in different relationships with environments. Multivariate functional diversity depends on the diversity of many different single traits, and only including a single multivariate functional diversity in the analysis may mask some key ecological processes in community assembly, thus niche axes should be considered in trait selection (Spasojevic & Suding Reference SPASOJEVIC and SUDING2012). However, other traits and environmental factors such as tree height, seed traits (Martínez-Vilalta et al. Reference MARTÍNEZ-VILALTA, MENCUCCINI, VAYREDA and RETANA2010, Poorter et al. Reference POORTER, WRIGHT, PAZ, ACKERLY, CONDIT, IBARRA-MANRIQUES, HARMS, LICONA, MARTINEZ-RAMOS, MAZER, MULLER-LANDAU, PENA-CLAROS, WEBB and WRIGHT2008, Wright et al. Reference WRIGHT, KITAJIMA, KRAFT, REICH, WRIGHT, BUNKER, CONDIT, DALLING, DAVIES, DIAZ, ENGELBRECHT, HARMS, HUBBELL, MARKS, RUIZ-JAEN, SALVADOR and ZANNE2010), light (King et al. Reference KING, DAVIES, SUPARDI and TAN2005) and aspect (Rubino & McCarthy Reference RUBINO and MCCARTHY2003) represent further avenues for evaluating the array of factors that moderate functional diversity.
ACKNOWLEDGEMENTS
We thank many individuals who contributed to the field survey of DHS plot, the Santiago Lab and Professor Jeff Diez at UC Riverside for comments that improved the manuscript, and “International Science Editing” for English editing. The study was funded by National Natural Science Foundation of China (31500338, 31370446, 41371078, 31011120470), China Postdoctoral Science Foundation (2015M582458), and Chinese Forest Biodiversity Monitoring Network.
Appendix 1. Abundance and sample size (leaf traits measurement) of 92 species in the Dinghushan (DHS) 20-ha Forest Dynamics Plot, China. Leaf samples were collected outside the plot if abundance of species is less than six.

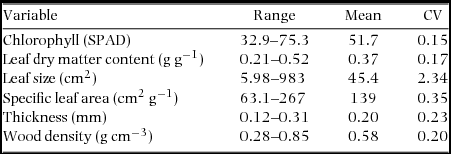
Appendix 2. Range, mean and coefficient of variation (CV) of plant functional traits (N = 92) in the Dinghushan (DHS) 20-ha Forest Dynamics Plot, China.
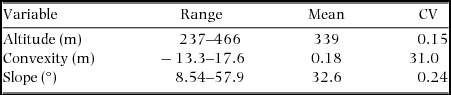
Appendix 3. Range, mean and coefficient of variation (CV) of topography (N = 500) in the Dinghushan (DHS) 20-ha Forest Dynamics Plot, China.
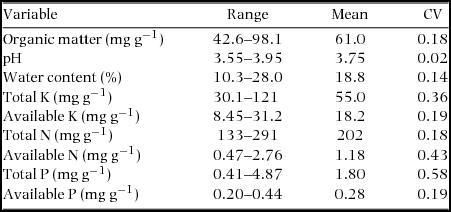
Appendix 4. Range, mean and coefficient of variation (CV) of soil variables (N = 500) in the Dinghushan (DHS) 20-ha Forest Dynamics Plot, China.
Appendix 5. Range, mean and coefficient of variation (CV) of functional dominance and functional diversity in the Dinghushan (DHS) 20-ha Forest Dynamics Plot, China.













