INTRODUCTION
The Brazilian Amazon is under the threat of more frequent and intense fires (Aragão & Shimabukuro Reference ARAGÃO and SHIMABUKURO2010). Forest clearing and degradation, and severe weather events, such as droughts related to the El Niño Southern Oscillation, increase fire frequency, resulting in negative consequences for habitat integrity and the biodiversity that they contain (Barlow & Peres Reference BARLOW and PERES2004, Reference BARLOW and PERES2006; Malhi et al. Reference MALHI, ROBERTS, BETTS, KILLEEN, LI and NOBRE2008). A positive feedback arises from more frequent ignition sources from human occupation, longer and drier dry seasons, and the increase of greenhouse gas emissions due to deforestation and related forest fires (Balch et al. Reference BALCH, MASSAD, BRANDO, NEPSTAD and CURRAN2013, Brando et al. Reference BRANDO, BALCH, NEPSTAD, MORTON, PUTZ, COE, SILVÉRIO, MACEDO, DAVIDSON, NÓBREGA, ALENCAR and SOARES-FILHO2014, Cochrane & Barber Reference COCHRANE and BARBER2009, Nepstad et al. Reference NEPSTAD, CARVALHO, BARROS, ALENCAR, CAPOBIANCO, BISHOP, MOUTINHO, LEFEBVRE, LOPES SILVA and PRINS2001). Studies show fire disturbance affecting forest structure, increasing tree mortality, depleting canopy cover (Balch et al. Reference BALCH, NEPSTAD, CURRAN, BRANDO, PORTELA, GUILHERME, REUNING-SCHERER and DE CARVALHO2011) and threatening local biodiversity (Andrade et al. Reference ANDRADE, BARLOW, LOUZADA, VAZ-DE-MELLO, SILVEIRA and COCHRANE2014, Mestre et al. Reference MESTRE, COCHRANE and BARLOW2013, Silveira et al. Reference SILVEIRA, BARLOW, ANDRADE, LOUZADA, MESTRE, LACAU, ZANETTI, NUMATA and COCHRANE2013). This fire dynamic is particularly significant in the south-east of the Brazilian Amazon, an agricultural frontier with intense land-use practices and markedly seasonal climate (Aragão et al. Reference ARAGÃO, POULTER, BARLOW, ANDERSON, MALHI, SAATCHI, PHILLIPS and GLOOR2014, Shimabukuro et al. Reference SHIMABUKURO, BEUCHLE, GRECCHI, SIMONETTI and ACHARD2014, Sombroek Reference SOMBROEK2001).
Although the impacts of fire on faunal diversity are well documented for fire-prone biomes (New Reference NEW2014), only a few studies have assessed this type of disturbance in humid tropical forests. Surveys in the Brazilian Amazon have shown fire disturbance affecting avian communities (Mestre et al. Reference MESTRE, COCHRANE and BARLOW2013), fruit production and large vertebrates (Barlow & Peres Reference BARLOW and PERES2006) and insect communities (Andrade et al. Reference ANDRADE, BARLOW, LOUZADA, VAZ-DE-MELLO, SOUZA, SILVEIRA and COCHRANE2011, Reference ANDRADE, BARLOW, LOUZADA, VAZ-DE-MELLO, SILVEIRA and COCHRANE2014; Barlow et al. Reference BARLOW, SILVEIRA, MESTRE, ANDRADE, D'ANDREA, LOUZADA, VAZ-DE-MELLO, NUMATA, LACAU and COCHRANE2012, Silveira et al. Reference SILVEIRA, BARLOW, ANDRADE, LOUZADA, MESTRE, LACAU, ZANETTI, NUMATA and COCHRANE2013). Little is known, however, about the specific effects of the increasing fire frequency associated with ignition sources and severe droughts in seasonal regions of the Brazilian Amazon.
Here, we surveyed fruit-feeding butterflies (Nymphalidae), known to be efficient indicators of forest integrity and diversity of other taxa. Studies show this guild responding to disturbance and environmental gradients (Brito et al. Reference BRITO, RIBEIRO, RANIERO, HASUI, RAMOS and ARAB2014, Filgueiras et al. Reference FILGUEIRAS, MELO, LEAL, TABARELLI, FREITAS and IANNUZZI2016, Ribeiro & Freitas Reference RIBEIRO and FREITAS2012, Sant'Anna et al. Reference SANT'ANNA, RIBEIRO, GARCIA and FREITAS2014) and correlating with diversity parameters of insects, spiders, vertebrates, lianas and trees (Barlow et al. Reference BARLOW, OVERAL, ARAUJO, GARDNER and PERES2007a, Gardner et al. Reference GARDNER, BARLOW, ARAUJO, AVILA-PIRES, BONALDO, COSTA, ESPOSITO, FERREIRA, HAWES, HERNANDEZ, HOOGMOED, LEITE, LO-MAN-HUNG, MALCOLM, MARTINS, MESTRE, MIRANDA-SANTOS, OVERAL, PARRY, PETERS, RIBEIRO-JUNIOR, DA SILVA, MOTTA and PERES2008). They include the subfamilies Biblidinae, Charaxinae, Nymphalinae and Satyrinae, and can comprise up to 75% of all Nymphalidae diversity (Brown Reference BROWN, Dick and Moritz2005). Additionally, they can be easily and consistently captured with traps baited with rotting fruits, allowing standardized sampling and comparable results across different surveys (Freitas et al. Reference FREITAS, ISERHARD, SANTOS, CARREIRA, RIBEIRO, MELO, ROSA, MARINI-FILHO, ACCACIO and UEHARA-PRADO2014). Previous studies have successfully used fruit-feeding butterflies to assess the effects of disturbance such as selective logging (Ribeiro & Freitas Reference RIBEIRO and FREITAS2012, Uehara-Prado et al. Reference UEHARA-PRADO, BROWN and FREITAS2007), fragmentation (Brito et al. Reference BRITO, RIBEIRO, RANIERO, HASUI, RAMOS and ARAB2014) and edge effects (Filgueiras et al. Reference FILGUEIRAS, MELO, LEAL, TABARELLI, FREITAS and IANNUZZI2016). As with other animal taxa, few studies have evaluated the impact of fires on tropical forest butterflies. ENSO-induced fires in Borneo, for instance, were shown to potentially decrease species richness and affect forest-specialist species (Charrette et al. Reference CHARRETTE, CLEARY and MOOERS2006, Cleary & Genner Reference CLEARY and GENNER2004), while burned-forest butterfly communities in India were shown to be compositionally distinct from communities in unburned forest (Kunte Reference KUNTE1997). Highly mobile insect taxa, such as butterflies, are expected to avoid first-order effects of fire (e.g. heat, smoke), but respond to long-term changes in microclimatic conditions and adult resource availability (New Reference NEW2014).
In this study, we assess the impacts of recurrent fires on communities of fruit-feeding butterflies in a south-eastern Amazon forest. We present a novel approach by sampling in a large-scale, long-term experimental burn area, with increasing fire frequency. More specifically, we test the following hypotheses: (1) Fire will affect diversity parameters (changing abundance and richness, and altering composition and structure) in local butterfly assemblages. We expect that a higher burn frequency will accentuate community changes associated with fire disturbance. (2) Butterfly species composition will change towards a state more similar to savanna assemblages as fire frequency increases. Forest specialist species will be excluded or negatively affected in burned forest, while species adapted to drier or more open environments will thrive. And (3) changes in microclimatic factors associated with fire disturbance (e.g. sunlight incidence, temperature, humidity) will drive butterfly community responses, favouring species better adapted to drier or more open habitats (e.g. less canopy cover). This pattern will also be accentuated as burning frequency increases.
METHODS
Study site
The study was conducted at Fazenda Tanguro (13o04.73′S 52o23.04′W), a private estate in Mato Grosso, Brazil, in the south-eastern part of the Amazon. The region was developed in the 1970s and, as far as we know and what we have been able to reconstruct from landowner maps and local knowledge, was not previously disturbed. At the Brazilian agricultural frontier, the landscape is dominated by different land uses, from soy monocultures to rubber plantations and pastures, while the remaining local vegetation is characterized by Amazonian forests that are bounded by savanna to the south-east. Compared with northern Amazonian forests, tree species richness in the forest is low (~100 species observed in inventory), dominated by Lauraceae and Burseraceae, as well as lower biomass and canopy average height (Balch et al. Reference BALCH, NEPSTAD, BRANDO, CURRAN, PORTELA, DE CARVALHO and LEFEBVRE2008). Mean annual precipitation is 1739 mm, with a dry season from May to September (Balch et al. Reference BALCH, NEPSTAD, BRANDO, CURRAN, PORTELA, DE CARVALHO and LEFEBVRE2008).
Three adjacent 50-ha (0.5 × 1.0 km) plots were established along the forest edge with pasture/soy plantation for fire experiments, as part of a collaborative study by Instituto de Pesquisa Ambiental da Amazônia (Ipam) and Woods Hole Research Center (Brando et al. Reference BRANDO, NEPSTAD, BALCH, BOLKER, CHRISTMAN, COE and PUTZ2012). One plot was used as an unburned control (Ctrl), one burned every 3 y (2004, 2007 and 2010) (B3y), and one burned every year (from 2004 to 2010, except 2008) (B1y) (Figure 1). All experimental burns in the vegetation were conducted during the late dry season (August–September). Butterfly sampling was conducted 4 y after the last burns, during the 2013/2014 wet season (November and January) and the 2014 early dry season (June). The study was conducted with the authorization of the landowner, with invertebrate sampling permission by ICMBio (#5896-1) and not involving endangered or protected species.
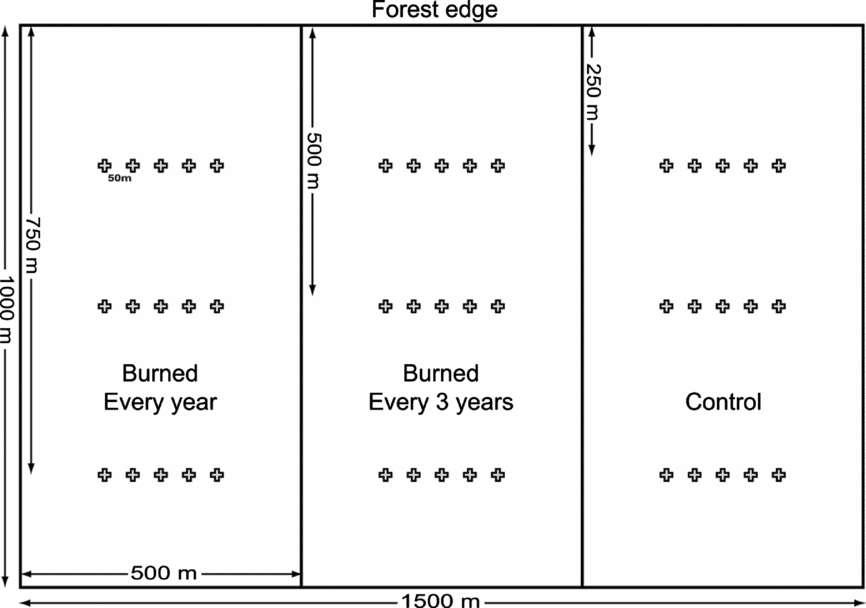
Figure 1. Burning experimental design and trap location for butterfly community sampling in a south-eastern Amazon forest. One control plot and two burned 50-ha plots. In each plot, three transects with five butterfly funnel traps each (‘+’ symbols).
Butterfly sampling design
In each plot, three transects were established at 250 m (avoiding most of the edge effects on butterflies, Ribeiro et al. Reference RIBEIRO, BATISTA, PRADO, BROWN and FREITAS2012), 500 m and 750 m parallel to the forest edge. Each transect comprised five non-independent butterfly traps (Freitas et al. Reference FREITAS, ISERHARD, SANTOS, CARREIRA, RIBEIRO, MELO, ROSA, MARINI-FILHO, ACCACIO and UEHARA-PRADO2014), baited with fermented sugar cane juice and banana, 50 m apart, totalling 45 traps (15 in each treatment plot) (Figure 1). Minimum distance between traps of different plots was 300 m. All traps were inspected and bait was replaced every 48 h, left open for 13 d in November 2013, 12 d in January 2014 and 12 d in June 2014, totalling 13,320 trap-h in each of the three treatments. Although studies show that additional funnel traps at ~25 m height can capture interesting, and often different, diversity patterns (Dumbrell & Hill Reference DUMBRELL and HILL2005, Ribeiro & Freitas Reference RIBEIRO and FREITAS2012), south-eastern Amazon forests have a lower canopy, and, in our case, the burned forest often had no canopy at all. For these reasons, we decided to standardize our sampling using only ground-level traps. Butterfly identification was carried out at Universidade Estadual de Campinas, and voucher specimens deposited at the resident collection. Species were classified (based on AVLF expertise) into (1) forest specialists, (2) savanna/open habitat specialist or (3) generalists.
Environmental parameters
To assess potential environmental factors affecting butterfly communities we measured Leaf Area Index (LAI, calculated as leaf area per ground area), temperature and relative humidity. LAI was measured at each trap using two Li-Cor 2000 Plant Canopy Analyzers, at 1 m and above, capturing also understorey density. Temperature and relative humidity were measured at transect level, using Onset Hobo U23 Pro v2 Temperature/Relative Humidity data loggers. Previous studies in the same experimental site showed changes in forest structure and microclimatic conditions due to fire, increased tree mortality increasing up to five times and depletion of almost two-thirds of canopy cover in burned forest (Brando et al. Reference BRANDO, BALCH, NEPSTAD, MORTON, PUTZ, COE, SILVÉRIO, MACEDO, DAVIDSON, NÓBREGA, ALENCAR and SOARES-FILHO2014).
Study design and sampling constraints
As there are no other large forested areas in the region that could be managed to act as ideal replicates, we considered sampling units within each sites as replicates of burn treatment. Although we are aware of the issues associated with pseudoreplication, similar sampling designs were successfully used previously in studies done on a site-specific basis, where no replication across the landscape is feasible (Block et al. Reference BLOCK, FRANKLIN, WARD, GANEY and WHITE2001, Sant'Anna et al. Reference SANT'ANNA, RIBEIRO, GARCIA and FREITAS2014, Uehara-Prado et al. Reference UEHARA-PRADO, BROWN and FREITAS2007). Even considering the known limitations of this design, the results obtained are a good approach to better understand the effect of fire on butterfly assemblages on tropical forests.
Data analysis
For all sample-based statistical analyses, one sample was considered as the pooled individuals collected in one transect (five non-independent traps). To test our first hypothesis, that fire disturbance influences butterfly diversity, we compared abundance, richness, community composition and structure between the three plots. We compared abundance and richness using individual-based species accumulation curves, with 100 permutations (Colwell et al. Reference COLWELL, MAO and CHANG2004). Analyses were made for dry, wet and both seasons pooled together. To compare community composition and structure, we used non-metrical multidimensional scaling (NMDS), based on Bray–Curtis similarity index matrices (Legendre & Legendre Reference LEGENDRE and LEGENDRE2012), and Anosim test (Clarke Reference CLARKE1993), both at transect level. Community structure was compared in Whittaker plots (Magurran Reference MAGURRAN2004). Bray–Curtis similarity index was calculated for dry and wet seasons at plot level. To test for spatial autocorrelation, we performed a Mantel test (Legendre & Legendre Reference LEGENDRE and LEGENDRE2012) between the community similarity matrix and a similarity matrix generated from trap geographical coordinates, across all nine sample transects (Dormann et al. Reference DORMANN, MCPHERSON, ARAÚJO, BIVAND, BOLLIGER, CARL, DAVIES, HIRZEL, JETZ, DANIEL KISSLING, KÜHN, OHLEMÜLLER, PERES-NETO, REINEKING, SCHRÖDER, SCHURR and WILSON2007).
For our second hypothesis, that savanna/dry habitat butterfly species will be favoured in burned forests, we categorized butterfly species, based on existing literature and field experience, into forest specialist, savanna specialist and generalist. We compared the abundance of species in each group, among the three plots, with Chi-squared test and post hoc pairwise comparisons with Bonferroni correction. We also performed Similarity Percentage (SIMPER) analysis, which provides the contribution of each species to the dissimilarity between two treatments (Clarke Reference CLARKE1993), in order to assess if abundance of forest or savanna specialist species are driving community changes.
To test our third hypothesis, that butterfly community changes are associated with microclimatic parameters, we used the BIO-ENV procedure (Clarke & Ainsworth Reference CLARKE and AINSWORTH1993) to correlate butterfly community composition and structure with (1) LAI (transect average), (2) average temperature, (3) average relative humidity and (4) number of burns. This procedure finds the best matching coefficient between Bray–Curtis similarity matrices generated from the habitat variables sampled and that generated from the butterfly data. Finally, we tested the influence of the same environmental variables on the abundance of forest specialists, savanna specialists and species with a dissimilarity contribution (from SIMPER analysis) above 5%, using generalized linear models (GLM) fitted by Poisson distribution. Variables were tested for collinearity using variance inflation factor. Reduced models were selected by backwards stepwise removal of variables with the lowest explanatory power (highest P values) and nested comparison of likelihood ratio tests, using the change in deviance as a Chi-square approximation. All analyses were performed using R and packages vegan, MASS (Venables & Ripley Reference VENABLES and RIPLEY2002) and car (Fox & Weisberg Reference FOX and WEISBERG2011).
RESULTS
Butterfly diversity in burned forests
We collected a total of 415 fruit-feeding butterfly individuals of 56 species. A total of 147 individuals of 34 species were collected in the control plot, 137 individuals of 37 species in forest burned every 3 y, and 131 individuals of 33 species in forest burned every year. Species accumulation curves per plot indicate a slightly lower abundance in burned forests and a higher species richness in the plot burned every 3 y (Figure 2). Accumulation curves by season indicate a more pronounced effect of fire on species richness during the dry season. NMDS plots show distinct community composition and structure between control and burned plots (Figure 3, Anosim R = 0.30; P < 0.05). We found no evidence of spatial autocorrelation in our community composition and structure analysis (Mantel test r = 0.09; P = 0.25). Whittaker plots (Figure 4) show burned forest with higher dominance, mostly due to the increase in relative abundance of Eunica pusilla (Bates, 1864). Community dissimilarity (to control plot) was higher in the wet season for forest burned every 3 y (Bray–Curtis index; wet season = 0.65; dry season = 0.52), and higher in the dry season for forest burned every year (wet season = 0.51; dry season = 0.62).
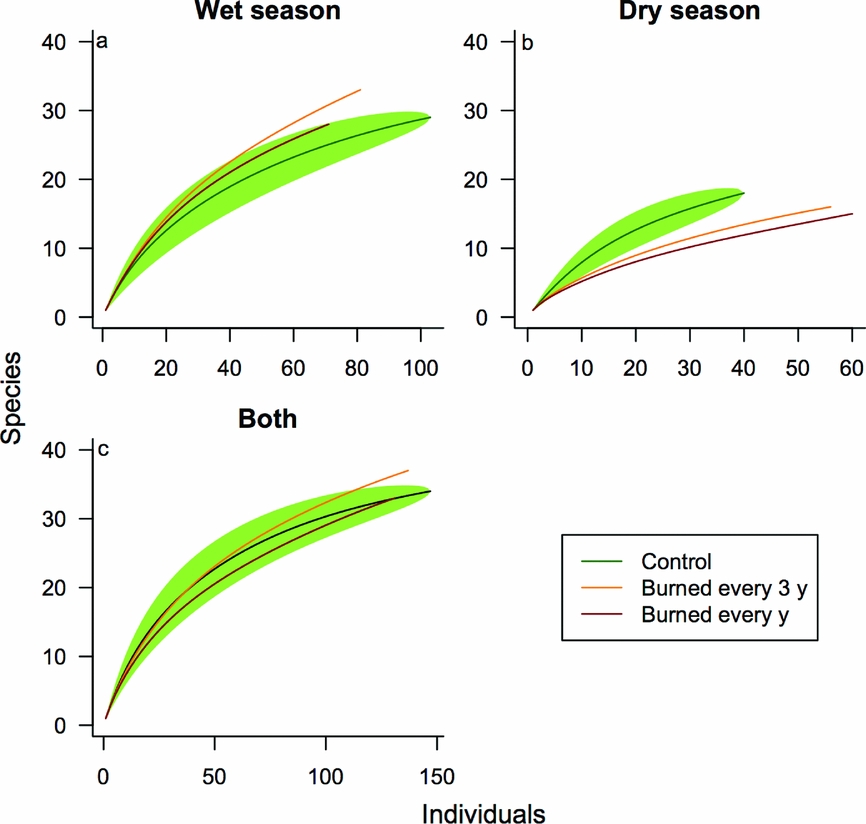
Figure 2. Randomized individual-based accumulation curves for butterfly species in one control and two burned experimental 50-ha plots in a south-eastern Amazon forest. Samples during wet season (a), dry season (b), and both pooled together (c). Green shaded area is 95% confidence interval for control (green) forest curve.

Figure 3. Non-metric multidimensional scaling (NMDS) plot and ellipses for butterfly community composition and structure (based on Bray–Curtis similarity index) in one control and two burned experimental 50-ha plots in a south-eastern Amazon forest. Each dot represents a five-trap sampling unit (Anosim R = 0.30; P < 0.05).

Figure 4. Percentage contribution to total abundance of species of butterflies ranked by abundance in one control and two burned experimental 50-ha plots in a south-eastern Amazon forest.
Forest, savanna and generalist species
Savanna specialist species were absent from our samples in the control forest and present in both burned treatments (Figure 5), indicating a significant change in species composition due to fire disturbance (χ2 = 17.5; df = 4; P < 0.01). Post hoc analyses showed a significant difference only between control and forest burned every 3 y (P < 0.01). SIMPER analysis shows the decrease in abundance of Zaretis itys (Cramer, 1777) and the increase of Eunica pusilla in burned forest as the two main contributors to community dissimilarity (Table 1). Additionally, species such as Nica flavilla (Godart, 1824) and Memphis polycarmes (Fabricius, 1775) appear as consistently affected by fire disturbance, absent or very rare in burned-forest samples.
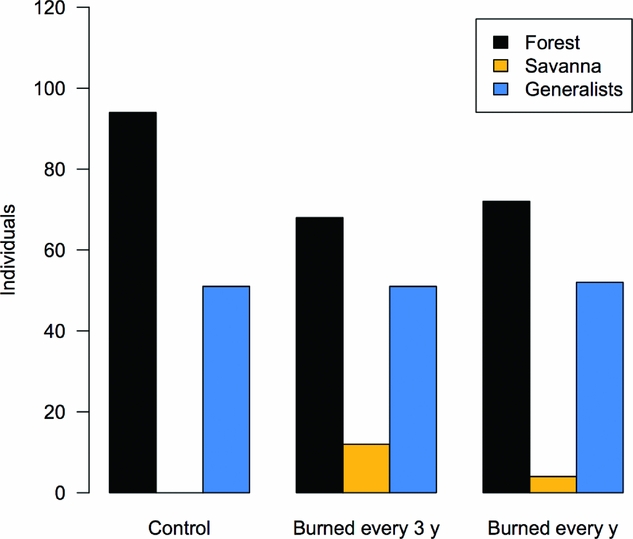
Figure 5. Abundance of forest, savanna, and generalist butterfly species in one control and two burned experimental 50-ha plots in a south-eastern Amazon forest (χ2 = 17.5, df = 4, P < 0.01).
Table 1. Species contribution to dissimilarities between butterfly communities in one control and two burned experimental 50-ha plots in a south-eastern Amazon forest. Associated habitat based on AVLF expertise. Contribution, standard deviation and average abundance per sample, based on SIMPER analysis.

Environmental parameters
Compared with the control, canopy cover (LAI, Leaf Area Index) decreased from 4.3 to 2.9 and 2.6 in burned every 3 y and burned every year, respectively (Table 2; averaged across transects). BIO-ENV analysis (Table 3) showed LAI as the environmental parameter that best correlated with spatial patterns of community composition, followed by the combination of LAI, average temperature and number of burns. GLM reduced models (Table 4) indicate LAI as a consistent predictor of community changes, with abundance of forest specialists and Zaretis itys responding positively to leaf area, and abundance of Eunica pusilla responding negatively. Relative humidity had a negative effect on Zaretis itys abundance.
Table 2. Average (± SD) environmental parameters in one control and two burned experimental 50-ha plots in a south-eastern Amazon forest.

Table 3. Bio-Env test results with Spearman's correlation r between combinations of explanatory environmental variables and spatial patterns of community composition and structure of butterfly communities in a south-eastern Amazon forest. Samples taken in one control and two experimentally burned forest plots.

Table 4. Reduced Poisson GLM models for abundance of forest specialist species, Eunica pusilla and Zaretis itys in one control and two burned experimental 50-ha plots in a south-eastern Amazon forest. Model selection was performed by backwards stepwise elimination of non-significant terms starting with terms that had the lowest explanatory power (highest P values)

DISCUSSION
Our study shows fire disturbance affecting a fruit-feeding butterfly community in a south-eastern Amazon forest. Our results did not fully support our first hypothesis, in which all diversity parameters would be affected and increasing fire frequency would exacerbate the effects. Compared to other studies on butterfly communities in burned forests (Charrette et al. Reference CHARRETTE, CLEARY and MOOERS2006, Cleary & Genner Reference CLEARY and GENNER2004), our samples indicate assemblages much more resilient to fire disturbance. Although we did not detect species richness changes in pooled samples, results indicate changes in community composition, with decreased occurrence of forest-specialist species and increase of savanna specialists. Species occurrence and abundance appear to correlate mostly to canopy cover, suggesting microclimatic alterations due to fire disturbance are important drivers of biodiversity loss. Our results, however, did not indicate additional effects of increasing fire frequency, with forest burned every 3 y and yearly burned harbouring similar butterfly assemblages. Our experimental design avoids issues that plague several disturbance and biodiversity surveys, such as spatial correlation and confounding factors (e.g. other disturbances outside the scope of the study) (Leather et al. Reference LEATHER, BASSET and DIDHAM2014). Most studies on the impacts of fire disturbance on tropical forest biodiversity used nearby unburned forests as baseline for parameters such as richness and species composition (Andrade et al. Reference ANDRADE, BARLOW, LOUZADA, VAZ-DE-MELLO, SILVEIRA and COCHRANE2014). However, in species-rich biomes such as the Brazilian Amazon, species turnover is expected to be high even in geographically close locations. Additionally, other types of disturbance, such as selective logging and edge effect, can often go undetected in non-ideal control sites. In our study design, with three adjacent experimental plots, we try to mitigate these issues and better isolate the effect of fire disturbance.
An obvious limitation of our experiment design, however, is the lack of replicated plots. Large-scale experimental manipulations such as ours and many others (e.g. the Smithsonian research station on Barro Colorado Island), are often constrained by the logistical trade-off between performing the recommended number of replicas or seizing the opportunity to capture ecological patterns of pressing matters such as fire disturbance in tropical forests. Although this implies our results must be extrapolated with prudence, our study agrees with a growing body of literature in this field (Brodie et al. Reference BRODIE, POST and LAURANCE2012, New Reference NEW2014). In fact, ours is the largest and longest-running burn experiment in Amazon forests (Fayle et al. Reference FAYLE, TURNER, BASSET, EWERS, REYNOLDS and NOVOTNY2015), comprising several studies from this exact location with novel and relevant results (summarized in Balch et al. Reference BALCH, BRANDO, NEPSTAD, COE, SILVÉRIO, MASSAD, DAVIDSON, LEFEBVRE, OLIVEIRA-SANTOS, ROCHA, CURY, PARSONS and CARVALHO2015).
Impacts on butterfly diversity
Our samples indicate no detectable impact of fire disturbance on overall richness and abundance, except for forest burned every 3 y, which presented a slightly higher number of species (Figure 2). This plot had several species that also occurred in at least one of the other two plots, as well as a few exclusive species, such as Callicore sorana (Goddart, 1824) and Hamadryas feronia (Table 1). Although a higher diversity in intermediate disturbance conditions can be expected in some cases (intermediate disturbance hypothesis; Connell Reference CONNELL1978), a larger and more comprehensive sample would be necessary to infer about the mechanism driving these patterns. Agreeing with previous studies on fire disturbance in seasonal tropical forests, the dry season exacerbated the effects on insect community (Andrade et al. Reference ANDRADE, BARLOW, LOUZADA, VAZ-DE-MELLO, SOUZA, SILVEIRA and COCHRANE2011, Silveira et al. Reference SILVEIRA, BARLOW, LOUZADA and MOUTINHO2010, Reference SILVEIRA, BARLOW, ANDRADE, MESTRE, LACAU and COCHRANE2012). With tropical insects, however, absolute richness and abundance are known to not necessarily decrease with disturbance (Lawton et al. Reference LAWTON, BIGNELL, BOLTON, BLOEMERS, EGGLETON, HAMMOND, HODDA, HOLT, LARSEN, MAWDSLEY, STORK, SRIVASTAVA and WATT1998), making analyses of species composition and structure more suitable to detect community changes.
Differences in community composition and structure indicate that forest changes associated with fire disturbance affect butterfly species in different ways. The lack of spatial autocorrelation indicates that the pattern is not a result of geographic distance between traps (Dormann et al. Reference DORMANN, MCPHERSON, ARAÚJO, BIVAND, BOLLIGER, CARL, DAVIES, HIRZEL, JETZ, DANIEL KISSLING, KÜHN, OHLEMÜLLER, PERES-NETO, REINEKING, SCHRÖDER, SCHURR and WILSON2007). Agreeing with previous studies on tropical insects, our results show that forest specialist species are sensitive to fire disturbance, even though other species seem to be more resilient (Andrade et al. Reference ANDRADE, BARLOW, LOUZADA, VAZ-DE-MELLO, SILVEIRA and COCHRANE2014, Cleary & Genner Reference CLEARY and GENNER2004). In our samples, changes in composition and structure due to fire disturbance can be consistently attributed to the decrease in abundance of Zaretis itys and Memphis polycarmes, and the increase of Eunica pusilla (Table 1). Even though all three species are associated with forest habitats, E. pusilla is commonly found in drier forests (AVLF pers. obs). Additionally, this species has a broad geographic distribution, found from Costa Rica to south-eastern Brazil, and is likely more tolerant of different habitat conditions (Austin Reference AUSTIN1992).
Our results indicate that different fire frequencies do not affect butterfly composition and structure. Tropical Nymphalidae are known for being able to travel hundreds of metres as adults (Andrade & Freitas Reference ANDRADE and FREITAS2005, Tufto et al. Reference TUFTO, LANDE, RINGSBY, ENGEN, SÆTHER, WALLA and DEVRIES2012), and the adjacent 50-ha experimental plots in our study can be easily crossed by many of the species we sampled. Possibly, the microclimatic conditions in the two burned plots are similar enough to allow the adult butterfly assemblages to mix, even if different during the larval stage. Interestingly, the control plot, equally close and within the range of most butterflies, showed a clearly distinct community composition, with complete absence of any species associated with savanna habitats. Although only a few individuals of savanna specialist species were captured in the burned plots, this pattern agrees with previous studies, indicating how the immediate surroundings are important in shaping fruit-feeding butterfly assemblages (Ribeiro et al. Reference RIBEIRO, BATISTA, PRADO, BROWN and FREITAS2012).
In our study, we have found that fire, through increased tree mortality and changes in canopy structure (Brando et al. Reference BRANDO, BALCH, NEPSTAD, MORTON, PUTZ, COE, SILVÉRIO, MACEDO, DAVIDSON, NÓBREGA, ALENCAR and SOARES-FILHO2014), is an important factor shaping butterfly diversity in south-eastern Amazonia, favouring species associated with savanna or more open habitats. Our results are, to some extent, similar to patterns found in different disturbances and environmental gradients such as logging, canopy gaps and successional stages (Filgueiras et al. Reference FILGUEIRAS, MELO, LEAL, TABARELLI, FREITAS and IANNUZZI2016, Nyafwono et al. Reference NYAFWONO, VALTONEN, NYEKO and ROININEN2014, Pardonnet et al. Reference PARDONNET, BECK, MILBERG and BERGMAN2010, Ribeiro & Freitas Reference RIBEIRO and FREITAS2012, Sant'Anna et al. Reference SANT'ANNA, RIBEIRO, GARCIA and FREITAS2014).
Microclimatic changes in burned forests
Changes in the microclimatic conditions of a forest can shape butterfly communities in different ways. Species can be directly affected by the physiological constraints caused by changes in temperature and humidity, inhibiting egg development, larval growth and hibernation (WallisDeVries & Van Swaay Reference WALLISDEVRIES and VAN SWAAY2006). High temperature variation can also cause dehydration and affect thermoregulation of butterflies (Bryant et al. Reference BRYANT, THOMAS and BALE2002, Checa et al. Reference CHECA, RODRIGUEZ, WILLMOT and LIGER2014). Indirectly, microclimate changes can restrict the occurrence of host plants, affecting larval distribution and development (Hellmann Reference HELLMANN2002). An altered plant community can also affect food sources for adults, especially for Nymphalidae butterflies that depend on rotting and fermenting fruits (Checa et al. Reference CHECA, RODRIGUEZ, WILLMOT and LIGER2014, Yamamoto et al. Reference YAMAMOTO, YOKOYAMA and KAWATA2007). Additionally, changes in the occurrence of predators and parasites (e.g. changes in bird communities due to fire disturbance, Mestre et al. Reference MESTRE, COCHRANE and BARLOW2013) can probably impact butterfly communities.
The results presented in this study suggest that, in seasonal forests of the Amazon, butterfly community composition can be affected by microclimatic changes associated with fires, even 4 y after the disturbance occurred (when our samples took place). When compared with other environmental variables, LAI was consistently better correlated with community composition and structure, and the better predictor of abundance of forest specialists and key species driving diversity patterns. Our results agree with other studies that found canopy openness as an important variable explaining the diversity patterns of insects and other groups in tropical forests (Andrade et al. Reference ANDRADE, BARLOW, LOUZADA, VAZ-DE-MELLO, SILVEIRA and COCHRANE2014, Barlow & Peres Reference BARLOW and PERES2004, Barlow et al. Reference BARLOW, GARDNER, ARAUJO, AVILA-PIRES, BONALDO, COSTA, ESPOSITO, FERREIRA, HAWES, HERNANDEZ, HOOGMOED, LEITE, LO-MAN-HUNG, MALCOLM, MARTINS, MESTRE, MIRANDA-SANTOS, NUNES-GUTJAHR, OVERAL, PARRY, PETERS, RIBEIRO-JUNIOR, DA SILVA, MOTTA and PERES2007b, Checa et al. Reference CHECA, RODRIGUEZ, WILLMOT and LIGER2014), including a study in the same area showing both fire and seasonality affecting leaf-litter arthropod abundance (Silveira et al. Reference SILVEIRA, BARLOW, LOUZADA and MOUTINHO2010). Canopy openness is associated with higher sun incidence and a consequent higher variance in temperature and humidity, imposing severe physiological constraints for forest-specialist species that depend on more stable microhabitats (Checa et al. Reference CHECA, RODRIGUEZ, WILLMOT and LIGER2014, Cleary & Genner Reference CLEARY and GENNER2004). In our case, the occurrence of species such as Zaretis itys appear to respond negatively to canopy openness, which is higher in burned forest. Also in the case of Z. itys, its larval host plant Casearia sp. (Beccaloni et al. Reference BECCALONI, HALL, VILORIA and ROBINSON2008) is known to have the above-ground portion killed by fire (Imatomi et al. Reference IMATOMI, SOUZA, GUALTIERI and FERREIRA2014), with probable impacts on larval distribution and development.
Conclusion
Increasing fire in south-eastern Amazonia can lead to an irreversible forest die-back and the replacement of primary humid vegetation by a drier forest or grassland state. Our study suggests that fire disturbance causes a series of habitat changes that threaten tropical insect diversity, negatively affecting rare, specialist species and introducing drought-tolerant species. Further, we did not detect marked differences in assemblages from the forest burned every 3 y and burned every y, indicating that it only takes a few burns to cause these alterations in the butterfly community. In addition, we found these changes in what was the original forest interior, greater than 250 m from the edge, suggesting that fire-induced forest degradation alone, and not the presence of invasive grasses (Silvério et al. Reference SILVÉRIO, BRANDO, BALCH, PUTZ, NEPSTAD, OLIVEIRA-SANTOS and BUSTAMANTE2013), can cause these changes. Our experimental design used short intervals between burns (1 and 3 y), compared with the 5–10 y between burns in highly affected areas of the Amazon (Cochrane Reference COCHRANE2003). The impacts of fire disturbance in our study can probably be underestimated, as longer intervals would allow more fuel accumulation and more intense fires (Balch et al. Reference BALCH, NEPSTAD, CURRAN, BRANDO, PORTELA, GUILHERME, REUNING-SCHERER and DE CARVALHO2011).
Overall, these fire-induced changes in arthropod communities can have substantial ecological consequences, such as the loss of pollination, reduction in nutrient cycling through herbivory, and changes in food-web dynamics (Nichols et al. Reference NICHOLS, SPECTOR, LOUZADA, LARSEN, AMEZQUITA and FAVILA2008). Butterflies, for instance, are important pollinators, herbivores and food sources for vertebrates (Samways Reference SAMWAYS1993). Further studies should investigate additional patterns in butterfly diversity shaped by fire disturbance, such as changes in community stratification (Ribeiro & Freitas Reference RIBEIRO and FREITAS2012), and how grass invasion (Silvério et al. Reference SILVÉRIO, BRANDO, BALCH, PUTZ, NEPSTAD, OLIVEIRA-SANTOS and BUSTAMANTE2013) may alter subfamily proportions (e.g. Satyrinae). Additionally, different taxa may reveal a broader picture on how terrestrial arthropods are affected by fire disturbance.
ACKNOWLEDGEMENTS
The authors thank IPAM, Woods Hole Research Center, and ‘Grupo Maggi’ for logistical support and access to experimental plots; Darlisson, Sandro, Sebastião, Ebes, Santarém and Dona Lúcia for making fieldwork possible; Marcos R. Lima for support with GLM analyses; Paul Lefebvre for GIS and mapping support. RBA was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (2013/00375-4 and 2013/19024-7). AVLF was funded by CNPq (302585/2011-7 and 303834/2015-3), RedeLep ‘Rede Nacional de Pesquisa e Conservação de de Lepidópteros’ – SISBIOTA Brasil/CNPq (563332/2010-7), National Science Foundation (DEB-1256742), and BIOTA-FAPESP Program (2011/50225-3 and 2012/50260-6).











