Introduction
Anthropogenic environmental change is often responsible for biodiversity loss (Dirzo et al. Reference Dirzo, Young, Galetti, Ceballos, Isaac and Collen2014, McKinney Reference McKinney2002), and ecologists increasingly seek to study the effects of human activity on wild-living animals. However, many animal taxa, including birds, invade human settlements and capitalize on the sympatry with humans, for example, because of lower predation (Díaz et al. Reference Díaz, Møller, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Markó and Tryjanowski2013) and lower parasitism risk (Calegaro-Marques & Amato Reference Calegaro-Marques and Amato2014), increased food availability (Tryjanowski et al. Reference Tryjanowski, Skórka, Sparks, Biaduń, Brauze, Hetmański, Martyka, Indykiewicz, Myczko, Kunysz, Kawa, Czyż, Czechowski, Polakowski, Zduniak, Jerzak, Janiszewski, Goławski, Duduś, Nowakowski, Wuczyński and Wysocki2015) and favourable microclimate (Stewart & Oke Reference Stewart and Oke2012). Anthropogenic activity considerably alters ecosystem patterns and processes in affected areas, hence appropriate behavioural adjustment may be an important factor that enables species to successfully invade urbanized areas and coexist with humans.
Animals often consider humans as a predatory threat and even non-lethal human activities affect animal behaviour equivalently to predation risk (Frid & Dill Reference Frid and Dill2002). Proximity to humans leads to frequent disturbances and recurrent escape responses that are costly, both directly (metabolic costs) and indirectly (e.g. reduced food intake) (Cooper & Blumstein Reference Cooper and Blumstein2014, Tatner & Bryant Reference Tatner and Bryant1986); hence it is advantageous to habituate and increase the degree of tolerance of human presence. Although behavioural adjustment to environmental settings is a very complex process, modified by a myriad of factors (Stankowich & Blumstein Reference Stankowich and Blumstein2005), birds consistently increase their tolerance to humans in urbanized areas across the world (Díaz et al. Reference Díaz, Møller, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Markó and Tryjanowski2013, Samia et al. Reference Samia, Nakagawa, Nomura, Rangel and Blumstein2015). However, most previous studies were conducted in and around long-established cities in Europe and North America, on well-established animal populations (Blumstein Reference Blumstein2019, Díaz et al. Reference Díaz, Møller, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Markó and Tryjanowski2013, Samia et al. Reference Samia, Nakagawa, Nomura, Rangel and Blumstein2015). Therefore, it is still unclear how animals respond to anthropogenic disturbance when only recently exposed to it, i.e. at the beginning of the process of urbanization.
Here, we used a field experiment to study the tolerance of wild birds (estimated as flight initiation distance, FID) to human disturbance in the tropical area of Pantanal, the largest freshwater wetland ecosystem in the world (a UNESCO World Heritage Site; Alho et al. Reference Alho, Lacher and Gonçalves1988). Similar to many other remote tropical areas around the world, the Pantanal ecosystems are threatened by increased anthropogenic land-use; habitat loss due to its conversion to farmland and other anthropogenic uses (Richards & VanWey Reference Richards and VanWey2015, Zalles et al. Reference Zalles, Hansen, Potapov, Stehman, Tyukavina, Pickens, Song, Adusei, Okpa, Aguilar, John and Chavez2019) and growing ecotourism (Bouton et al. Reference Bouton, Frederick, Rocha, Dos Santos and Bouton2005). However, annual flooding negatively affects the local economic activities of humans, causing the local human population to still be relatively small and penetration into many remote areas by humans is very recent, within the last 50 years (Alho et al. Reference Alho, Lacher and Gonçalves1988, Silva et al. Reference Silva, Prasad and Diniz-Filho2017). We studied the tolerance of humans of birds that occupy areas near small, recently established farms and surrounding undisturbed grassland areas. We predicted that birds associated with human settlements were expected to exhibit higher tolerance of human disturbance than birds in natural areas.
Methods
Study site
The study took place near Porto Jofre (17°21′51.8″S, 56°46′24.6″W), Pantanal, Mato Grosso, Brazil. The study site is a part of the Poconé subregion in the northern Pantanal which is characterized mainly by open areas, such as grasslands and temporal wetlands. Human settlements have only occurred here since the 1970s. The human population is concentrated in one city, Poconé, otherwise occupying single farms or small villages, hotels and resorts distributed close to the 140 km main road Transpantaneira to Porto Jofre. Human activities, such as farming, occur in and close to human settlements. Moreover, waste food and occasional deliberate bird feeding occur on farms. Hunting is not practiced very close to human settlements; in general, hunting pressure is greater in more remote tropical areas (Benítez-López et al. Reference Benítez-López, Alkemade, Schipper, Ingram, Verweij, Eikelboom and Huijbregts2017). Altogether, opportunities for habituation by birds to non-lethal interactions with humans are greater near human settlements, such as farms, than in surrounding natural areas.
Data collection
Flight initiation distance. Tolerance of birds to human disturbance was estimated by a widely used technique, termed flight initiation distance (FID), that represents a trade-off between the benefits of staying put and the costs of escape (Díaz et al. Reference Díaz, Møller, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Markó and Tryjanowski2013, Samia et al. Reference Samia, Nakagawa, Nomura, Rangel and Blumstein2015, Stankowich & Blumstein Reference Stankowich and Blumstein2005). FID estimates were collected during two seasons (October–November, 2017 and 2018). Birds were approached during favourable weather conditions (sunny days with no rain or strong wind) during the morning (6:00–10:00). We approached birds in two habitat types significantly differing in the level of human activity. First, we collected data near recently established farms; the term ‘farm’ refers to area near human settlements used for crop production and pasture. Second, we focused on natural open areas, such as grasslands and temporal wetlands. In both farms and natural areas, we approached birds at sites characterized by low vegetation which were >100 hectares in size.
FID data were collected using a standard procedure (Blumstein Reference Blumstein2006) adopted in our previous studies (Díaz et al. Reference Díaz, Møller, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Markó and Tryjanowski2013, Mikula et al. Reference Mikula, Díaz, Albrecht, Jokimäki, Kaisanlahti-Jokimäki, Kroitero, Møller, Tryjanowski, Yosef and Hromada2018). In brief, when a target bird (either a singleton or a randomly selected bird in a single species flock) was spotted by a researcher (usually using binoculars), the researcher moved at a normal walking speed directly towards the target bird, while recording the number of ~1 m paces. The FID was then estimated as the distance (equal to the number of paces) from the researcher to the bird when the bird first started to escape. When the target bird was in vegetation or on an artificial structure, the researcher estimated its height above ground to the nearest metre; FID was then calculated as the Euclidean distance based on horizontal and vertical distances. Large FIDs indicate low tolerance, whereas small FIDs indicate a high tolerance of humans. FID estimates were sampled by systematic searches of the study area but simultaneously we avoided re-sampling by moving to another site (≥300 m away) after each sampling. We focused only on bird individuals engaged in foraging and comfort behaviour (e.g. roosting or preening). We did not approach birds sitting on nests or caring for fledglings or birds exhibiting highly vigilant behaviour. Almost all data were collected by a single person (PT = 90% and MH = 10% of all estimates) to avoid a multiple collector effect. When collecting FID data, researchers wore standardized dull outdoor clothing. We collected data also on starting distance (defined as the distance between the researcher’s position and the bird when first spotted) and flock size in which the target bird occurred.
Starting distance. Starting distance has often been found to be strongly positively correlated with FID (Blumstein Reference Blumstein2006, Mikula et al. Reference Mikula, Díaz, Albrecht, Jokimäki, Kaisanlahti-Jokimäki, Kroitero, Møller, Tryjanowski, Yosef and Hromada2018). Hence, we measured the starting distance, defined as the distance between the researcher’s position and the bird when first spotted.
Flock size. When approached by humans, birds in flocks often behave differently compared with single birds, for example, because of ‘dilution’ or ‘many eyes’ effects (Mikula et al. Reference Mikula, Díaz, Albrecht, Jokimäki, Kaisanlahti-Jokimäki, Kroitero, Møller, Tryjanowski, Yosef and Hromada2018, Pulliam Reference Pulliam1973, Roberts Reference Roberts1996). Hence, when estimating FID, we also collected data on flock size in which the target bird occurred.
Statistical analysis
Animal behaviour, including escape behaviour, is influenced by phylogenetic relatedness of taxa because related taxa have a higher probability of sharing similar characteristics from a common ancestor than from distant taxa. First, we tested for differences in mean FID (log10-transformed) of natural and farm-associated populations of same species (where available) by a phylogenetic paired two-tailed t-test, using the ‘phyl.pairedttest’ function implemented in the phytools R-package (Lindenfors et al. Reference Lindenfors, Revell and Nunn2010).
However, potential differences in FID between birds in natural and farm areas can be affected by confounding factors such as starting distance, flock size, taxonomy or phylogenetic relationships between species. First, we built a multivariable linear mixed model (LMM) to explore variation in FID (response variable) in relation to habitat type (natural/farm), starting distance and flock size (predictors). We used lme4 R-package (Bates et al. Reference Bates, Maechler, Bolker and Walker2014) for LMM with species introduced as a random factor; fitted by maximum likelihood. We also built a model where species was replaced by bird family but the conclusions were the same; hence, we present only results for the model with species as a random factor. All continuous variables were log10-transformed before analysis. The response variable and all continuous fixed effects were centred and scaled to allow comparison of estimated effect sizes. We assessed the degree of multicollinearity among the predictor variables by calculating variation inflation factors (VIFs) using the ‘vif’ function in car R-package (Fox & Weisberg Reference Fox and Weisberg2016). In general, a VIF > 4 indicates a possible collinearity, and a VIF > 10 indicates strong collinearity (Neter et al. Reference Neter, Kutner, Nachtsheim and Wasserman1996). The VIFs were < 1.42 in all cases, suggesting weak multicollinearity between predictors. Variances explained by the fixed and random effects (conditional R2) and by the fixed effects (marginal R2) were calculated using the ‘r.squaredGLMM’ in MuMIn R-package (Nakagawa & Schielzeth Reference Nakagawa and Schielzeth2013). A LMM revealed that birds near farms were more tolerant than birds in natural areas; tolerance of birds also positively correlated with starting distance and flock size (Table 1). This approach produced the same results as the phylogenetically informed mixed model (see below), hence, we report only the results from the latter model in the Results section.
Table 1. Associations between flight initiation distance (FID; response variable) and habitat type (natural/farm), starting distance and flock size (predictors) in birds of Pantanal, Brazil. We modelled FID–predictor associations by a linear mixed model (LMM) with species identity introduced as a random factor. Response and predictor variables were log10-transformed before analysis. A LMM explained > 80% of the variation present in the data (conditional R2 = 0.84; marginal R2 = 0.81)

Then, we investigated association between FID (response variable) and habitat type (natural/farm), starting distance and flock size (predictors) by a phylogenetic generalized linear mixed-effect model (PGLMM) (Ives & Helmus Reference Ives and Helmus2011). All continuous variables were log10-transformed before analysis. We performed PGLMM by Bayesian inference using the Markov chain Monte Carlo technique (MCMCglmm; Hadfield Reference Hadfield2010, Hadfield & Nakagawa Reference Hadfield and Nakagawa2010). To control for phylogenetic relationships among species, we entered a single maximum credibility phylogenetic tree as a random factor in the model. First, we generated 1000 trees using the Hackett backbone at the online platform available at http://birdtree.org/ (Jetz et al. Reference Jetz, Thomas, Joy, Hartmann and Mooers2012). The maximum credibility tree was then reconstructed using the TreeAnnotator tool v. 1.8.2 in BEAST software v. 1.8.2 (Drummond & Rambaut Reference Drummond and Rambaut2007). We used an uninformative inverse-gamma prior for random effects and an uninformative prior for the residual variance. The model was run for 1 millioniterations with a burn-in of 20 000 iterations and thinning of 100. We then checked convergence of model parameters (fixed and random effects) using the Gelman–Rubin statistic that compares within- to between-chain variance and calculates the potential scale reduction factor (Gelman & Rubin Reference Gelman and Rubin1992). The posterior fixed effect distribution was examined for overlap with zero (i.e. the significance test), using a 95% highest posterior density as a credible interval. Finally, we estimated a value of the phylogenetic signal (lambda) to estimate the proportion of variance in FID in a multivariable model which is explained by the effect of phylogenetic relatedness.
All primary data (Supplementary Material 1), phylogenetic tree (Supplementary Material 2) and R code (Supplementary Material 3) are deposited as supplementary material. All statistical analyses were conducted in RStudio 0.98.1103 (R Development Core Team 2019).
Results
Altogether, we collected 1120 flight initiation distances (FIDs) from 132 bird species. The number of estimates per species ranged from 1 to 64 (mean ± SD = 8.49 ± 10.45, median = 4). FIDs of sampled birds were highly variable (raw mean ± SD = 23.31 ± 25.97 m; range = 1–221 m; median = 15 m). Sampling effort was similar in both habitat types (527 and 593 estimates for natural and farm areas, respectively).
The phylogenetically informed analysis of paired same-species populations revealed that birds showed a consistently larger tolerance (shorter FID) to humans in disturbed (farms) than in natural areas (phylogenetic paired t-test, t = 21.64, 95% CI (0.38, 0.46), df = 82, λ = 0, P < 0.0001, N = 85 species; raw mean ± SD (m) = 36.84 ± 25.79 for natural and 14.07 ± 10.08 for farm birds, respectively) (Fig. 1). The MCMCglmm supports these results and revealed significant associations between degree of tolerance of birds and habitat type, but also starting distance and flock size (Table 2). Birds approached near farms were more tolerant of approaching humans than birds in natural areas. Simultaneously, birds were more tolerant when approached from a shorter starting distance and when in smaller flocks.
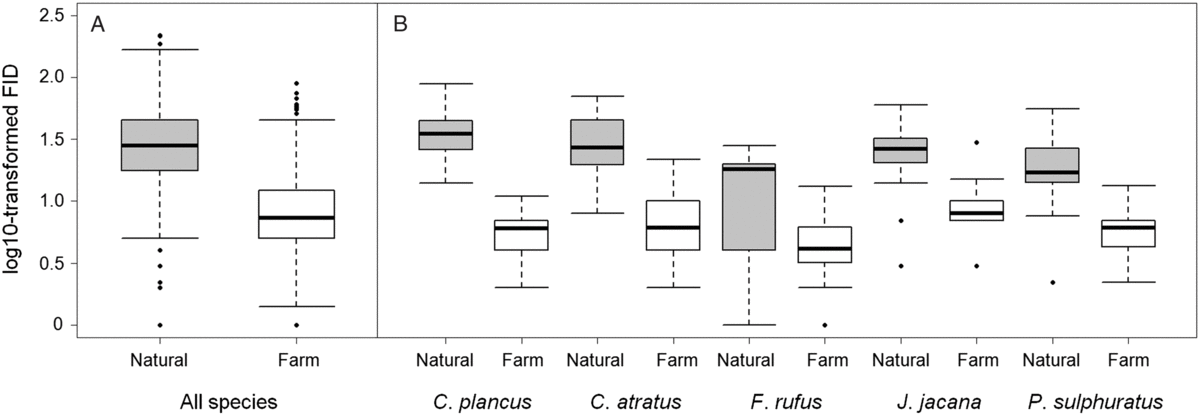
Figure 1. Flight initiation distance (FID) of birds approached in natural areas (grey) or near farms (white) in Pantanal, Brazil. (A) represents overall FID for all sampled bird species (N = 1120 FID estimates), (B) represents a subset of five most frequently examined species: Caracara plancus (N = 48), Coragyps atratus (N = 41), Furnarius rufus (N = 64), Jacana jacana (N = 37) and Pitangus sulphuratus (N = 43) (ordered in alphabetical order). Box plots show the median (horizontal line in the middle of the boxes), upper (75%) and lower (25%) quartiles (top and bottom of the box), 1.5 times the inter-quartile range (whiskers) and outliers (points outside of 1.5 times the inter-quartile range; black dots).
Table 2. Associations between flight initiation distance (FID; response variable) and habitat type (natural/farm), starting distance and flock size (predictors) in birds of Pantanal, Brazil. Associations were examined using a multivariable model based on a Markov chain Monte Carlo technique for generalized linear mixed-effect models with phylogeny (the maximum credibility tree) as a random effect. Response and predictor variables were log10-transformed before analysis. We report estimates of the posterior mean with 95% credible intervals (lower and upper CI), P values and posterior mode of the phylogenetic signal (lambda)

Discussion
We found that birds approached around farms exhibited consistently higher tolerance toward humans than birds in natural areas. Although such a pattern is widely reported from elsewhere (Díaz et al. Reference Díaz, Møller, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Markó and Tryjanowski2013, Samia et al. Reference Samia, Nakagawa, Nomura, Rangel and Blumstein2015, Reference Samia, Blumstein, Díaz, Grim, Ibáñez-Álamo, Jokimäki, Tätte, Markó, Tryjanowski and Møller2017), we document that the emergence of tolerance toward human disturbance is widespread also in bird populations which were only recently disturbed by anthropogenic activity. Therefore, our results may shed light on the behavioural adjustments of animals necessary for a successful exploitation of human-disturbed areas during the initial stage of their synurbanization. Moreover, this study may be useful for a more general understanding of the impact of urbanization on animal behaviour because our knowledge on this phenomenon is strongly skewed towards temperate regions (Blumstein Reference Blumstein2019, Díaz et al. Reference Díaz, Møller, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Markó and Tryjanowski2013, Ortega-Álvarez & MacGregor-Fors Reference Ortega-Álvarez and MacGregor-Fors2011).
Tolerance of animals toward humans is a gradual process with animal populations that have a longer history of inhabiting urbanized areas having a greater tolerance of humans (Møller et al. Reference Møller, Díaz, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Mänd, Markó and Tryjanowski2012, Symonds et al. Reference Symonds, Weston, van Dongen, Lill, Robinson and Guay2016). However, the majority of previous studies focused on populations around long-established urban areas and the majority of birds became urbanized several decades ago (e.g. on average > 50 years ago in Europe; Møller et al. Reference Møller, Díaz, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Mänd, Markó and Tryjanowski2012). We have shown that individuals of numerous wild-living bird species in Pantanal respond flexibly to human-altered environmental conditions as indicated by a dramatic decrease of FID among synurbic populations. Flexible and perhaps rapidly emerging changes in behaviour can be attributed to behavioural plasticity which seems to be particularly important during the initial stages of establishment of populations in urbanized areas (Sol et al. Reference Sol, Lapiedra and González-Lagos2013). However, although behavioural plasticity is surely important at this stage, one has to bear in mind that species occupying invading urban areas usually represent a non-random subset of species available in the regional pool (Sol et al. Reference Sol, Lapiedra and González-Lagos2013, Reference Sol, González-Lagos, Moreira, Maspons and Lapiedra2014). Nevertheless, the ability of birds to modify their degree of tolerance of human disturbance may represent an important mechanism enabling wildlife–human coexistence. This may be crucial for their survival at a time of rapid human-induced environmental changes in the tropics in general, and in Brazil in particular (Richards & VanWey Reference Richards and VanWey2015, Zalles et al. Reference Zalles, Hansen, Potapov, Stehman, Tyukavina, Pickens, Song, Adusei, Okpa, Aguilar, John and Chavez2019).
Large wild areas where human activity was not detectable until recently, such as many tropical regions, offer a valuable opportunity to study how and when birds and other animals begin to invade human settlements. For instance, it is almost impossible to precisely identify a time and place when and where some animal populations started to do this in long-term and heavily urbanized regions such as in Western Europe. Møller et al. (Reference Møller, Díaz, Flensted-Jensen, Grim, Ibáñez-Álamo, Jokimäki, Mänd, Markó and Tryjanowski2012) have shown that a year of urbanization among same-species populations across Europe is highly repeatable, suggesting that the initial establishment of an urbanized population in one area is followed by a rapid spread of this behaviour across other populations. Extrapolating from this, small and recently established human settlements may, at least in some world regions such as Pantanal, represent starting points where synurbanization begins, facilitating subsequent penetration of animals into other urbanized areas. Moreover, given that most human settlements in our study site were built <40 years ago and are already widely exploited by birds indicates that the history of animal exploitation of urbanized areas may be significantly older in many world regions than previously thought.
In conclusion, we have demonstrated that individuals of many bird species, until recently occurring in intact areas with regard to human-driven habitat exploitation, exhibited increased tolerance of humans. This indicates that birds of at least some species are able to cope with human-altered conditions very flexibly by behavioural adjustments. Since our study was conducted in a region where urbanization is very recent, it may extend our knowledge of the fundamental aspects of behavioural adjustment in animals at the beginning of the synurbanization process. Finally, identification and understanding of behavioural processes and mechanisms which enable birds and other animals to cope with anthropogenically altered environments may contribute to designing effective conservation strategies that reduce the negative effects of human disturbance on biodiversity in a rapidly urbanizing world.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467419000282
Acknowledgements
We are very thankful to A. Tószögyová for help with statistical analysis, and to F. Angioletto and Brazilian friends from Universidade Federal de Mato Grosso, Rodovia Rondonópolis/Guiratinga for their considerable support during the field trip. We were permitted to work in a private area Fazenda Jofre Velho owned by NGO Panthera Brazil, within Pantanal and we worked in close cooperation with local conservationists. Data collection was designed to cause only brief and minimal disturbance to birds, and this method does not differ from standard background disturbance caused by other visitors.
Financial support
This research was supported by the Slovak Research and Development Agency under the contract No. APVV-16-0411. None of the funders had any input into the content of the manuscript, nor required approval of the manuscript before submission or publication.





