INTRODUCTION
Frugivores are ubiquitous in tropical forests, where both unripe and ripe fruit represent a key resource for a wide range of vertebrate taxa (Fleming & Kress Reference FLEMING and KRESS2011). In addition to birds (Kissling et al. Reference KISSLING, BÖHNING-GAESE and JETZ2009), frugivory has evolved within bats (Muscarella & Fleming Reference MUSCARELLA and FLEMING2007), carnivores (Ray & Sunquist Reference RAY and SUNQUIST2001), fish (Correa et al. Reference CORREA, WINEMILLER, LÓPEZ-FERNANDEZ and GALETTI2007, Goulding Reference GOULDING1980, Horn et al. Reference HORN, CORREA, PAROLIN, POLLUX, ANDERSON, LUCAS, WIDMANN, TJIU, GALETTI and GOULDING2011), primates (Hawes & Peres Reference HAWES and PERES2013), reptiles (Valido & Olesen Reference VALIDO, OLESEN, Dennis, Schupp, Green and Westcott2007) and ungulates (Bodmer Reference BODMER1990). Earlier organism-based studies (typically focused on either consumer or resource species) are now shifting towards a more complete understanding of community networks and the mechanistic processes driving this suite of interactions (Carlo & Yang Reference CARLO and YANG2011, Jordano et al. Reference JORDANO, FORGET, LAMBERT, BÖHNING-GAESE, TRAVESET and WRIGHT2011).
The most comprehensive assessments of fruit–frugivore networks to date, however, have been conducted in temperate environments (Herrera Reference HERRERA1998), and/or focused only on birds and bird-dispersed plants (Carlo et al. Reference CARLO, COLLAZO and GROOM2003, Snow Reference SNOW1981). There are still few studies on the degree of dietary overlap or partitioning of available fruit resources among members of a large coterie of phylogenetically independent but co-occurring frugivores (Donatti et al. Reference DONATTI, GUIMARÃES, GALETTI, PIZO, MARQUITTI and DIRZO2011, Kitamura et al. Reference KITAMURA, YUMOTO, POONSWAD, CHAUILUA, PLONGMAI, MARUHASHI and NOMA2002, Schleuning et al. Reference SCHLEUNING, BLÜTHGEN, FLÖRCHINGER, BRAUN, SCHAEFER and BÖHNING-GAESE2011). Indeed, the distinct fruit morphological partitioning identified amongst frugivores at Makokou, Gabon (Gautier-Hion et al. Reference GAUTIER-HION, DUPLANTIER, QURIS, FEER, SOURD, DECOUX, DUBOST, EMMONS, ERARD, HECKETSWEILER, MOUNGAZI, ROUSSILHON and THIOLLAY1985) remains one of the most comprehensive assessments of trophic interactions within a broad guild of tropical forest frugivores. A suite of plant traits, including fruit morphology, presentation mode, colour and nutritional content, are suggested to collectively represent a ‘dispersal syndrome’ that broadly matches a functional group of fruit consumers (Fischer & Chapman Reference FISCHER and CHAPMAN1993, Janson Reference JANSON1983, Jordano Reference JORDANO1995, van der Pijl Reference VAN DER PIJL1982).
Determining the variation in fruit trait selection and degree of dietary overlap in coexisting consumers is critical to understanding fruit evolution (Lomáscolo & Schaefer Reference LOMÁSCOLO and SCHAEFER2010) and potential frugivore resilience to disturbance. For example, large frugivores are more at risk from selective hunting, which could threaten the demographic viability of large-fruited or large-seeded plants (Peres & van Roosmalen Reference PERES, VAN ROOSMALEN, Levey, Silva and Galetti2002, Wheelwright Reference WHEELWRIGHT1985) unless alternative frugivores can effectively provide substitutional roles as dispersal agents. Several tropical forest studies have examined differences in fruit trait selection within a single frugivore assemblage (Bollen et al. Reference BOLLEN, ELSACKER and GANZHORN2004, Flörchinger et al. Reference FLÖRCHINGER, BRAUN, BÖHNING-GAESE and SCHAEFER2010, Kitamura et al. Reference KITAMURA, YUMOTO, POONSWAD, CHAUILUA, PLONGMAI, MARUHASHI and NOMA2002, Voigt et al. Reference VOIGT, BLEHER, FIETZ, GANZHORN, SCHWAB and BÖHNING-GAESE2004). Surprisingly few studies, however, have been attempted in lowland Amazonia (Link & Stevenson Reference LINK and STEVENSON2004), even though this region holds both the highest diversity of terrestrial and aquatic frugivorous vertebrates (Fleming et al. Reference FLEMING, BREITWISCH and WHITESIDES1987) and the widest spectrum of morphological fruit types (Gentry Reference GENTRY1996, van Roosmalen Reference VAN ROOSMALEN1985) anywhere on Earth. This is particularly the case for seasonally flooded várzea forests, which account for ~5% of Amazonia but as much as 20% of its woody flora (Junk Reference JUNK and Junk1997).
Using a novel synthesis of field and interview methods, we compare the plant diets of medium- to large-bodied terrestrial, arboreal and aquatic frugivorous vertebrates in both várzea and unflooded (terra firme) forest, and examine the relative contribution of fruit traits, including fruit morphology and colour, to their diet selection in terms of fruit resources. To our knowledge, this represents the first systematic attempt to comprehensively document the tropical fruit–frugivore networks of two adjacent, yet radically different, forest types. Tropical fruit–frugivore networks tend to be diffuse and characterized by low levels of specialization or high connectance, whereby individual fruiting species may be attended by a large number of generalist frugivores (Bascompte & Jordano Reference BASCOMPTE and JORDANO2007, Schleuning et al. Reference SCHLEUNING, FRÜND, KLEIN, ABRAHAMCZYK, ALARCÓN, ALBRECHT, ANDERSSON, BAZARIAN, BÖHNING-GAESE, BOMMARCO, DALSGAARD, DEHLING, GOTLIEB, HAGEN, HICKLER, HOLZSCHUH, KAISER-BUNBURY, KREFT, MORRIS, SANDEL, SUTHERLAND, SVENNING, TSCHARNTKE, WATTS, WEINER, WERNER, WILLIAMS, WINQVIST, DORMANN and BLÜTHGEN2012). However, the lower plant diversity and the influence of the flood pulse on both the seasonal composition of the frugivore assemblage and adaptations in fruit morphology may reduce overall connectance in várzea forests compared with terra firme forests. We tested the a priori hypotheses that (1) neighbouring terra firme and várzea forest support contrasting fruit–frugivore networks, with a lower level of connectance in várzea forests; and (2) heterogeneity in fruit resource use between frugivore guilds is based on fruit traits including morphology.
METHODS
Study area
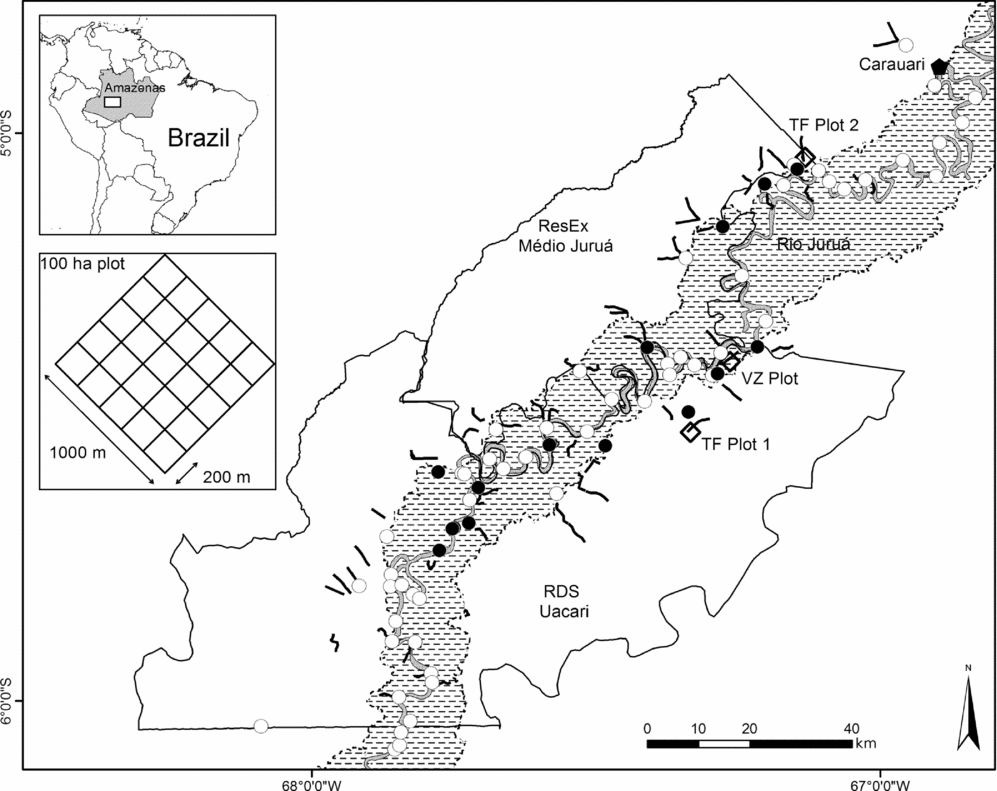
This study was conducted within two contiguous forest reserves in the State of Amazonas, Brazil, namely the 253227-ha Médio Juruá Extractive Reserve (ResEx Médio Juruá) and the 632949-ha Uacari Sustainable Development Reserve (RDS Uacari) (Figure 1). These reserves border the Juruá river, a major white-water tributary of the Solimões (= Amazon) river, and contain large expanses of upland unflooded terra firme forest (80.6% of combined reserve area) and, closer to the river channel, seasonally flooded várzea forest (17.9%) (Hawes et al. Reference HAWES, PERES, RILEY and HESS2012), which can be inundated for up to 210 d y−1, rising to a depth of 10–15 m (Parolin et al. Reference PAROLIN, FERREIRA, ALBERNAZ and ALMEIDA2004).

Figure 1. Map of the Médio Juruá region of western Brazilian Amazonia showing locations of 67 transects of 5 km in length (lines) and three 100-ha plots (squares) in terra firme (white) and várzea forest (dashed). Local communities within the two forest reserves are indicated by solid circles (where interviews were conducted) and open circles (where interviews were not conducted). Solid lines represent reserve boundaries; dashed lines represent the total extent of the várzea floodplain in this region as measured by ALOS ScanSAR images © JAXA/METI 2009 (Hawes et al. Reference HAWES, PERES, RILEY and HESS2012).
Such extreme environmental conditions result in substantial differences in terms of plant composition, forest structure (Hawes et al. Reference HAWES, PERES, RILEY and HESS2012), plant phenology and fruit production (Haugaasen & Peres Reference HAUGAASEN and PERES2005a, Parolin et al. Reference PAROLIN, WITTMANN, SCHÖNGART, Junk, Piedade, Wittmann, Schöngart and Parolin2010). The resident frugivore assemblage in várzea forests is also strongly affected by the seasonal flood pulse (Haugaasen & Peres Reference HAUGAASEN and PERES2005b, Reference HAUGAASEN and PERES2008), which physically excludes terrestrial vertebrates during the aquatic phase, but remains accessible to arboreal and scansorial mammals and canopy birds and bats. This terrestrial frugivore assemblage, however, is seasonally replaced by the highly predictable incursion of frugivorous fish, including characids and catfish, which abandon the river channel and oxbow lakes with the rising flood waters to take advantage of canopy resources, including seeds, fruit pulp and arthropods (Goulding Reference GOULDING1980).
The Juruá region has a wet, tropical climate with a mean annual temperature of 27.1°C and annual rainfall, calculated from daily records over three consecutive years (2008–2010) at the Bauana Ecological Field Station (S 5°26′19″, W 67°17′12″), averaging 3679mmy−1. The altitudinal range within the reserves is 65–170 m asl. Terra firme soils are typically heavily leached and nutrient-poor compared with the eutrophic alluvial soils of pre-Andean origin in várzea forests. All sites surveyed consisted of primary forest, although commercially valuable timber species along the Juruá river had experienced small-scale selective logging from 1970 to 1995, especially in várzea forest (Scelza Reference SCELZA2008).
Frugivore surveys
We conducted surveys of medium- to large-bodied diurnal vertebrates (birds and mammals) in three 100-ha plots (two in TF and one in VZ), each consisting of a trail grid of 12 1-km transects at 200-m intervals (Figure 1). Monthly surveys were conducted in accordance with a standardized line-transect census protocol (Peres & Cunha Reference PERES and CUNHA2011), between 06h30 and 11h00, and were discontinued whenever necessary during rain. The 100-ha plots were surveyed during the first 2 wk of every month (April 2008–July 2010), over the course of four consecutive days (three 1-km transects d−1, depending on weather conditions). Várzea forest transects were surveyed by dugout canoe during the aquatic phase. For all encounters, we recorded species, detection cue, distance along the transect, perpendicular distance from the transect and animal group size. We also recorded any observations of fruit feeding behaviour, including identification and/or collection of plant vouchers of whole fruits or fruit parts. Target frugivore species included primates, ungulates, caviomorph rodents, squirrels, some frugivorous Carnivora, and larger-bodied terrestrial and canopy birds. Bats and small-bodied avian frugivores, including Cotingidae, Pipridae and Tyranidae, were excluded from our surveys.
Fruit surveys
We conducted monthly ground surveys of residual fruit-fall in three 100-ha plots (two TF, one VZ), as described above. Surveys were completed concurrently with frugivore surveys, recording the presence of all patches of fallen fruit occurring within a 1-m wide strip along the transect (total transect length = 12 km per plot, total survey area = 1.2 ha per plot). For each fruit patch encountered we recorded its location along the transect, and collected a fresh specimen for our reference fruit collection. In each case we also located the fruiting stem bearing fruits, including both trees and high-climbing woody lianas, and measured its dbh and perpendicular distance from the transect. Similar ground surveys were also conducted on an intermittent monthly basis by 22 trained local field assistants who walked a network of 67 transects of 5 km in length (41 TF, 26 VZ; Figure 1) which were widely distributed across the two study reserves.
Fruit identification and traits
Further voucher collections were made of fallen fruit from tagged trees monitored for phenology records (Hawes Reference HAWES2012), which were identified in situ by a trained technician from the Botany Department of the Instituto Nacional de Pesquisas da Amazônia (INPA, Manaus). All fruit and seed specimens were also identified at INPA before being deposited at the EAFM Herbarium of the Instituto Federal de Educação, Ciência e Tecnologia do Amazonas (IFAM, Manaus). Additional identification of trees and fruits was aided by the following sources: Cornejo & Janovec (Reference CORNEJO and JANOVEC2010), Gentry (Reference GENTRY1996), Ribeiro et al. (Reference RIBEIRO, HOPKINS, VICENTINI, SOTHERS, COSTA, BRITO, SOUZA, MARTINS, LOHMAN, ASSUNÇÃO, PEREIRA, SILVA, MESQUITA and PROCÓPIO1999), van Roosmalen (Reference VAN ROOSMALEN1985) and Wittmann et al. (Reference WITTMANN, SCHÖNGART, DE BRITO, WITTMANN, PIEDADE, PAROLIN, JUNK and GUILLAUMET2010a). Fruits and seeds were weighed to the nearest 0·01g using an electronic balance and their length, width and depth were measured using callipers (10 fruits/seeds per sample whenever possible). Fruit type, colour, dehiscence and number of seeds were also recorded. Fruit type was classified into four functional groups (Fleming & Kress Reference FLEMING and KRESS2011, van der Pijl Reference VAN DER PIJL1982): (1) berries and berry-like fruit, (2) drupes and syncarps, (3) arillate fruits and (4) dry fruits. Fruit colour was described using five categories: green, brown, yellow, red and purple/black. The number of seeds per fruit was assigned into four classes as single-seeded, several (2–5), numerous (6–15) and many seeds (>15).
Fruit–frugivore interactions
In addition to feeding observations made during frugivore surveys within the 100-ha plots and along the 5-km transects, we include all feeding observations recorded by JEH during other parallel field activities over an 18-mo period, conducted across the same array of plots and transects and distributed equally between terra firme and várzea forest. We do not attempt to infer the demographic consequences of fruit–frugivore interactions to the fate of seeds, and thereby define frugivory (sensu lato) as simply feeding on fruit parts, including both immature/mature seeds and ripe fruit pulp consumed by granivores and frugivores (sensu stricto), respectively.
We supplemented these records with local knowledge of fruit-frugivore interactions from 18 semi-structured interviews conducted at 16 local communities located within the two study reserves (Figure 1), during July–August 2011. Interviewees were selected non-randomly in each community to target the most knowledgeable informants, typically experienced adult hunters, fishermen and older women who had examined stomach contents of hundreds/thousands of fish. Colour photographs of fruits with known identity from our reference collection were shown to two or three interviewees simultaneously who were invited to list their respective vertebrate consumers whenever those were known. Colour photographs of frugivorous mammal, bird and fish species were available as a prompt in all cases. Local informants interviewed were free to contribute jointly, and records were made for the combined group. A total of 188 photographs of fruit species/genera were shown (103 TF, 79 VZ), including six additional photographs of non-native (exotic) fruit to check for any tendency to report type II errors (i.e. false trophic interactions), with each interview typically lasting 90 min. Finally, an unstructured portion of the interview invited informants to match all várzea forest fruits known to be consumed by 22 bony and cartilaginous fish taxa and three freshwater turtle species.
Data analyses
Data from monthly frugivore surveys were pooled across the two terra firme plots and converted into number of sightings per 10 km walked to compare between forest types. Sightings of closely related species were typically pooled at the genus level, including capuchin monkeys (Cebus spp.), tamarins (Saguinus spp.) and brocket deer (Mazama spp.), although ambiguous identifications also required pooling observations across congeners for parrots, pigeons and tinamous.
Fruit–frugivore interactions recorded from all methods (direct observations from 100-ha plots and transects, and local knowledge) were combined to create a single binary matrix of frugivore consumers and fruit resources, with undocumented and confirmed positive interactions represented by the values 0 and 1, respectively. We examined the total number of species in each network, i.e. ‘network size’ (S), the average number of plant genera per animal functional group, i.e. ‘plants per animal’ (Ppa), and the proportion of realised interactions or links (L) in relation to the total number possible, i.e. ‘connectance’ (C). Connectance can be considered a surrogate for specialization (Mello et al. Reference MELLO, MARQUITTI, GUIMARÃES, KALKO, JORDANO and DE AGUIAR2011); more sophisticated analyses of specialization/generalization would require a standardized metric of interaction frequencies across different methods (Blüthgen et al. Reference BLÜTHGEN, MENZEL and BLÜTHGEN2006) which is unavailable in this study. Independent networks were generated for each forest type using Pajek 2.05 (http://pajek.imfm.si), and presented as bipartite graphs, excluding consumers with fewer than 10 trophic resources identified in both forest types. Non-metric multidimensional scaling (NMDS) ordinations, based on the Bray–Curtis similarity index, were produced from the same binary matrices, and we used ANOSIM to further explore differences in dietary composition between functional groups of frugivores.
All plant species and genera were assigned mean values for fruit and seed mass, length, width and depth, with field measurements of at least 10 fruits/seeds supplemented by values from the literature where necessary (Cornejo & Janovec Reference CORNEJO and JANOVEC2010, van Roosmalen Reference VAN ROOSMALEN1985, Wittmann et al. Reference WITTMANN, SCHÖNGART, DE BRITO, WITTMANN, PIEDADE, PAROLIN, JUNK and GUILLAUMET2010a). This approach is appropriate as both fruit type (Casper et al. Reference CASPER, HEARD and APANIUS1992) and seed size (Kelly Reference KELLY1995, ter Steege & Hammond Reference TER STEEGE and HAMMOND2001) tend to be morphologically conservative and consistently uniform within Amazonian tree and woody liana genera, so that most of the variation in these traits occurs between genera. As a result of strong correlations between morphometric variables (Fruit mass ~ Fruit length: R2 = 0.35, P < 0.001; Fruit mass ~ Fruit width: R2 = 0.55, P < 0.001; Seed mass ~ Seed length: R2 = 0.44, P < 0.001; Seed mass ~ Seed width: R2 = 0.59, P < 0.001), we used only fruit and seed mass in the subsequent analyses.
In addition to the continuous variables fruit and seed mass, we used fruit type, fruit colour, a ranked classification of number of seeds (as categorical variables) and whether or not fruits were dehiscent (a binary variable) to examine the role of fruit traits on the heterogeneity in fruit resource use across all functional groups of frugivores. We used a classification and regression tree (CART) approach (Breiman et al. Reference BREIMAN, FRIEDMAN, OLSHEN and STONE1984, Loh Reference LOH2011) that successfully incorporates the combination of continuous, categorical and binary variables, which is not conducive to ordination techniques. All analyses were conducted in R (v 3.0.1, 2013): NMDS and ANOSIM used the ‘vegan’ package (http://cran.r-project.org/web/packages/vegan/); CART analysis used the ‘rpart’ package (http://cran.r-project.org/web/packages/rpart/).
RESULTS
Frugivores
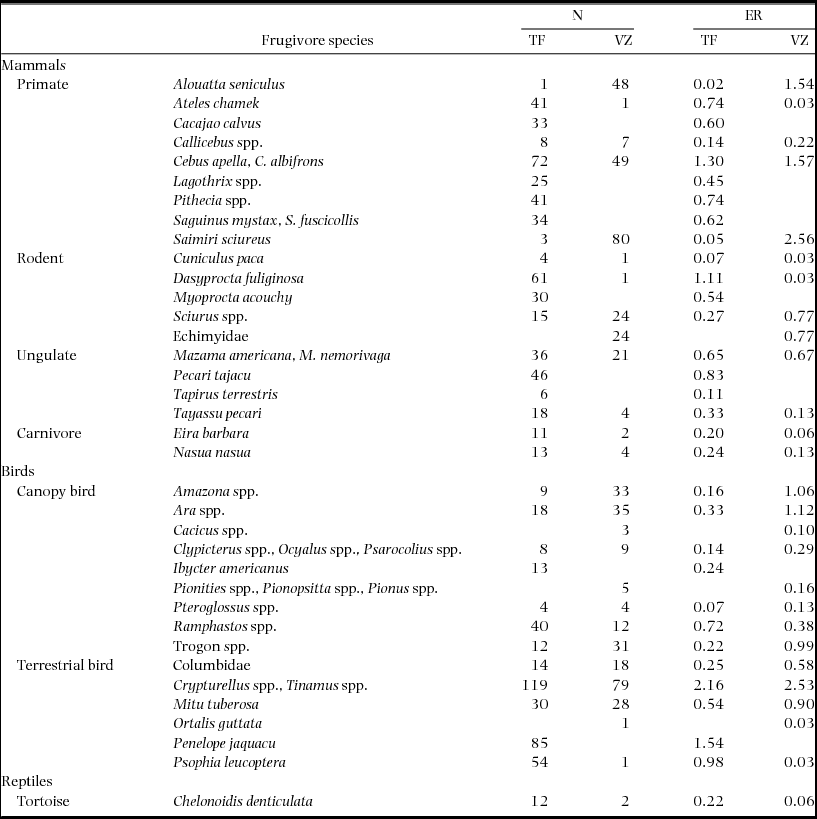
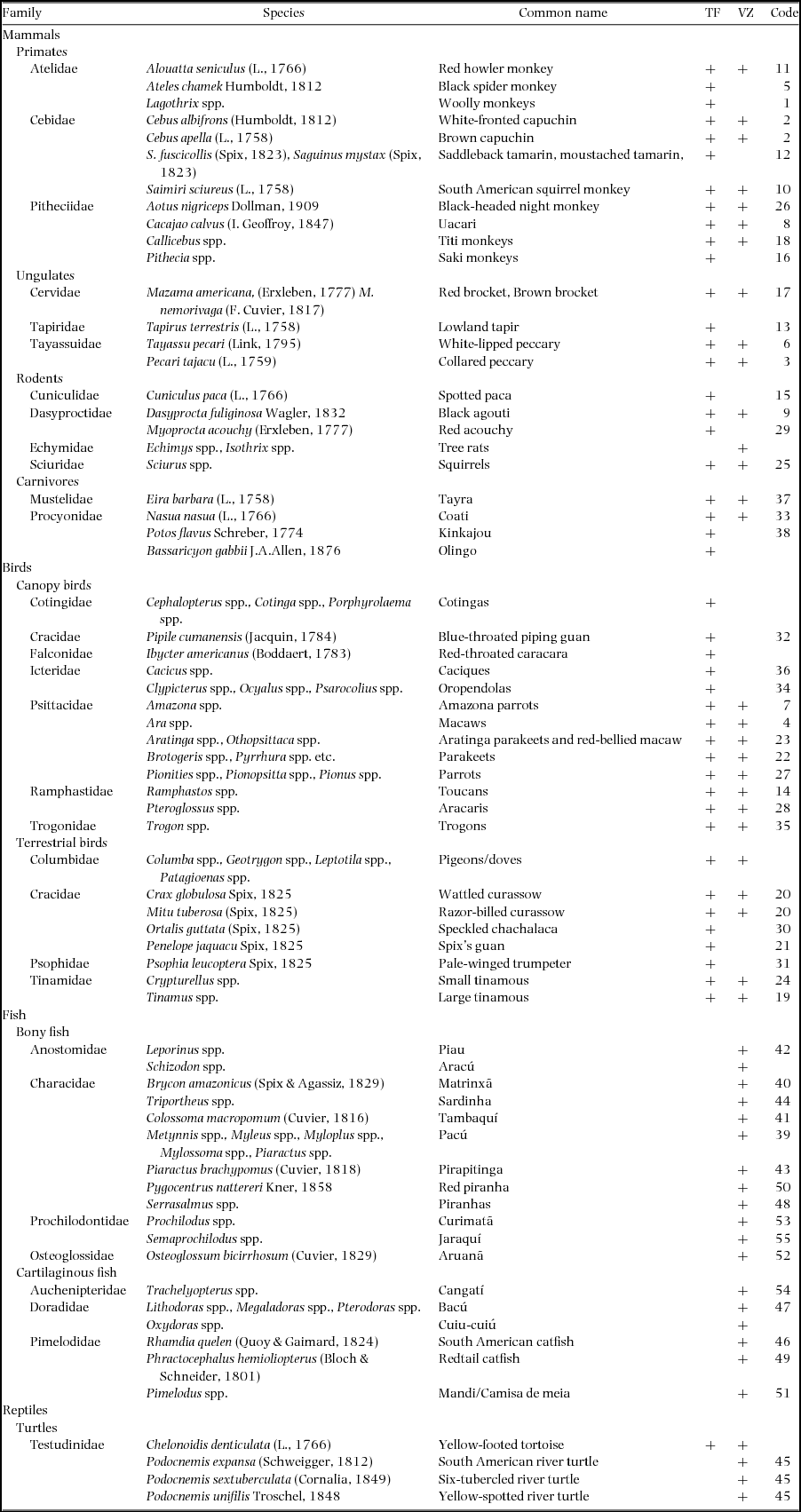
Total survey effort of the 100-ha plots was 552 km in terra firme (Plot 1 = 24 mo: 11 mo wet season, 13 mo dry; Plot 2 = 22 mo: 7 mo wet season, 15 mo dry) and 312 km in várzea forest (26 mo: 13 mo aquatic phase, 13 mo terrestrial). We detected 36 functional groups of medium- to large-bodied terrestrial and/or arboreal frugivorous vertebrates, including nine primate, four ungulate, five rodent, two carnivore, nine canopy bird, six terrestrial bird and one reptile taxa (Table 1). These surveys failed to detect the wattled curassow (Crax globulosa), night monkey (Aotus nigriceps) and two arboreal procyonids (kinkajou, Potos flavus and olingo, Bassaricyon gabbii), although their presence was confirmed outside surveys in the Médio Juruá region. The complete list of medium- to large-bodied frugivores of the Médio Juruá region also includes aquatic taxa represented by 12 bony fish functional groups, six cartilaginous fish functional groups and three freshwater turtle species (Appendix 1). We do not report on the interactions of frugivorous bats or small-bodied birds.
Table 1. Sightings (N) and encounter rates (ER, sightings per 10 km surveyed) of frugivorous vertebrates during monthly line-transect surveys within three 100-ha plots in terra firme (TF) and várzea (VZ) forest along the Juruá river, Amazonas, Brazil.

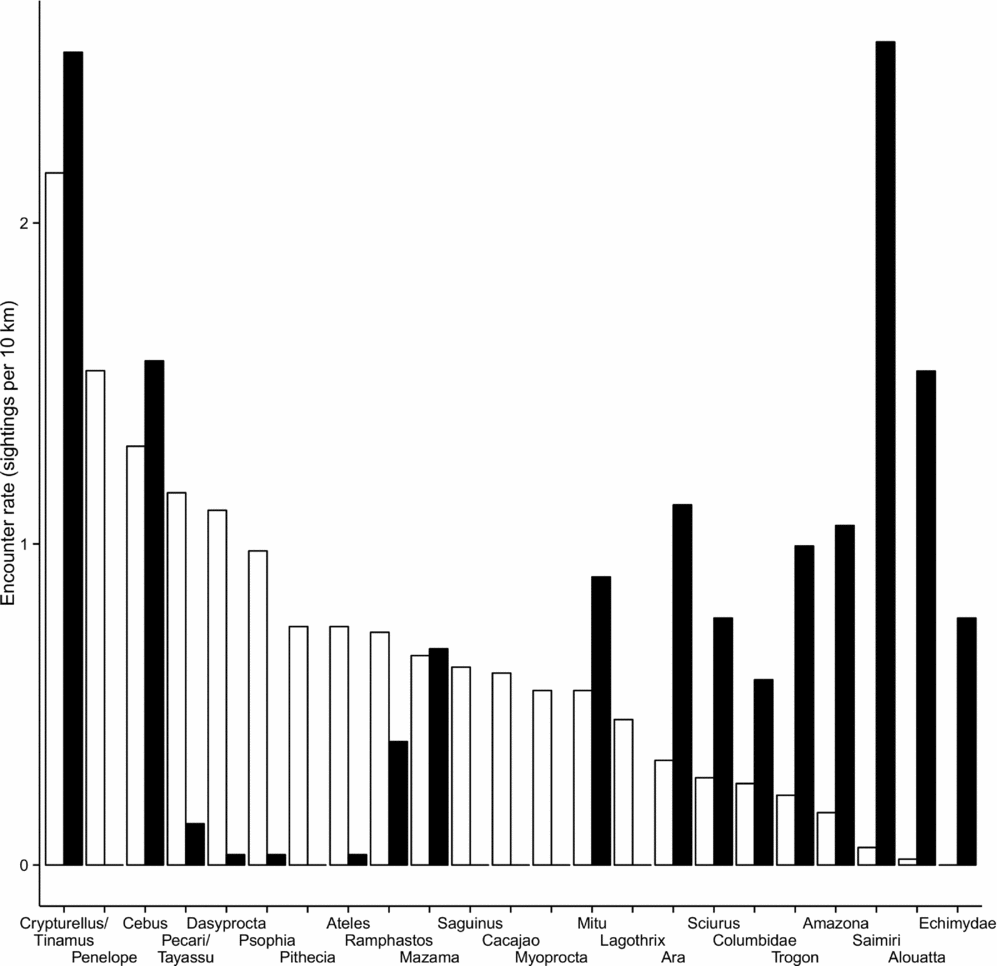
There were clear differences between the frugivore assemblages in terra firme and várzea forests (Figure 2). Primates such as woolly monkeys (Lagothrix spp.), saki monkeys (Pithecia spp.) and tamarins (Saguinus spp.) were absent from várzea forest. Uacari (Cacajao calvus) and spider monkey (Ateles chamek) are known to occur in várzea forest but are patchy in their distribution across the Médio Juruá region and were absent from our várzea study plot. In contrast, howler monkey (Alouatta seniculus) and squirrel monkey (Saimiri sciureus) were much more frequently sighted in várzea than terra firme forest. Within the ungulates, lowland tapir (Tapirus terrestris) and collared peccary (Pecari tajacu) were absent from várzea forest, while within the rodents, agouti (Dasyprocta fuliginosa) and acouchi (Myoprocta acouchy) were also almost exclusively sighted in terra firme. Conversely, arboreal echimyid rodents (Dactylomys spp. and Isothrix spp.) and large squirrels (Sciurus spp.) were largely restricted to, or more common in várzea, respectively. This strong turnover in community composition is exacerbated when considering the additional inclusion of frugivorous fish and turtles during the prolonged aquatic phase when floodwaters invade the várzea forest.

Figure 2. Encounter rates of frugivorous vertebrates (> 10 sightings per plot in at least one forest type, mean per 10 km) during line transect surveys conducted within 100-ha plots in terra firme (open bars) and várzea forest (solid bars) along the Juruá river, Amazonas, Brazil.
Fruits
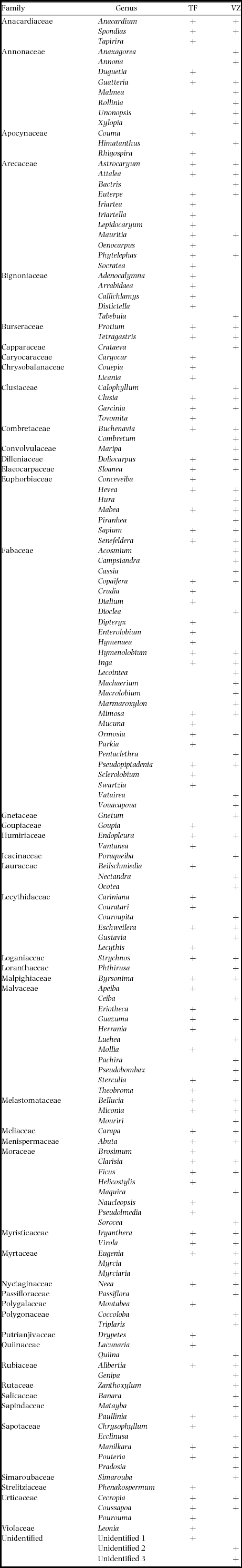
In addition to the survey effort within the three 100-ha plots, information on fruit resource availability was supplemented by fruit surveys along the 5-km transects. Total effort comprised 498 surveys (312 TF, 186 VZ) over 29 mo and an average of 78.9 km mo−1 walked along transects (50.5 km mo−1 TF, 28.4 km mo−1 VZ). Of the 152 plant genera considered in this study, 50 and 54 genera were restricted to either terra firme or várzea forest, respectively, whereas the other 48 genera occurred in both forest types (Appendix 2).
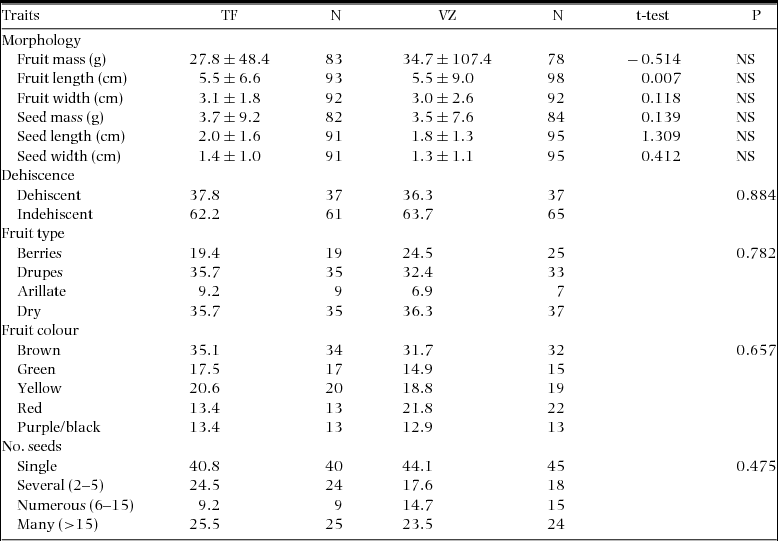
Fruit and seed mass were measured for over 75% of sampled plant genera in both terra firme and várzea forests (Table 2). Fruit and seed dimensions and mass did not differ significantly between terra firme and várzea plant genera, but fruit mass and size were more evenly distributed over a wider range in várzea forest. The proportion of plant genera within mutually exclusive categories of fruit type, fruit colour, fruit dehiscence and number of seeds per fruit were also comparable across the two forest types (Table 2).
Table 2. Summary of fruit morphology measures per plant genus (mean ± SD) and other fruit traits (% of plant genera) in terra firme (TF) and várzea (VZ) forest along the Juruá river, Amazonas, Brazil. Difference tested by t-test for morphology variables and Fisher's exact tests (two-sided) for all other fruit traits.

Fruit–frugivore interactions
Fruit–frugivore interactions, excluding functional groups with insufficient data (<10 interaction records), yielded a sample of 55 frugivore consumers targeting 152 fruit resources across the two forest types (TF: 38 × 98; VZ: 48 × 103), resulting in a slightly larger overall network size (S, TF: 136; VZ: 151) with slightly fewer plants per animal (Ppa, TF: 2.58, VZ: 2.15) in várzea forest. We recorded an almost equal number of interactions or links (L) in each forest type (TF: 956; VZ: 958), resulting in a lower number of links per node in várzea forest (Total – TF: 7.0, VZ: 6.3; Links per animal – TF: 25.2, VZ: 19.6; Links per plant – TF: 9.8, VZ: 9.3) and the overall filling or connectance (C) of 25.7% and 19.4% of all potential fruit–frugivore interactions in terra firme and várzea forest, respectively.
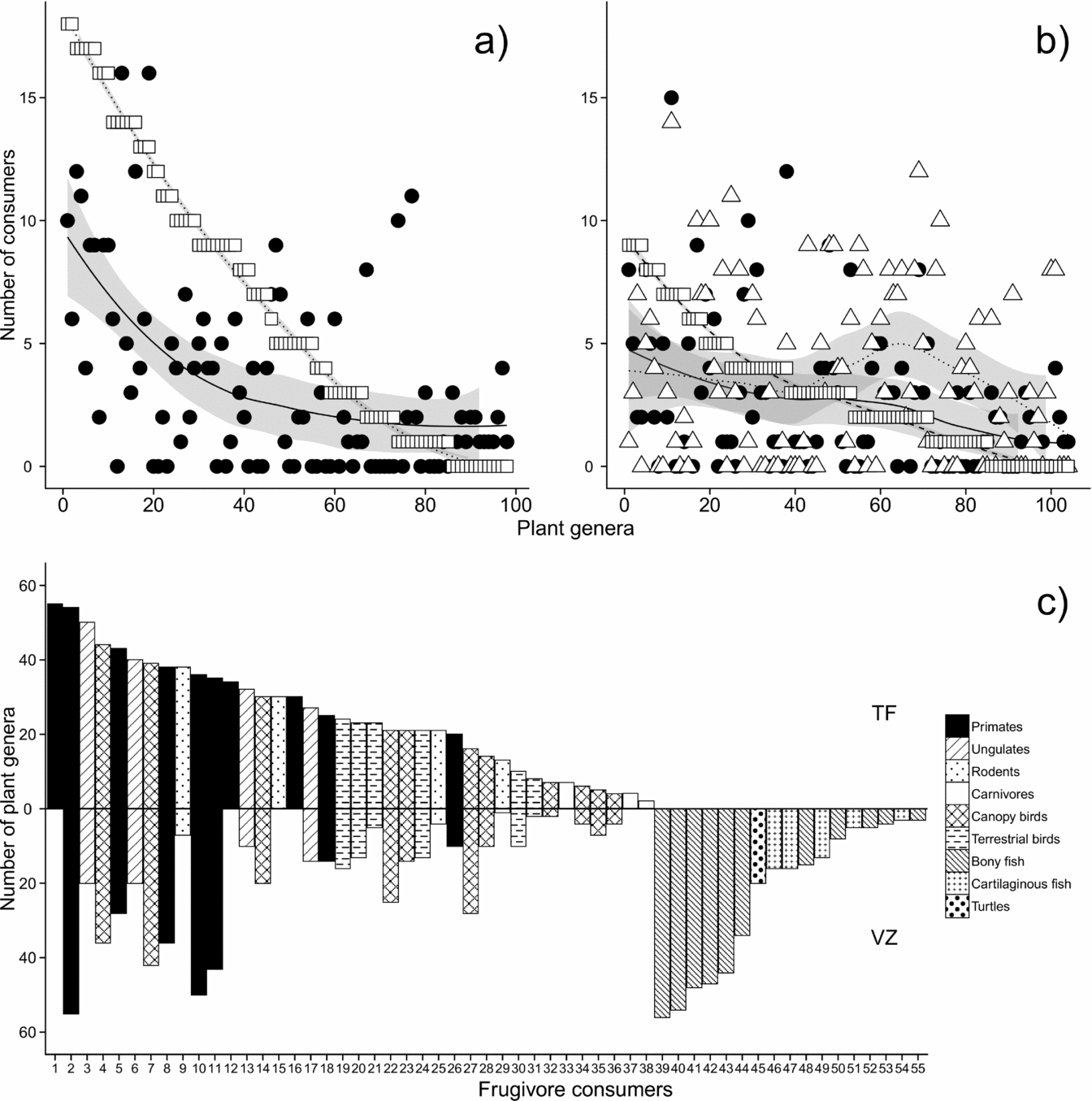
These fruit–frugivore interactions were distributed very unevenly between both fruit resources and fruit consumers (Figure 3). Mammals were the principal fruit consumers for most plant genera in terra firme forest, in contrast to várzea where more plant genera had their fruit consumed by a combination of mammals, birds and fish. Primates featured prominently amongst mammals with the highest number of unique interactions, especially in terra firme. With the exception of four primate (Cebus spp., Cacajao calvus, Saimiri sciureus, Alouatta seniculus) and three canopy bird taxa (Ara spp., Amazona spp., Pionus spp. etc.), almost all frugivores occurring in both forest types exhibited fewer interactions in várzea forest than in terra firme forest. Six bony fish functional groups were recorded as consumers for as many plant genera as primates in várzea forest.

Figure 3. Numbers of fruit consumers identified per plant genus in terra firme (a) and várzea forest (b), and corresponding numbers of plant genera identified as fruit resources per frugivore consumer (c). Symbols in (a) and (b) represent mammals (squares), birds (circles) and fish (triangles); plant genera are ranked by number of mammalian consumers; curves represent smoothed means; shading represents 95% confidence intervals. Numbers along the x-axis in (c) refer to frugivore codes listed in Appendix 1; bars above and below the zero line represent terra firme and várzea forest, respectively.
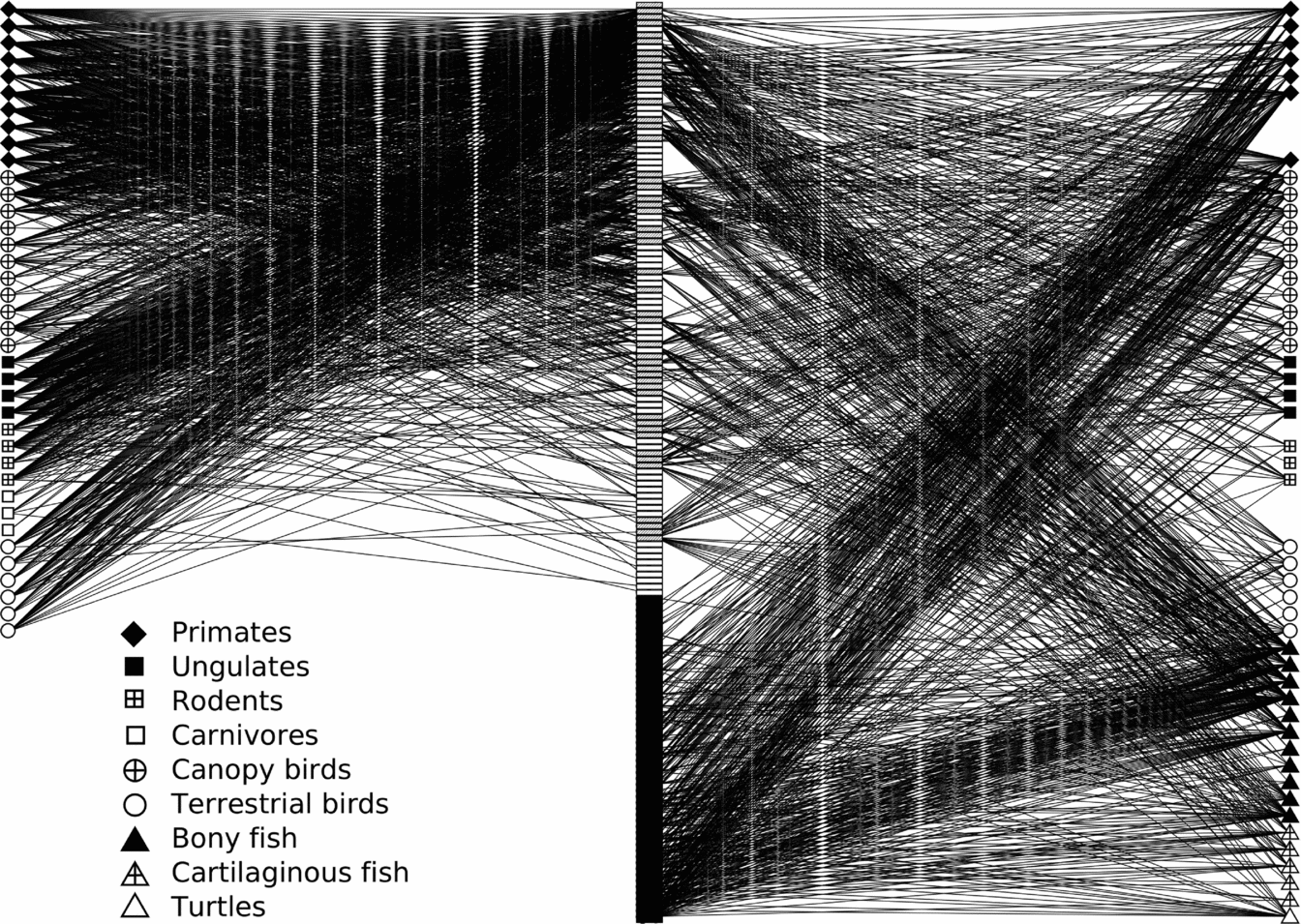
Bipartite graphs show that fruit–frugivore networks in both terra firme and várzea forest were highly diffuse, with most frugivores exhibiting a generalized diet including fruit resources from a wide range of plant genera (Figure 4). Similarly, most plant genera bear fruits consumed by a diverse coterie of frugivores. Beyond these general observations, however, the networks for the studied taxa appear to differ substantially between the two forest types. The interactions in terra firme forest were heavily dominated by arboreal frugivores, and primates in particular. Primates remained important in várzea but, in addition to a number of plant genera common to terra firme, their fruit resources were notably comprised of plant genera unique to várzea forests, which were also heavily consumed by frugivorous fish. Accordingly, there was a notably smaller contribution to the várzea forest network from terrestrial frugivores, including ungulates, rodents and terrestrial birds, as these taxa are not year-round residents in this forest type.

Figure 4. Bipartite networks of fruit–frugivore interactions in terra firme and várzea forests along the Juruá river, Amazonas, Brazil. Fruit consumers are ordered by taxonomic group. Fruit resources are plotted in the centre, in descending order of the number of interactions detected in terra firme forest. White, black and hatched rectangles represent plant genera occurring uniquely in either terra firme, várzea, or shared between both forest types, respectively.
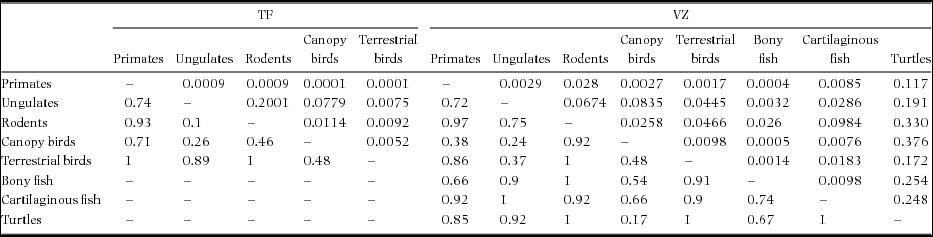
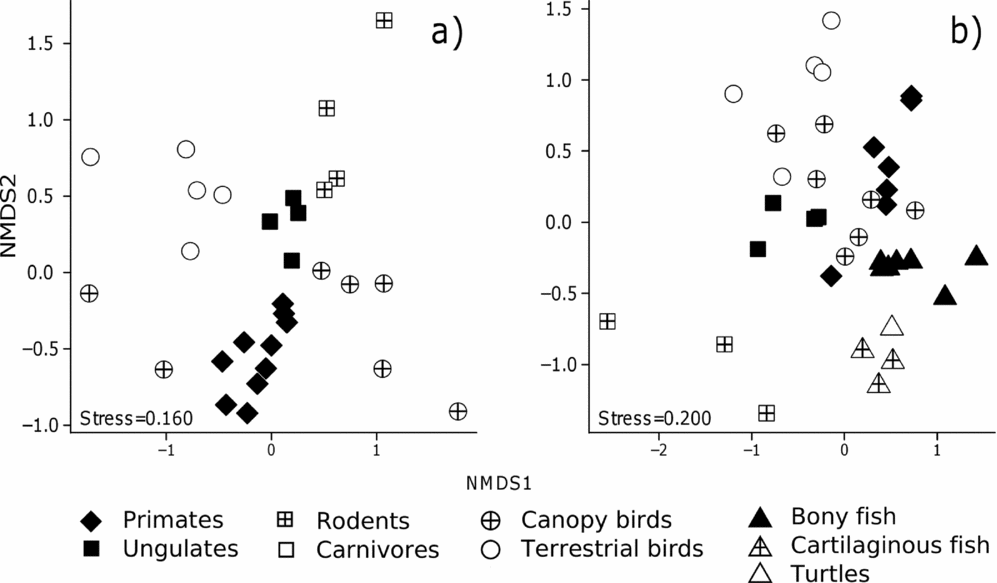
Despite such apparent overlap in fruit resources across frugivorous vertebrates of widely different life histories, the two-dimensional NMDS ordination plots, based on the binary interaction matrices, show a distinct habitat-based grouping of frugivores according to major functional groups (Figure 5; ANOSIM, TF: R = 0.70, P < 0.001, VZ: R = 0.66, P < 0.001). Variation in dietary fruit composition is generally lower within major functional groups than between pairs of groups (Table 3). There was also a noticeable separation between arboreal and terrestrial frugivores in terra firme forest, and between arboreal, terrestrial and aquatic frugivores in várzea forest.
Table 3. ANOSIM results showing partitioning of fruit resources between frugivore consumer groups in terra firme (TF) and várzea (VZ) forest along the Juruá river, Amazonas, Brazil. Below the diagonal: R, above the diagonal: P.


Figure 5. NMDS ordinations based on binary matrices describing the genus-level plant composition of fruit diets in terra firme (a) and várzea forest (b) along the Juruá river, Amazonas, Brazil.
The heterogeneity in fruit resource use amongst frugivores was not clearly explained by the CART analysis of fruit traits, although the relative importance of fruit traits indicates that fruit and seed size, and to some degree fruit dehiscence, were the most important traits in the overall fruit partitioning across the frugivore assemblages in both terra firme and várzea forest. In contrast, other categorical traits, such as fruit colour and fruit type, explained the least amount of trait-partitioning variation. Finally, forest type was the most important variable when included in the analysis, likely because of the high degree of turnover in plant genera providing fruit resources in either terra firme or várzea forest.
DISCUSSION
This study provides one of the first multitaxon assessments of tropical fruit–frugivore networks from adjacent but highly contrasting forest types. Our use of direct feeding observations from extensive frugivore and fruit surveys, coupled with knowledge of interactions obtained through interviews with long-term residents, allowed us to construct binary matrices for seasonally flooded and unflooded forest from the Rio Juruá region of western Brazilian Amazonia. Our study reports three important observations: (1) taxonomic turnover was high between terra firme and várzea forests, in terms of both medium- to large-bodied vertebrate frugivores and fruit resources available; (2) fruit–frugivore networks in both forest types consisted of a large and diffuse set of interactions whose structure varied markedly between forest types, with lower connectance in várzea forests; and (3) partitioning of fruit resources among functional consumer groups was clear but not well explained by our data on fruit morphology and presentation.
High turnover in frugivore assemblages and fruit resources
Even without considering the seasonal occupancy of várzea forests by fish (Horn et al. Reference HORN, CORREA, PAROLIN, POLLUX, ANDERSON, LUCAS, WIDMANN, TJIU, GALETTI and GOULDING2011) and freshwater turtles (Balensiefer & Vogt Reference BALENSIEFER and VOGT2006), we recorded considerable differences in the medium- to large-bodied vertebrate assemblages of flooded and unflooded forests. In addition, frugivores common to both forest types also differed substantially in their abundance expressed as encounter rates. These findings are consistent with previous studies comparing the vertebrate communities of flooded and unflooded forests (Ayres Reference AYRES1986, Haugaasen & Peres Reference HAUGAASEN and PERES2005b, Reference HAUGAASEN and PERES2008; Patton et al. Reference PATTON, DA SILVA and MALCOM2000, Peres Reference PERES1997), which tend to report a relatively depauperate fauna in várzea in comparison to terra firme, although mammal biomass is higher in the former (Haugaasen & Peres Reference HAUGAASEN and PERES2005b, Peres Reference PERES, Eisenberg and Redford1999).
These differences owe much to the physical barrier to terrestrial frugivores imposed by the seasonal floodwaters. Most arboreal and scansorial vertebrates, including primates, squirrels, generalist carnivores such as tayra (Eira barbara) and coati (Nasua nasua), and canopy birds retain accessibility to várzea forests all year-round. In contrast, caviomorph rodents, ungulates, terrestrial birds and tortoises are almost completely excluded from this forest type during the aquatic phase for up to half the year. The annual lateral migration patterns between flooded and unflooded forests have not yet been comprehensively explored but the seasonal use of flooded forests by a range of terrestrial rodents and marsupials, ungulates, primates and birds has been documented (Bodmer Reference BODMER1990, Boubli Reference BOUBLI1999, Fragoso Reference FRAGOSO1998, Haugaasen & Peres Reference HAUGAASEN and PERES2007, Malcolm et al. Reference MALCOLM, PATTON, DA SILVA, Lacey and Myers2005, Peres Reference PERES1996). In particular, terrestrial frugivores excluded during the aquatic phase are potentially attracted to the renewed supply of fruits and seeds exposed or deposited on the forest floor by the receding floodwaters, in addition to the burst of fresh undergrowth foliage (Haugaasen & Peres Reference HAUGAASEN and PERES2007), all of which are sustained by the nutrient-rich soils of várzea forests.
The species composition of consumed fruit resources is similarly divergent between flooded and unflooded forests. The plant communities of Amazonian floodplain forests have received less research attention than their upland counterparts, but have consistently been shown to have lower species richness (Campbell et al. Reference CAMPBELL, DALY, PRANCE and MACIEL1986, Haugaasen & Peres Reference HAUGAASEN and PERES2006, ter Steege et al. Reference TER STEEGE, PITMAN, SABATIER, BARALOTO, SALOMAO, GUEVARA, PHILLIPS, CASTILHO, MAGNUSSON, MOLINO, MONTEAGUDO, NUNEZ VARGAS, MONTERO, FELDPAUSCH, CORONADO, KILLEEN, MOSTACEDO, VASQUEZ, ASSIS and TERBORGH2013) as a result of the extreme conditions of stress imposed by the flood pulse. Yet Amazonian várzea forests are the most species-rich floodplain forests worldwide (Wittmann et al. Reference WITTMANN, SCHÖNGART, MONTERO, MOTZER, JUNK, PIEDADE, QUEIROZ and WORBES2006), partly as a result of their internal habitat heterogeneity, the relentless process of natural forest succession, and the relative geoclimatic stability of Amazonian floodplains since the Andean uplift (Hoorn & Wesselingh Reference HOORN and WESSELINGH2010, Wittmann et al. Reference WITTMANN, SCHÖNGART, JUNK, Junk, Piedade, Wittmann, Schöngart and Parolin2010b).
The high species richness of várzea forests can also be partly attributed to the ability of some terra firme plant species to tolerate varying degrees of inundation, thereby expanding their ecological distribution into floodplain forests on higher ground (Wittmann et al. Reference WITTMANN, SCHÖNGART, JUNK, Junk, Piedade, Wittmann, Schöngart and Parolin2010b). However, the unique environmental pressures within várzea forests are reflected in very low levels (10–30%) of floristic similarity with terra firme forests (Wittmann et al. Reference WITTMANN, SCHÖNGART, JUNK, Junk, Piedade, Wittmann, Schöngart and Parolin2010b). These general patterns are consistent with the high proportion of plant genera recorded in our fruit surveys that were unique to either terra firme or várzea forests, with a smaller fraction occurring in both forest types. Moreover, this floristic dissimilarity further increases at the species level as many parapatric congeners are restricted to either terra firme or várzea forest (Junk Reference JUNK, Holm-Nielsen, Nielsen and Balslev1989).
Forbidden or missing interactions
The high diversity in the frugivore and fruit resource assemblages in our study landscape results in a large number of potential interactions. Our field observations, combined with repeatedly verified cognitive information from local informants, suggest that a large proportion of these interactions are not realized. It is important to understand that these unobserved interactions may truly not occur (forbidden), or may alternatively have just passed undetected during sampling (missing) (Olesen et al. Reference OLESEN, BASCOMPTE, DUPONT, ELBERLING, RASMUSSEN and JORDANO2011). These unobserved interactions are of general concern to network studies as the problem in discerning forbidden from missing links makes it difficult to assess the degree of completion in the matrix, and any number of sampling artefacts resulting in incomplete matrices will affect a variety of network metrics (Blüthgen et al. Reference BLÜTHGEN, FRÜND, VÁZQUEZ and MENZEL2008). Our networks are likely to contain both sorts of unobserved interactions.
To reduce missing observations we supplemented field observations using in-depth knowledge from local residents with decades of personal experience from hunting, fishing and examining gut contents of terrestrial game vertebrates and frugivorous fish, both of which are typical of the local subsistence diets. However, there are biases in this approach as local knowledge is likely to favour those species most targeted by hunters and fishermen, and fruits from the best-known plant species. For example, the diets of primates, ungulates and caviomorph rodents are likely to be more comprehensively reported than those of non-game mustelids and procyonids, which have broadly omnivorous diets that can include high levels of frugivory (Alves-Costa & Eterovick Reference ALVES-COSTA and ETEROVICK2007, Kays Reference KAYS1999). Similarly, consumers are likely to be more readily reported for plant species that are prominent in the local ethnobotany, including those that are abundant, large-girthed or more heavily used by people as valuable extractive resources, such as fruits, seeds, latex and timber (Peterson Reference PETERSON2010). The patchy distribution and rarity of many plant species in tropical forests, and the often ephemeral nature of their fruiting strategies, means that many rare interactions are likely to remain undetected. In our study area, local knowledge is likely more extensive within várzea forests, which are more heavily settled and more frequently ‘sampled’ by hunters/fishermen than adjacent terra firme forest (Figure 1).
Despite the high likelihood of many missing links in our dataset, a large proportion of zero values in our matrix almost certainly represent forbidden interactions for a number of reasons. Firstly, the spatial turnover of fruits and frugivores between terra firme and várzea simply prohibits certain interactions from taking place. Secondly, any asynchrony between the temporal cycles of fruit production and accessibility of flooded forests to terrestrial or aquatic frugivores (at different times of year) precludes otherwise possible interactions. Finally, the repeated absence of any given interaction in the aggregate data pool from 2288 km of census walks along 371 km of transects, sampled over 29 mo by 25 local field assistants likely reflects either forbidden or very rare interactions, which are unlikely to be ecologically important. Despite lumping our interaction data within frugivore functional groups and plant genera, suggesting that much of our networks remain unknown at the species level, we are confident that the networks presented here effectively portray the broad patterns of medium- to large-bodied frugivory in both flooded and unflooded forests.
Partitioning of large, diffuse networks
Networks in both forest types showed a large number of diffuse interactions, but the number of links and overall connectance (the proportion of total potential links realized) was higher in terra firme for almost all frugivore groups occurring in both forest types. This suggests more frugivory generalization in terra firme forests, compared with greater specialization in várzea forests, although further testing of weighted networks with greater coverage of rarely observed species is required (Blüthgen et al. Reference BLÜTHGEN, MENZEL and BLÜTHGEN2006, Reference BLÜTHGEN, FRÜND, VÁZQUEZ and MENZEL2008). Primates in várzea forest exhibited ecological plasticity in retaining a large number of links, including interactions with plant genera unique to this forest type, but the overall dominance of primates in the várzea network was weaker than that in terra firme. This was partly due to the absence of three major terra firme fruit consumers (Lagothrix spp., Pithecia spp. and Saguinus spp.). The high number of interactions associated with frugivorous fish also provided a major contribution to the more even distribution of fruit resources among várzea consumers. Despite their wide recognition as important frugivores (Goulding Reference GOULDING1980), we still have little detail on the diet of many fish species including their relative generalization/specialization (Correa et al. Reference CORREA, WINEMILLER, LÓPEZ-FERNANDEZ and GALETTI2007, Horn et al. Reference HORN, CORREA, PAROLIN, POLLUX, ANDERSON, LUCAS, WIDMANN, TJIU, GALETTI and GOULDING2011).
The suggestion that the diet of frugivorous fish may overlap substantially with other consumers (Horn et al. Reference HORN, CORREA, PAROLIN, POLLUX, ANDERSON, LUCAS, WIDMANN, TJIU, GALETTI and GOULDING2011) is supported by evidence from várzea forest that fish consume fruits that are widely used by both mammals and birds. This overlap could potentially reduce the selective pressure on fruit traits; with trait matching being hardly detectable compared with more specialized networks such as many flowering plants and their pollinators (Blüthgen et al. Reference BLÜTHGEN, MENZEL, HOVESTADT, FIALA and BLÜTHGEN2007). While we found clear partitioning of fruit resources among major frugivore groups in both forest types, this could not be immediately attributed to particular fruit traits, which may be related to the considerable levels of overlap recorded. We also note the overriding influence of forest type in our study, demonstrating the important role of the annual flood pulse in determining the traits of fruit resources consumed by arboreal, terrestrial and aquatic frugivores in várzea forests.
CONCLUSIONS
Both seasonally flooded and unflooded forests of the Médio Juruá region of western Brazilian Amazonia contain large, complex assemblages of frugivorous vertebrates, although turnover is high and the temporal sequence of frugivores and their fruit resources in várzea forests is strongly determined by floodplain water level. Terrestrial vertebrates are excluded by the prolonged flood pulse, when access is permitted to frugivorous fish and freshwater turtles. In combination with the variable fruit resources available in terra firme and várzea forests throughout the year, the binary networks of fruit–frugivore interactions we constructed from field observations and local knowledge differed substantially in structure between the two forest types. Fruit resources were clearly partitioned among broad taxonomically coherent groups of medium- to large-bodied frugivores but this could not be clearly attributed to fruit traits.
Our networks were characterized by a large proportion of unobserved potential interactions, suggesting a high probability of missing data due to sampling effects in addition to the identification of truly ‘forbidden links’. However, we hope that this study will highlight the importance of community-wide assessments of fruit–frugivore networks, particularly in tropical forests where a large proportion of the vertebrate species richness and biomass is sustained by immature and mature fruits and seeds. We also hope to highlight the potential roles of poorly studied frugivores, particularly frugivorous fish in flooded forests. Finally, we emphasize the valuable role that traditional ecological knowledge can play in studies of species-rich ecosystems, including the assembly of complex fruit–frugivore networks.
ACKNOWLEDGEMENTS
This study was funded by a NERC doctoral studentship to JEH and a DEFRA Darwin Initiative (UK) grant (Ref. 16–001) to CAP. We thank the Secretaria do Estado do Meio Ambiente e Desenvolvimento Sustentável of Amazonas (SDS) and the Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis (IBAMA) for authorizing this research. We are grateful to P. Apóstolo and A. Azevedo (both INPA) for assisting with fruit/seed and tree identification respectively, V. Kinupp (IFAM) for specimen curation, and all resident communities of the Médio Juruá region for their generous hospitality. We thank Paul Dolman, Pia Parolin and Tomás Carlo for constructive comments on an earlier version of the manuscript. This is publication no. 9 of the Projeto Médio Juruá series on Resource Management in Amazonian Reserves.
Appendix 1. Frugivore species of the Medio Juruá region of Brazilian Amazonia, and their occurrence in terra firme (TF) and várzea (VZ) forests. Species codes refer to numbers along the x-axis in Figure 3. Lagothrix poeppigii Schinz, 1844 (Poeppig's woolly monkey) and L. cana E. Geoffroy, 1812 (Geoffroy's woolly monkey) occur on the left and right banks of the Rio Juruá, respectively; Callicebus cupreus (Spix, 1823) (coppery titi monkey) plus C. regulus Thomas, 1927 (red-headed titi monkey) and C. purinus Thomas, 1927 (Rio Purus titi monkey) occur on the left and right banks of the Rio Juruá, respectively; Pithecia monachus (E. Geoffroy, 1812) (monk saki monkey) and P. irrorata Gray, 1842 (bald-faced saki monkey) occur on the left and right banks of the Rio Juruá, respectively.

Appendix 2. Plant genera recorded in fruit surveys of the Medio Juruá region of Brazilian Amazonia, and their occurrence in terra firme (TF) and várzea (VZ) forests.