Introduction
Seasonally dry tropical forests (SDTF), in contrast to wet tropical forests, alternate contrasting periods of abundant water supply and periods of drought with annual precipitation from 250 to 2000 mm and a prolonged dry season of 4–6 months (Dirzo et al. Reference Dirzo, Young, Mooney and Ceballos2011). Because of this, precipitation seasonality becomes a key environmental factor that determines and orchestrates the biological activity of plants such as growth, leaf production and reproduction (Eamus Reference Eamus1999, Murphy & Lugo Reference Murphy and Lugo1986).
SDTF trees display several physiological mechanisms to avoid water stress during drought periods (Murphy & Lugo Reference Murphy and Lugo1986). The most evident of these mechanisms are leaf senescence and leaf fall but water stress could be likewise followed by a decline in root and leaf hydraulic conductance, stomatal closure and hormonal alterations within vascular tissues (Giraldo & Hoolbrook Reference Giraldo, Holbrook, Dirzo, Young, Mooney and Ceballos2011). A variety of leaf phenology patterns have evolved in response to this seasonality, including leafless periods during the dry season or during the wet season, or year-round leaf retention (Borchert Reference Borchert1994a, Bullock & Solís-Magallanes Reference Bullock and Solís-Magallanes1990).
Leaf fall of deciduous species typically increases with the onset of the dry season and reduced soil water availability (Bullock & Solís-Magallanes Reference Bullock and Solís-Magallanes1990), with flowering between the mid-dry season and the end of the wet season; these are often regulated by day length (Borchert & Riviera Reference Borchert and Rivera2001, Borchert et al. Reference Borchert, Rivera and Hagnauer2002). However, the synchronicity of leaf fall may vary both within and among species, and tree water status may be a determining factor, varying with the availability of subsoil water, and intrinsic biotic factors such as structure and lifespan of leaves, time of leaf shedding, wood density and capacity for stem water storage, and depth and density of root systems (Borchert Reference Bullock1998). For instance, high wood density species, usually restricted to upland sites with no access to the water table, exhibit high stem water potentials (<4 MPa) and remain dormant the entire dry season until rehydration of stem tissues (Borchert Reference Borchert1994a). Meanwhile, trees with residual water storage in tree trunks and access to subsoil water may flower and flush during the dry season. Thus, seasonal water stress is likely to determine the timing of leaf shedding in SDTFs (Borchert Reference Borchert1994a, b, Bullock & Solís-Magallanes Reference Bullock and Solís-Magallanes1990, Murphy & Lugo Reference Murphy and Lugo1986).
Phenological variation and stem water status in SDTF trees show a strong relationship that explains the common pattern of leaf production after the first rainfalls and leaf shedding during the beginning of the dry season (Méndez-Alonzo et al. Reference Méndez-Alonzo, Paz, Cruz Zuluaga, Rosell and Olson2012). Because soil water potential is negatively correlated with altitude, insolation and water flow accumulation (Méndez-Alonzo et al. Reference Méndez-Alonzo, Pineda-García, Paz, Rosell and Olson2013), deciduous species might avoid seasonal drought and respond to water pulses at the beginning of the rainy season (Borchert Reference Borchert1994a, b; Bullock & Solís-Magallanes Reference Bullock and Solís-Magallanes1990). However, the heliophilous tree species Bonellia nervosa (Theophrastaceae, formerly Jacquinia pungens) in Mesoamerican SDTFs exhibits an inverted deciduous leaf phenology, maintaining its leaves during the dry season (Bullock & Solís-Magallanes Reference Bullock and Solís-Magallanes1990, Janzen Reference Janzen1970): new leaves emerge at the beginning of the dry season when most of the forest canopy is leafless and are dropped about 2 weeks after the rains begin, remaining leafless and dormant throughout the rainy season (Janzen Reference Janzen1970). Understanding the responses of plants with different phenologies to water availability thus becomes a critical need, in light of the increasing occurrence of extreme or erratic climatic regimes (Cleland et al. Reference Cleland, Chuine, Menzel, Mooney and Schwartz2007, Walther et al. Reference Walther, Post, Convey, Menzel, Parmesan, Beebee, Fromentin, Hoegh-Guldberg and Bairlein2002). Here, we examine the responses to water availability of the inverse-phenology tree Bonellia nervosa. Specifically, we study, via experimental dry-season irrigation, the consequences of unpredictable water availability for an inverted deciduous leaf phenology from a plastic-response perspective. We tested whether providing water during the dry season might trigger phenotypically plastic responses of vegetative and reproductive traits. If water is made available, we predicted that this inverted deciduous species would: (1) cease leaf activity and initiate leaf abscission, or (2) increase reproductive success in response to additional water and nutrient availability.
Methods
Study site
This work was conducted in the SDTF located within the Estación de Biología Chamela (Universidad Nacional Autónoma de México) in Jalisco, Mexico (19°29'N, 105°01'W). The region receives mean annual rainfall of 788 mm (1977–2000) concentrated (80%) between July and October, with a dry season from February to the end of June (Bullock Reference Bullock1986). The dominant vegetation is tropical deciduous forest with a leafless physiognomy during the dry season, and semi-deciduous riparian forest along creeks (Lott et al. Reference Lott, Bullock and Solis-Magallanes1987). Although precipitation patterns are relatively constant among years, sporadic rains may occur after the end of the rainy season (December–January), important for facultative leaf flushing species (Bullock & Solís-Magallanes Reference Bullock and Solís-Magallanes1990).
Species of study
Bonellia nervosa C. Presl (Theophrastaceae) is a heliophilic understorey shrub with coriaceous, simple and alternate leaves with a needle-like tip, occurring from southern Jalisco, Mexico, to north-eastern Costa Rica (Janzen Reference Janzen1970, Ståhl & Källersjö Reference Ståhl and Källersjö2004). Theophrastaceae species are typically evergreen, but Bonellia nervosa is the only known species with inverted phenology (Ståhl Reference Ståhl2010, Ståhl & Källersjö Reference Ståhl and Källersjö2004).
Experimental design
We experimentally manipulated water availability in the field for 45 reproductive trees (Figure 1a) in an upland area about 6 km2 and occupying similar morpho-edaphic conditions (Cotler et al. Reference Cotler, Duran, Siebe, Noguera, Vega, García and Quesada2002). To minimize ontogenetic variation within treatments we selected reproductive trees from 3–5 m height with similar trunk diameter at breast height (dbh). Experimental trees (n = 15 per treatment) were randomly assigned to three water irrigation treatments: low (60 mm), high (100 mm) and control. Trees in the three treatments did not initially differ for dbh (F = 1.22; P = 0.30). Irrigation treatments simulated intra-annual precipitation events observed during 1977–2007 in the Chamela SDTF (data from meteorological station at La Estación Biología de Chamela). Water volume for each tree in the low water irrigation treatment simulated the lowest monthly precipitation record (in 2005; 384 mm), and the total volume per tree for the high water irrigation treatment matched the highest monthly precipitation (in 1992; 1125 mm) (Bullock Reference Bullock1986, Hayden et al. Reference Hayden, Greene and Quesada2010).

Figure 1. Bonellia nervosa experimental trees during the dry season in the SDTF in western Mexico: (a) adult tree with leaves during the dry season; other species are leafless; (b–c) Tap root system with lateral roots; (d) water trench around the trunk perimeter in all experimental trees.
Irrigation set-up
Prior to irrigation we determined crown width, lateral root presence and root characteristics of two individuals of 3–5 m height and 64.9 mm and 83.8 mm dbh. After exploratory excavations into limestone (Figure 1b–c, 1.24 m depth), we found that lateral roots did not exceed crown width (2.55 m and 3.2 m, respectively), and varied in number from 5–10 with maximum lengths of ~90 cm. The primary root was similar to a taproot, maintaining the same trunk-width observed above ground. These observations suggest that lateral roots might be involved in superficial water uptake and that primary roots might reach the water table.
In March 2008, we started the experimental irrigation with an initial standardized intensity (25 mm), simulating the mean precipitation from the first rains of the wet season that trigger vegetative activity in deciduous species (Bullock Reference Bullock1986). All experimental trees were irrigated with the total amount of water previously assigned by treatment. To avoid water run-off, a perimeter trench (1.5 m from the trunk base) was excavated around all experimental and control trees (Figure 1d). Low and high irrigation regimes started on the same date (21 March), with a lag time of 2 d between each watering, until the total water for each treatment was achieved: low (384mm, April 4) and high (1125 mm, April 10). Previous to the experimental irrigations, soil moisture was assessed for each tree from five measurements (5–10 cm depth) using an Onset Soil Moisture Sensor with a 5-cm Probe Soil Moisture Smart Sensor (Onset HOBOTM S-SMC-M005).
Leaf traits
Leaf phenological response of all experimental trees was quantified monthly (March–October) using the Fournier index, based on a scale of 0–4 to determine the fraction of potential leaves in the canopy; with 0 = 0%; 1 = 1–25%; 2 = 25–50%; 3 = 50–75%; and 4 = 100% leaf canopy (for details see Fournier Reference Fournier1974). Monthly leaf survival was determined by monitoring a group of marked leaves on five branches of each tree; leaf survival was obtained as the number of standing leaves minus the standing leaves of the previous census for each treatment.
Whole-plant traits
Mean annual growth was estimated as the difference of trunk dbh between the beginning of irrigation (March 2008) and the end of the next dry season (May 2009). Water stress on plants was estimated for five randomly selected stems per treatment from different heights and positions of each tree. Stems were cut slantwise from every tree at the beginning and the end of the irrigation treatments (October 2008). Material was immediately placed within sealed plastic bags to avoid water loss and measured in situ with a Scholander pressure bomb (Model 600 Pressure Chamber Instrument, PMS Instrument Company) (Scholander et al. Reference Scholander, Hammel, Bradstreet and Hemmingsen1965).
Reproductive phenology was recorded using the same criteria as for vegetative phenology. Flowers and fruits as a percentage of canopy cover were calculated monthly, using the Fournier index. Because carbohydrate reserves decrease during the wet season when the plant is dormant (Janzen & Wilson Reference Janzen and Wilson1974), we assumed that nutrient acquisition to produce new leaves, flowers and fruits occurred in the previous season. Thus, the effect of water manipulations on reproductive traits is expected to be measurable in the next dry season after irrigation. We randomly selected and marked five branches from each individual in all treatments and counted the total flowers produced in the dry season following irrigations. We counted the total number of fruits produced after 4 mo during the dry season, and estimated fruit-set as the proportion of the total number of fruits divided by the total number of flowers.
To determine non-structural carbohydrate (NSC) content in twigs, fruits and seeds, we randomly collected 3–5 terminal twigs and mature fruits from different heights from the canopy from five different trees in each treatment. Mean concentrations of fructose, sucrose and glucose were determined by a modification of the Sigma® Fructose assay kit (Lara-Núñez et al. Reference Lara-Núñez, Sánchez-Nieto, Anaya and Cruz-Ortega2009).
Data analysis
To compare the effect of anomalous water availability during the dry season on vegetative and reproductive phenology, we quantified yearly phenological patterns for the three treatments using circular statistics. The frequency of individuals at each phenological stage within each month was calculated, frequency data were transformed to circular percentage and analyses were carried out using Oriana v.4 (Kovach Reference Kovach2011). We determined whether mean angles of phenological patterns differed significantly between irrigation treatments with non-parametric Watson U2 tests that compared the mean vector lengths for each treatment with those for the pooled phenological data. The effect of irrigation on leaf longevity was analysed through a proportional hazards regression model fitted to a Weibull distribution. Annual growth and mid-day twig water potential were analysed with factorial analyses of variance with treatment and year as main effects and repeated-measures design with between-subject factors. To analyse the effect of experimental irrigation on fruit-set we conducted a general linear model (GLM) with maximum verisimilitude function and a Poisson distribution fit where individual tree variation was set as weight factor within the model test. Non-structural carbohydrate (glucose, starch and sucrose) content within fruits and seeds was analysed using a two-way ANOVA followed by Tukey tests. All data analyses with exception of phenological records were performed with JMP® 11.0.0 (SAS Institute, Inc., Cary, NC).
Results
Leaf traits
Eight months following experimental irrigation, leaf phenology showed no significant differences between irrigation treatments. Leaf canopy cover among water manipulations showed similar patterns of decline until the beginning of the wet season (Figure 2a). There were no significant leaf canopy cover differences between control and experimental irrigations or between the irrigation treatments (control vs. low: U2 = 0.074, P > 0.5; control vs. high: U2 = 0.07, P > 0.5; low vs. high: U2 = 0.043, P > 0.5).
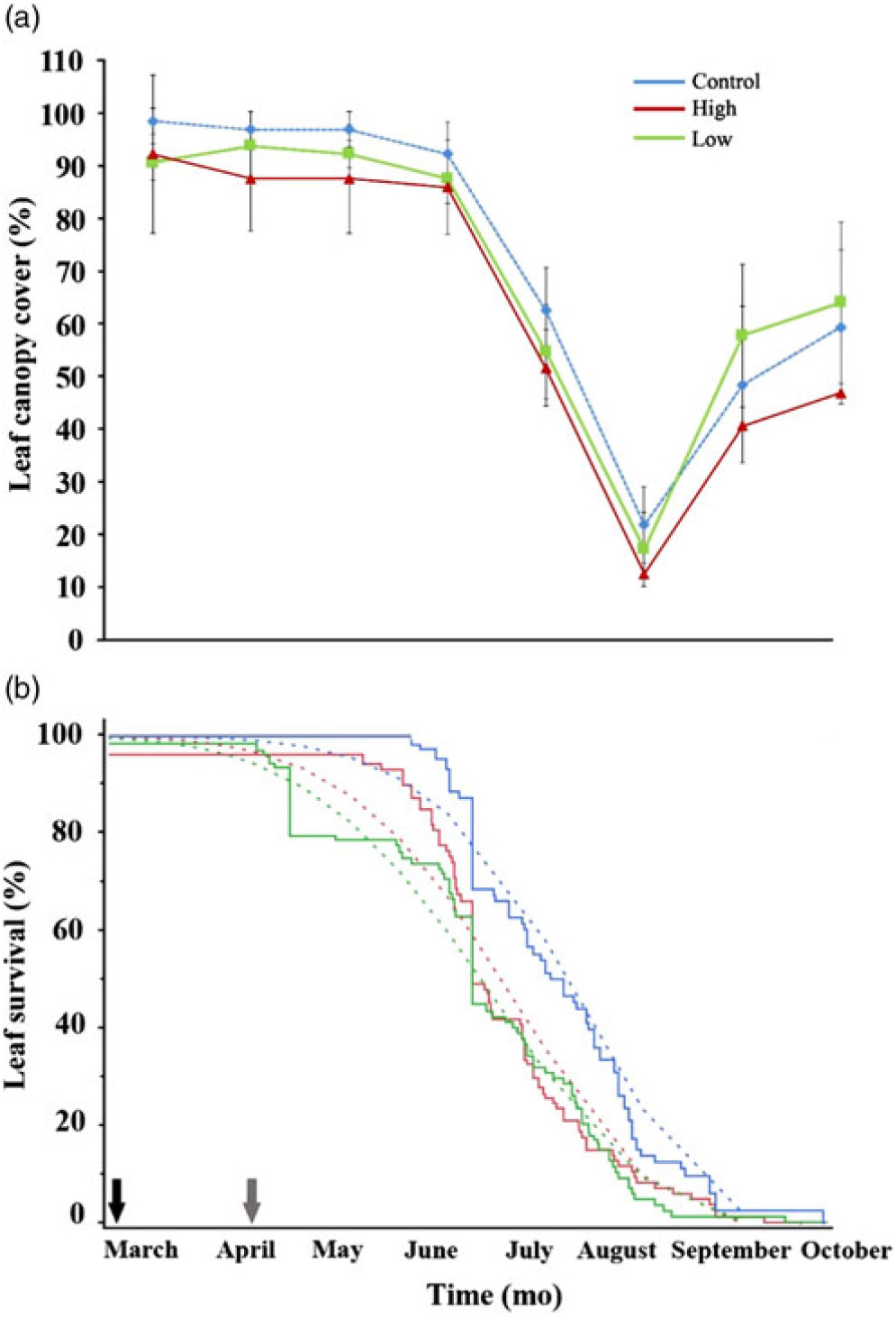
Figure 2. Vegetative responses after irrigation treatments in the inverted deciduous species Bonellia nervosa in the SDTF in western Mexico. (a) Monthly leaf phenological records (± SD); (b) Kaplan–Meier plot of survival probability on marked leaves through irrigations. Dotted line curves show data fitted to the Weibull distribution. Arrows marked the beginning (black) and end (grey) of irrigation treatments (21 March and 10 April 2008, respectively).
Leaf survival probability decreased during the months following experimental irrigation (χ2 = 39,992, P < 0.0001). Marked leaves in the low-irrigation treatment exhibited the most negative trend (April) followed by leaves from the high-irrigation treatment that showed a further decline (June) after the beginning of the wet season (Figure 2b). Leaves from control trees had higher survival after 8 mo, compared with both high- (χ2 = 2,837, P < 0.0001) and low- (χ2 = 3,347, P < 0.0001) irrigation treatments. There was also a significant difference between low- and high-irrigation treatments (χ2 = 41.1, P < 0.0001). During June, leaf survival decreased abruptly for all trees, however Weibull-fitted curves showed that low- and high-irrigation treatments still had lower survival than leaves on control trees during the months following irrigation (Figure 2b; dotted-lines). Finally, experimental trees from all treatments dropped all marked leaves at the onset of the rainy season (Figure 2b).
Whole-plant traits
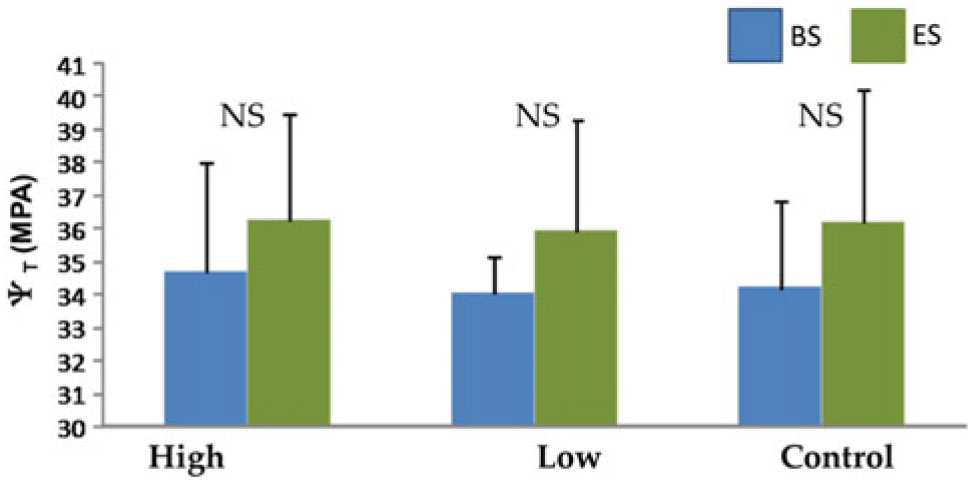
Both control and experimental trees showed significant annual growth (F = 3.0, P = 0.034), but there were no differences among irrigation treatments (F = 1.37, P = 0.25) or any year×treatment interaction (F = 0.33, P = 0.71). Contrary to expectations, mid-day twig water potential also showed no differences between the initiation and the end of irrigation (F = 1.72, P = 0.17).
After 8 mo of experimental irrigation, reproductive phenological patterns varied among treatments. Flower canopy percentage tended to decrease gradually until the wet season began, with control trees having higher percentages (Figure 3), but the Watson’s U2 paired-comparisons showed no significant differences among treatments: control vs. low (U2 = 0.052, P > 0.5); control vs. high (U2 = 0.085, P > 0.5); high- vs. low-irrigation (U2 = 0.02, P > 0.5).

Figure 3. Flower and fruit canopy percentage (%) in Bonellia nervosa reproductive trees under experimental water irrigations during the dry season: (a) flowers and (b) fruits. Each point represents the mean value observed for that month (± SD).
Fruit canopy percentage showed a significant effect of experimental irrigation, where control trees had higher fruit canopy percentage than both low (U2 = 0.19, P < 0.05) and high (U2 = 0.20, P < 0.05) irrigation treatments. Fruit-set was significantly lower in the low-irrigation treatment (X2 = 145.5, P < 0.0001) (Figure 4).

Figure 4. Mean fruit-set (± SE) in Bonellia nervosa adult trees after 1 year of irrigation treatments in the SDTF in Chamela, western Mexico.
Overall, NSC concentrations showed different patterns among different tissues from irrigated trees (Figure 5). Twigs of irrigated trees showed a lower glucose concentration compared with control trees (F = 3.7, P = 0.02), but high-irrigation trees had a significantly higher starch concentration compared with both control and low-irrigated treatments (F = 17.9, P < 0.0001; Figure 5a). There were no differences in sucrose content between treatments (F = 1.46, P = 0.24). Glucose concentration of fruits and seeds showed different trends (Figure 5c): while there were no differences for fruits (F = 2.33, P = 0.10), seeds showed higher concentration in both irrigation treatments (F = 23.5, P < 0.0001). Starch concentration within fruits and seeds was higher in the low and high irrigation treatments (F = 5.43, P = 0.007; and F = 10.32, P = 0.0002, respectively; Figure 5b) compared with controls. Sucrose concentration decreased in fruits from high irrigation trees (F = 4.21, P = 0.021), while seeds from low irrigation trees had a higher concentration than control and high-irrigation treatments (F = 4.18, P = 0.022; Figure 5d).
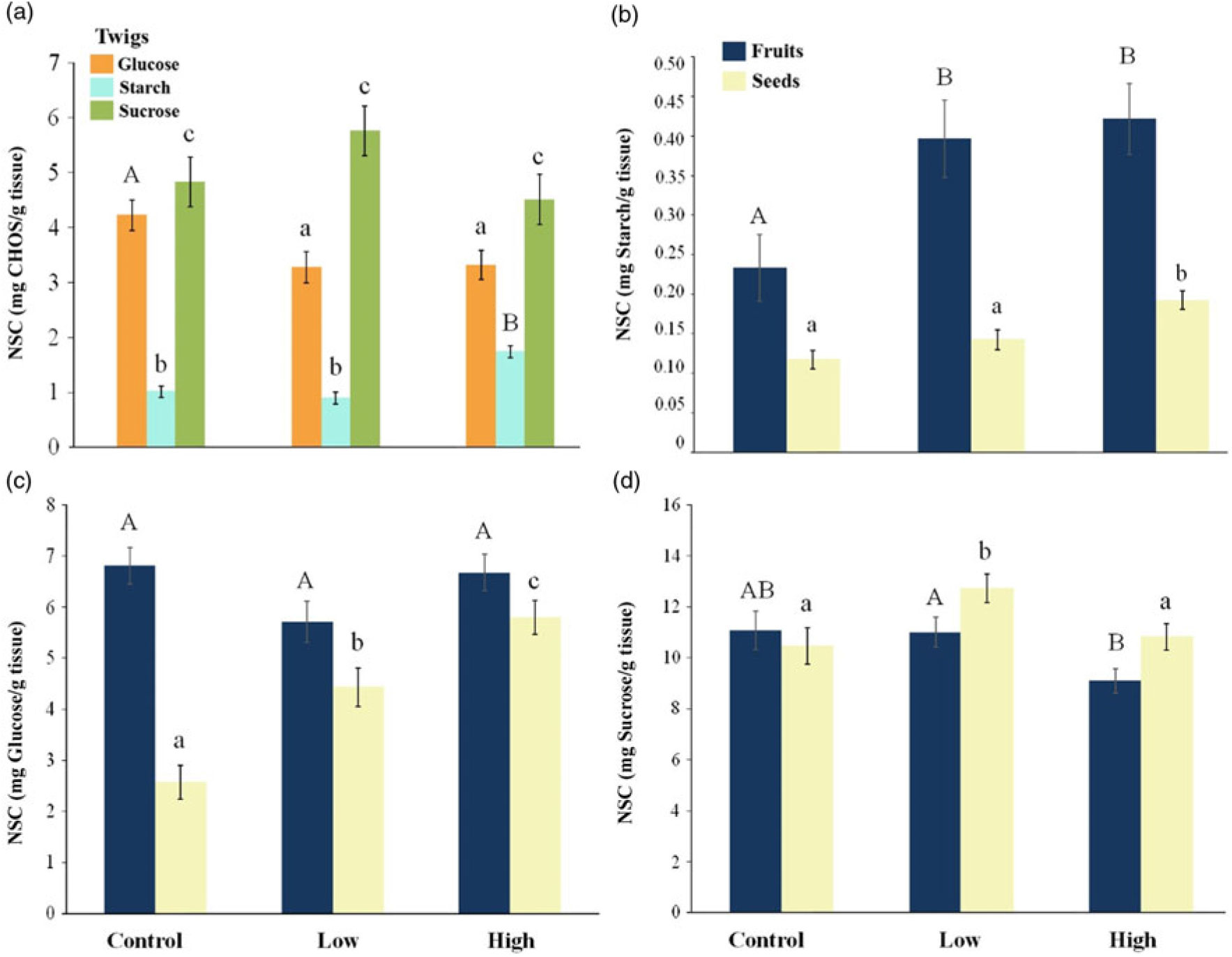
Figure 5. Mean concentration of non-structural carbohydrates (NSC) in Bonellia nervosa tissues (± SE) (twigs, fruits and seeds) after experimental irrigation during the dry season in the SDTF in western Mexico. (a) Differences in twigs NSC content. (Capital vs. lowercase letters show significant differences among treatments). Fruit and seed (b–d) differences are marked with capital and lowercase letters, respectively.
Discussion
We predicted that experimental irrigation of Bonellia nervosa during its typical dry season should alter its leafing phenology or perhaps increase its reproductive output. Overall, our results supported neither of these predictions. Perhaps most surprisingly for this species with inverted phenology, leaf phenology was unaffected, and although some whole-plant traits (e.g. fruit and seed composition) did show differences across treatments, there was no increase in reproductive output relative to the control as a result of watering.
Leaf phenology
Experimental irrigations during the dry season did not alter the normal occurrence of leaf drop following the first rains of the wet season. Contrary to our expectations, we did not find a significant difference between leaf abscission among irrigation treatments (Figure 2b). We expected that precipitation, as a key environmental factor in vegetative activity, should affect inverse vegetative activity in Bonellia nervosa. However, our results suggest that leaf abscission in this species may be under genetic control rather than governed by environmental factors (Mojica et al. Reference Mojica, Mullen, Lovell, Monroe, Paul, Oakley and McKay2016), despite evidence that many phenological responses of deciduous species in SDTFs are driven by water availability or photoperiod (Borchert Reference Borchert1994a, b; Bullock & Solís-Magallanes Reference Bullock and Solís-Magallanes1990).
Sánchez et al. (in preparation) have also found that inverted leaf phenology in Bonellia nervosa remains unaltered after experimental shading, suggesting that the inverse phenological behaviour has no plasticity (i.e. is genetically fixed). This species also presents no phenological plasticity to environmental light variability in Costa Rican populations (Chaves & Avalos Reference Chaves and Avalos2008). Together, these findings on immutable leaf phenology support the idea that inverted leaf phenology may have been genetically accommodated in this species.
Whole-plant traits
Our findings integrating off-season water availability and whole-plant traits do not explain the benefits of inverted phenology with activity during drought conditions. The effect of dry-season irrigation on inverted leaf phenological species has not previously been described, but our findings are consistent with other research that found little effect of irrigation on leaf longevity, stem water status or storage (Myers et al. Reference Myers, Williams, Fordyce, Duff and Eamus1998), nor differences in subsequent relative growth rate after one-time experimental irrigation of four (non-inverted) deciduous species in the SDTF of western Mexico (Hayden et al. Reference Hayden, Greene and Quesada2010).
These results could be explained through four different lines of reasoning: (1) primary roots are storing water or (2) directly accessing the water-table, or (3) unusual water availability during the dry season produces plastic responses to reallocate nutrients to reproductive traits. In general, deciduous trees with no access to soil or stem water storage in the dry season are not in a state of dormancy, but rather in a drought-induced rest period whereby they remain inactive until rehydration (Borchert Reference Borchert1994 b). In contrast to most dry season deciduous trees, Bonellia nervosa exhibits a root system composed of long deep roots that could potentially store water or reach the groundwater table (Figure 1b, c). In Faidherbia (Acacia) albida, an inverse phenology species in semi-arid Africa, water uptake comes from roots distributed to a depth of 7 m, probably extending to the permanent water-table (Roupsard et al. Reference Roupsard, Ferhi, Granier, Pallo, Depommier, Mallet, Joly and Dreyer1999). Such a mechanism may explain water status during the dry season in Bonellia nervosa and the lack of any effects of irrigation treatments on mid-day stem water potentials (Appendix 1).
Typically, SDTF tree roots have no access to the groundwater table and soil water reserves are depleted early in the dry season in dense tree stands (Borchert Reference Borchert1994a). However, Bonellia nervosa roots could act as water reservoirs during the dry season for reproduction and vegetative growth. Flower and fruit phenology are restricted to the utilization of residual water, allowing rehydration of stem tissues and the subsequent flowering and flushing (Borchert Reference Borchert1994b). Typically, Bonellia nervosa flowers at the same time as it flushes new leaves during the shift between wet and dry season, suggesting that reactivating phenology does not require the first rains, but rather utilizes stored water within stem wood or the root system. We lack quantification of soil water oxygen (δ18O and δ16O) or carbon (δ13C) isotopic composition that could identify specific patterns of water-relations and potential evapotranspiration (Fardusi et al. Reference Fardusi, Ferrio, Comas, Voltas, Resco De Dios and Serrano2016).
Janzen & Wilson (Reference Janzen and Wilson1974) demonstrated that carbohydrate allocation among roots, stems and twigs of Bonellia nervosa occurs during the dry season, depleting one-half of the stored reserves while the tree is leafless during the rainy season. Our results suggest that some non-structural carbohydrate reallocation occurred within fruits and seeds following dry-season irrigations, although irrigation had no quantitative effects on flower and fruit production. It is possible that water from the root system is being redistributed to reproductive components. Although the evolutionary pathways of inverted phenology are unclear, the occurrence of different adaptive strategies exhibited by Bonellia nervosa such as physiological plasticity, efficient internal cycling, conservative utilization of soil nutrients and water use efficiency, are probably necessary to endure drought conditions in SDTFs.
Conclusions
This study provides a first experimental examination of mechanisms underlying an exceptional inverted leaf phenological pattern during the dry season in SDTF, evaluating phenotypic plasticity in response to supplying water out of phase. Although our findings suggest that phenology is not affected by experimental irrigation, mean fruit-set in trees from high-irrigation seem to produce more fruits than low treatment, and non-structural carbohydrates are reallocated among twigs, fruits and seeds. The reason for these contradictory results is unclear now, but these differences can be explained in part by the plasticity of hydraulic redistribution of water from deep layers by roots (where deep soil nutrients are mobilized and taken up by plants; McCulley et al. Reference McCulley, Jobbágy, Pockman and Jackson2004), and by the physiological plasticity of reproductive traits (Valladares et al. Reference Valladares, Gianoli and Gómez2007). In addition, our results could indicate the adaptive mechanisms used by plants in seasonal environments to reallocate nutrients from senescent tissues to the production of new leaves, flowers or fruits, when water supply is limited (Lal et al. Reference Lal, Annapurna, Raghubanshi and Singh2001), specifically reallocation of non-structural carbohydrates to seeds may occur in high-irrigation treatments.
The overall results of this study do not delineate the occurrence of inverted leaf phenology of Bonellia nervosa. However, our findings provide the first attempt to elucidate water uptake, nutrient allocation and phenotypic plasticity response to unusual water availability from a species with an exceptional inverted leaf phenological pattern during the dry season, when drought conditions and leaf deciduousness are mostly the norm for SDTF trees.
Acknowledgements
We are grateful for logistical support provided by the Estación de Biología Chamela of the Instituto de Biología, Universidad Nacional Autónoma de México. We also thank H. Mooney and J. Ehleringer for comments and discussion previous to this manuscript. We are especially grateful to P. Meli, D. Vilchis, J.M. Contreras-Sánchez and B. Ruiz-Guevara for valuable assistance in the field and Gumersindo Sánchez-Montoya for technical support and J.M. Lobato-García for assistance in preparing the figures.
Financial support
This study was supported by grants from Universidad Nacional Autónoma de México (PAPIIT # IN212714-3, IV200418), CONACyT (# 2009-131008 and # 155016) SAGARPA-CONACYT 291333, CONACYT-UNAM-UAGro to LANASE (2015-LN250996, 2016-LN271449, 2017-LN280505, 2018-LN293701) and grant No. 48440, CONACYT, to OS). This study was performed in partial fulfillment of the requirements for the PhD degree of O.S. at Posgrado en Ciencias Biológicas, UNAM.
Appendix 1
Mean mid-day twig water potentials of Bonellia nervosa trees in the SDTF in western Mexico (15 individuals per treatment) in response to three watering treatments. Measurements (± SE) were made at the beginning (BS) and end of the dry season (ES).