In southern African savannas, the occurrence of geoxylic suffrutices or ‘underground trees’ in genera which normally have a tree growth habit has long fascinated botanists (Burtt Davy Reference BURTT DAVY1922, De Wildeman Reference DE WILDEMAN1933, Duvigneaud Reference DUVIGNEAUD1951, White Reference WHITE1977). The transition from the tree to the suffrutescent life form has been interpreted as an adaptation to fire (Burtt Davy Reference BURTT DAVY1922, De Wildeman Reference DE WILDEMAN1933). However, other limiting factors might be involved as well, especially frost and nutrients (Davies et al. Reference DAVIES, DARU, VANDER BANK, MAURIN and BOND2016, Finckh et al. Reference FINCKH, REVERMANN and AIDAR2016, Maurin et al. Reference MAURIN, DAVIES, BURROWS, DARU, YESSOUFOU, MUASYA, VAN DER BANK and BOND2014). Much of the discussion on the difference between trees and their suffrutescent counterparts has focused on the dramatic difference in canopy height and position of dormant buds. Surprisingly, other traits have received little attention (but see Maurin et al. Reference MAURIN, DAVIES, BURROWS, DARU, YESSOUFOU, MUASYA, VAN DER BANK and BOND2014). Many plant traits are correlated to size (Niklas Reference NIKLAS1994). The 10- to 100-fold difference in height could conceivably be correlated to alterations in other quantitative traits in geoxyles. Selection for decreased height (whatever selective force is actually involved) can potentially alter a cascade of quantitative traits, as for instance in alpine plants (Körner et al. Reference KÖRNER, NEUMAYER, PELAEZ MENENDEZ-RIEDL and SMEETS-SCHEEL1989a). Furthermore, the particular ecological conditions of the savannas compared with the tree layer might also select particular trait values. The possibility for suffrutices to express trait values that are not attainable by their tree counterparts has never been investigated.
I have compared morphological characters of geoxyles and their respective congeneric trees. I expect geoxyles to have smaller values of a suite of quantitative traits, in line with their smaller habit. More specifically, my working hypothesis is that geoxyles exhibit phenotypes that are either a subset of the range of trait values of their tree congener (hereafter referred to as the contracted phenetic space hypothesis), or alternatively, that their range of trait values is shifted towards trait values situated out of the range of their congener (the shifted phenetic space hypothesis).
In order to minimize confounding effects of different climatic and biogeographic contexts, all the species studied occur in the same area i.e. Katanga (southern Democratic Republic of the Congo) and neighbouring regions of Zambia. Katanga is situated at the northern limit of the Zambezian region (8–13°S, 22–30°E). The climate is characterized by a 5–6-mo dry season and the annual rainfall ranges from 1000 to 1500 mm. The mean annual temperature (Lubumbashi) is 20.5°C. The vegetation mostly consists of miombo woodland. However, savannas cover large areas especially on Kalahari sand and in seasonally waterlogged soil and metal-enriched soil (Duvigneaud Reference DUVIGNEAUD1958, Schmitz Reference SCHMITZ1971). Geoxylic suffrutices are a prominent component of vegetation of savannas in Katanga (Duvigneaud Reference DUVIGNEAUD1958, Schmitz Reference SCHMITZ1971).
Thirty-six species of geoxylic suffrutex distributed in 35 genera and 23 families have at least one tree congener in the regional flora. A pair of species was considered in each genus, with one exception (Ochna, in which two pairs were considered in different taxonomic groups). The selection of tree congeners was based on Meerts (Reference MEERTS2015) and Meerts & Hasson (Reference MEERTS and HASSON2016). When several tree congeners existed in the regional flora, the closest relative was selected based either on existing phylogenies (Combretum: Maurin et al. (Reference MAURIN, CHASE, JORDAAN and VAN DER BANK2010), Syzygium: Byng (Reference BYNG2013)), or on taxonomic relatedness, based on the Flore d'Afrique centrale (FAC) (Multiple authors 1948–1987) and, when available, more recent taxonomic revisions (Garcinia: Jones (Reference JONES1980), Protea: Chisumpa & Brummitt (Reference CHISUMPA and BRUMMITT1987), Psychotria: Lachenaud (Reference LACHENAUD2013)). Seven genera comprised several geoxylic suffrutices; in such cases, the most widespread one was selected. Trait values were obtained from the electronic version of the FAC, the most comprehensive flora for the study region (http://www.br.fgov.be/RESEARCH/DATABASES/FOCA/). The FAC provides extensive descriptions of species based on the critical revision of copious herbarium material originating mostly from our study region. For taxa not yet covered by the FAC, or in the case of missing trait values, the Flora Zambesiaca (FZ) (Multiple authors 1960–2006) (http://apps.kew.org/efloras/search.do) was used instead. More recent taxonomic revisions were used when available (Syzygium: Byng (Reference BYNG2013), Heteromorpha: Winter & van Wyk (Reference WINTER and VAN WYK1996), Psychotria: Lachenaud (Reference LACHENAUD2013)). Trait values were extracted from the abovementioned references. The selected traits include vegetative traits (petiole length, leaf blade length and width) and reproductive traits (inflorescence length or number of flowers, petal length, stamen length, fruit length). Leaf size influences light capture, transpiration, and leaf temperature. Inflorescence size is a proxy of reproductive output. Petal length and stamen length are correlated to floral display, pollination and breeding system. Fruit size influences propagule dispersibility. However, the traits available in floras do not capture the entire spectrum of plant life strategies; in particular, nutrient capture and conservation, and phenology are not covered. Trait values are generally reported in floras as a range (minimum–maximum). Both the minimum and the maximum trait values were considered; extreme values reported in brackets were not considered.
As the difference between geoxyles and trees was not normally distributed, geoxyles and congeneric trees were compared by Wilcoxon signed-rank test, the non-parametric counterpart of the Student's paired t-test. The ratio of trait values of trees and geoxyles was calculated. Under the null hypothesis that geoxyles are equally likely to have higher and lower values respectively compared with congeneric trees, the expected value of the median of that ratio is unity. Median values greater than unity indicate higher trait values for trees. A Principal Component Analysis was also conducted to explore the multivariate phenetic relationships between trees and geoxyles (correlation matrix of log-transformed data, height excluded).
For maximum height, trees had on average 14-fold higher values compared with geoxyles.
For the 14 morphometric traits, the median value of the tree/geoxyle ratio ranged from 0.92 (minimum petal length) to 1.67 (maximum petiole length). In 12 of 14 trait comparisons, that ratio was greater than 1.00, pointing to a systematic tendency for trees to have larger trait values than geoxyles. However, the difference was significant in only 5 of 14 comparisons (max petiole length, max leaf length, max leaf width, max fruit length, min leaf length).
Interestingly, the tree/geoxyle ratio was markedly closer to unity for floral and fruit traits (median ranging from 0.92 to 1.14) compared with leaf traits (median ranging from 1.14 to 1.67). For reproductive traits, the difference was significant in only one case, i.e. maximal fruit length. Maximal fruit length of trees is 14% larger compared with geoxyles, an apparently negligible difference, which however corresponds to almost 50% higher volume (assuming spheroid shape). These results are in line with the well-known phylogenetic conservatism of floral traits. Corolla and stamen size are linked to pollination and breeding system. Thus, the results do not provide evidence for a systematic alteration of reproductive strategy in the transition from the tree to the geoxylic habit. This suggests that selection for short stature is not driven by disruptive selection by pollinators or dispersal agents. No significant difference was found in inflorescence size. This suggests that, relative to their overall size, inflorescence represents a proportionately larger fraction of total size in geoxyles. Whether this implies higher allocation to reproduction deserves investigation.
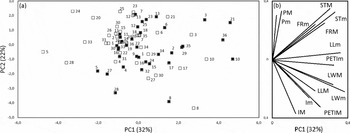
Vegetative traits seem to be more responsive to the transition to geoxylic life form. The maximal value of leaf traits (lamina length, lamina width, petiole length) was 33–67% greater in trees and Wilcoxon test was significant in all three traits. In contrast, the minimum value of petiole length and leaf width was not significantly different between trees and geoxyles. Thus, for these traits, the phenetic space of geoxyles conforms to the contracted phenetic space hypothesis. In other words, trees can express trait values that are not attainable to geoxyles, but the opposite is not true. In the case of leaf length, however, the minimum trait value of trees is on average 25% higher (i.e. median of tree/geoxyle ratio = 1.25) compared with geoxyles and the difference is significant (P = 0.02). Thus, for leaf length, geoxyles can actually express (slightly) lower values compared with trees, thus conforming to the shifted phenetic space hypothesis. Principal Component 1 accounts for 32% of variation, being essentially correlated to vegetative traits. PC2 (22%) is positively correlated to petal length, stamen length and fruit size, and negatively correlated to inflorescence size. The scatter plots of trees and geoxyles show a considerable overlap, with a slight tendency for geoxyles to be shifted towards more negative values of PC1 (Figure 1).

Figure 1. Principal Component Analysis of traits of 36 pairs of geoxyles and congeneric trees. Projection of species on PC1 and PC2 (a). Geoxyles: empty squares; trees: filled squares. Species labels: see legend of Table 1. Projection of variables on PC1 and PC2 (b). Traits included are minimum and maximum value of: fruit length (FRm, FRM), inflorescence length (Im, IM), leaf length (LLm, LLM), leaf width (LWm, LWM), petal length (Pm, PM), petiole length (PETIm, PETIM), stamen length (STm, STM). Height is not included.
Table 1. Comparison of geoxyles and congeneric trees for the minimum and maximum value of seven morphological traits. The expected median of the ratio tree/geoxyle is unity under the null hypothesis that geoxyles are equally likely to have lower or higher trait values compared with congeneric trees. Trees and geoxyles are compared by Wilcoxon signed rank test. Species included (the first species in each pair is the geoxyle; genus number in parentheses is the same as in Figure 1; nomenclature follows the African Plant Database): Anisophyllea quangensis/A. boehmii (1); Annona stenophylla subsp. nana/A. senegalensis (2); Brachystegia russelliae/B. boehmii (3); Bridelia eranalis/B. micrantha (4); Clutia timpermaniana/C. abyssinica (5); Combretum platypetalum/C. paniculatum (6); Cryptosepalum exfoliatum subsp. suffruticans/C. exfoliatum subsp. pseudotaxus (7); Cussonia corbisieri/C. arborea (8); Diospyros virgata/D. mespiliformis (9); Entadopsis nana/E. abyssinica (10); Eugenia malangensis/E. nyassensis subsp. capensis (11); Garcinia buchneri/G. huillensis (12); Gardenia subacaulis/G. ternifolia (13); Grewia decemovulata/G. flavescens (14); Ozoroa marginata/O. insignis (15); Heteromorpha kassneri/H. trifoliata (16); Lannea edulis/L. discolor (17); Magnistipula sapinii/M. butayei (18); Ochna manikensis/O. pulchra (19); Ochna pygmaea/O. schweinfurthiana (20); Oncoba suffruticosa/O. subtomentosa (21); Parinari capensis subsp. capensis/P. curatellifolia (22); Paropsia brazzeana/P. guineensis (23); Pavetta radicans/P. crassipes (24); Protea trichophylla/P. manikensis (25); Psorospermum mechowii/P. febrifugum (26); Psychotria plantaginoidea/P. punctata (27); Rauvolffia nana/R. caffra (28); Ritchiea pygmaea/R. quarrei (29); Salacia bussei/S. rhodesiaca (30); Sapium oblongifolium/S. cornutum (31); Searsia kirkii/S. tenuinervis (32); Sericanthe suffruticosa/S. andongensis (33); Steganotaenia hockii/S. araliacea (34); Syzygium guineense subsp. huillense/S. guineense subsp. macrocarpum (35); Trichilia quadrivalvis/T. emetica (36).

In conclusion, the results show that the morphological traits of geoxyles are remarkably similar to their tree counterparts, especially for reproductive traits. Trait values of geoxyles are generally nested within the range of their tree congeners. For vegetative traits, geoxyles express a restricted part of the phenetic space of trees, being unable to attain trait values as high as those of their tree congeners. Unlike bonsais or alpine dwarfs (Körner et al. Reference KÖRNER, NEUMAYER, PELAEZ MENENDEZ-RIEDL and SMEETS-SCHEEL1989a, Reference KÖRNER, PELAEZ MENENDEZ-RIEDL and JOHNb), the leaves of geoxyles are not much smaller compared with normal trees. However, geoxyles and trees might still differ for dimensions of the life strategy not covered in the present paper, notably nutrient capture and conservation, and phenology. Future work should extend the comparison to functional leaf traits and biomass allocation patterns, which cannot be assessed based on literature data.