INTRODUCTION
Activity patterns in animals are influenced by and change in response to many factors, including resource availability and predators (Brown Reference BROWN1999, Manor & Saltz Reference MANOR and SALTZ2003, Tadesse & Kotler Reference TADESSE and KOTLER2011). For example, non-lethal effects such as the fear generated from the possibility of being attacked may be enough to change prey behaviours (Brown & Kotler Reference BROWN and KOTLER2004, Cresswell Reference CRESSWELL2008, Lima Reference LIMA1998, McCauley et al. Reference MCCAULEY, ROWE and FORTIN2011). Activity patterns of animals are generally regarded at least in part as the outcome of two conflicting demands: the activity required to maximize nutritional, social and reproductive objectives, and the need to minimize the costs and risks imposed by the environment (Rosenzweig Reference ROSENZWEIG1974, Sih Reference SIH1980, Stephens & Krebs Reference STEPHENS and KREBS1986). Previous studies noted that risk of predation can be directly inferred from the time-budget where animals in a risky environment allocate most of their time to safety-related behaviours, such as increased level of vigilance at the expense of foraging, nesting and resting activities (Brown Reference BROWN1999, Gill et al. Reference GILL, SUTHERLAND and WATKINSON1996).
Behavioural modelling based on time-budget of focal individuals helps in understanding how animals perceive their environment (Hochman & Kotler Reference HOCHMAN and KOTLER2006, Manor & Saltz Reference MANOR and SALTZ2003, Tadesse & Kotler Reference TADESSE and KOTLER2011) and trade-off between food acquisition and safety (Brown & Kotler Reference BROWN and KOTLER2004, Lima & Dill Reference LIMA and DILL1990, Stephens & Krebs Reference STEPHENS and KREBS1986). It also allows inferences about the quality of the environment where an organism lives (Druce Reference DRUCE2005, Siegfried Reference SIEGFRIED1980). This can be accomplished in practice by correlating behavioural time-budget with relevant environmental variables (Reid Reference REID2005, Tadesse & Kotler Reference TADESSE and KOTLER2011). Changes in the behaviour of individuals can indicate a change in environmental conditions and can provide leading indicators of habitat quality (Morris et al. Reference MORRIS, KOTLER, BROWN, SUNDARARAJ and ALE2009). The objectives of this study were to: (1) analyse the time-budget allocated by mountain nyala (Tragelaphus buxtoni) to different activities (i.e. proportion of time vigilant, feeding and moving) during the wet and dry season, and (2) explore the environmental conditions that affect the behaviours of mountain nyala. To do so, we developed behavioural models based on time-budget of focal mountain nyala as functions of environmental variables, group-size, sex-age class and season.
Previous studies demonstrated that the behaviours of wild animals are generally affected by habitat type (Druce Reference DRUCE2005, Hochman & Kotler Reference HOCHMAN and KOTLER2006, Lagory Reference LAGORY1986), season (Shettleworth et al. Reference SHETTLEWORTH, HAMPTON and WESTWOOD1995, Tadesse & Kotler Reference TADESSE and KOTLER2011), group-size (Brown Reference BROWN1999, Manor & Saltz Reference MANOR and SALTZ2003, Roberts Reference ROBERTS1996, Treves Reference TREVES2000) and sex-age class (Laundré et al. Reference LAUNDRÉ, HERNANDEZ and ALTENDORF2001, Rieucau & Martin Reference RIEUCAU and MARTIN2008, Ruckstuhl Reference RUCKSTUHL1998, Reference RUCKSTUHL2007). In line with those studies, we hypothesized that the behavioural responses of mountain nyala (i.e. activity time-budget allocated to vigilance, feeding and moving) should vary with habitat type, season, group-size and sex-age class. We predicted that mountain nyala should spend more time on vigilance or moving in habitats with high risk of predation. In contrast, they should spend more time on feeding in habitats with low risk of predation. We also predicted that adult females should more strongly select habitats with lower risk of predation than do adult males. This should be true especially for females with young because bearing and raising young during the calving season (i.e. the mountain nyala has a defined calving peak during the wet season) may increase the predation cost to females and thereby limit their use of habitat types with high predation risk (Childress & Lung Reference CHILDRESS and LUNG2003).
MATERIALS AND METHODS
Study species
The mountain nyala is a species endemic to Ethiopia (Brown Reference BROWN1969). Listed as endangered, the mountain nyala is only found in a few locations in the Ethiopian highlands (Evangelista et al. Reference EVANGELISTA, NORMAN, LAKEW, KUMAR and ALLEY2008). It is a sexually dimorphic antelope in which adult males are much larger than adult females (Refera & Bekele Reference REFERA and BEKELE2004). The mountain nyala is a social animal with females congregating in family units of three to eight individuals and males forming small bachelor groups outside the mating season (Hillman Reference HILLMAN1985). They occur in a variety of high-elevation habitat types requiring access to seasonal forage and cover from risk of predation (Hillman & Hillman Reference HILLMAN and HILLMAN1987, Mamo Reference MAMO2007). The mountain nyala mostly grazes on grass and herbs during the wet season; however, it commonly browses on shrubs during the dry season when palatable grass and herbs become scarce in its natural habitats (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Hillman Reference HILLMAN1985, Mamo Reference MAMO2007, Refera & Bekele Reference REFERA and BEKELE2004). Potential predators of the mountain nyala include leopard (Panthera pardus), spotted hyena (Crocuta crocuta) and African lion (Panthera leo) (Brown Reference BROWN1969). However, we note that the lion is not found in Munessa.
Study area
The study was conducted in Munessa, south-eastern Ethiopia. Munessa is situated in Oromiya Administrative Regional State at 7°13′N, 38°37′E. The altitude range extends from 2100 to 2700 m asl. Munessa has a distinct wet and dry season. The mean annual rainfall is about 1250 mm. The mean annual temperature varies from 15 °C to 20 °C. The vegetation is composed of natural and plantation forests where the main forest blocks are found on the escarpment and associated plateau lying between the Rift Valley lakes and the eastern edge of the Rift Valley (Teketay Reference TEKETAY1992, Teketay & Granström Reference TEKETAY and GRANSTRÖM1995). Munessa has an area of 111 km2 of forests, of which the natural forests approximately cover 85 km2 while the remaining 26 km2 are plantation forests (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007). Munessa is a Controlled Hunting Area with limited trophy sport-hunting. Mountain nyala is one of the legally hunted wild game species in Munessa. We identified three major types of habitat that are used by mountain nyala over our study site: natural forest, plantation and cleared vegetation habitats.
The natural-forest habitat: the natural forest is situated in undulating terrain providing good escape and refuge for the mountain nyala from risk of predation especially during the daylight hours because leopard usually prey in the natural forest during the night-time (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007). It also serves as valuable cover for thermal regulation and provides a good source of palatable forage. However, there are various human- and livestock-induced impacts on the natural forests especially during the crop-growing season (Tadesse & Kotler Reference TADESSE and KOTLER2013). For example, some natural forests have been totally cleared and converted into agricultural fields, while others suffered from heavy grazing and selective logging of economically important tree species (Abebe Reference ABEBE2008, Teketay Reference TEKETAY1992).
The plantation habitat: the plantations are potentially good habitat for the mountain nyala. Although the plantations offer a sparse herbaceous understorey, they provide a very important habitat by offering escape refuge from risk of predation during daylight hours, valuable cover for thermal regulation, and travel corridors especially in areas where natural forests are extremely disturbed and/or limited (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007). Illegal tree-cutting activities by local people for fuel-wood and construction materials as well as free-range livestock grazing and browsing were observed to be common threats to the plantation habitat especially in the crop-growing season (Tadesse & Kotler Reference TADESSE and KOTLER2013).
The cleared-vegetation habitat: the cleared vegetation habitat is characterized by relatively freely draining areas that are rich in grass and other palatable herbs. So, it serves as good feeding habitat for the mountain nyala especially in the rainy season. There are also some salt licks that attract the mountain nyala at night in the rainy season when people and livestock are not present. Lack of cover could allow the mountain nyala to detect approaching potential predators at greater distances, for example, by providing less stalking cover for leopard. In addition, the leopard is absent in the cleared vegetation habitat so that the mountain nyala can safely access forage there during the night-time. However, due to lack of cover, risks of human- and livestock-induced disturbances are highest in the cleared vegetation during the daytime (Tadesse & Kotler Reference TADESSE and KOTLER2013). As most of the cleared vegetation habitat is surrounded by natural forests and plantations, it is fairly easy for the mountain nyala to escape from human and livestock disturbances. Previous studies also noted that dense vegetation provides the mountain nyala with good cover from such disturbances (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Mamo Reference MAMO2007, Tadesse & Kotler Reference TADESSE and KOTLER2013).
Data collection
To collect the mountain nyala time-budget data from major habitat types found in Munessa, we randomly set out permanent walking transects with the aid of a GARMIN 75 GPS device, with each transect sampling a major habitat type within the study site. We established a total of 12 transects, i.e. four transects in each habitat type. Transects varied in length from 0.8 km to 2.3 km, i.e. the length of each transect varied with the size of each habitat patch. The field data were collected from May to August 2010 for the wet season, while the dry season data were collected from December 2010 to April 2011.
Focal-animal observations
We applied focal-animal sampling (Altmann Reference ALTMANN1974, Martin & Bateson Reference MARTIN and BATESON1993) to quantify how the activity time-budget of the mountain nyala are related to habitat type, environmental variables, season, group-size and sex-age class. Following Hochman & Kotler (2006) and Tadesse & Kotler (2011), we classified the behavioural activities of each focal animal as vigilance, feeding, moving and resting. Vigilance referred to when the animal had its head up, neck erect, ears oriented forward and eyes wide open for watching and appeared alert. Feeding was defined as the entire process of food searching, locating, biting and ingestion as characterized by slight movements with the head down or foraging with head in a shrub. Moving was defined as when the animal showed steady movement with the head held horizontally or was running. Resting referred to when the animal was lying down or sleeping, standing in the shade, standing still with head held horizontally and ears drooping, but not appearing alert.
We took field observations on individual focal mountain nyala encountered while we walked along each transect in each habitat. In order to minimize any effect caused by the presence of the observer, we waited for a minimum of 10 min before collecting information allowing time for the animals to become acclimated and resume feeding (Hochman & Kotler Reference HOCHMAN and KOTLER2006, Tadesse & Kotler Reference TADESSE and KOTLER2011). The total number of individuals in a group was recorded. Individuals were considered to be in the same group if the separation distance from edge individual was approximately less than 50 m (Hillman & Hillman Reference HILLMAN and HILLMAN1987). Solitary individuals farther than 50 m from another mountain nyala were considered groups of one. Following Brown (Reference BROWN1969) and Refera & Bekele (2004), we divided observations according to sex-age class (i.e. adult male, adult female, sub-adult or juvenile) of the focal individual.
Adult males are easily distinguished by their long twisting horns, large body size and dark brown/grey colour (Brown Reference BROWN1969). Sub-adult males are identified by their straight spiked horns (but not twisted), medium body size and brownish colour (Mamo Reference MAMO2007). Adult females are differentiated by their absence of horns, red-brown appearance and smaller body size than adult males (Gebrekidan Reference GEBREKIDAN1996). Sub-adult females are differentiated from adult females by their smaller body size and reddish colour (Refera & Bekele Reference REFERA and BEKELE2004). However, previous studies revealed that sub-adult males and sub-adult females more or less exhibit similar activity behaviour (Hillman Reference HILLMAN1985, Hillman & Hillman Reference HILLMAN and HILLMAN1987). Thus, we merged sub-adult males and sub-adult females together for this study. Juveniles are defined by their small body size and absence of horns.
If the group contained a mix of ages and genders, stratified random sampling was employed to select focal individuals. The stratification was based on sex-age class while random sampling was achieved through use of random numbers in selection of the focal-animal. As sex-age category of the mountain nyala includes four classes (i.e. adult male, adult female, sub-adult or juvenile), card numbers representing each class (i.e. 1 = adult male; 2 = adult female; 3 = sub-adult; 4 = juvenile) were prepared and randomly drawn when a group of mountain nyala was observed along each transect before starting the focal-animal observations. For example, if a card with 3 is randomly drawn, then this implies that the first focal-animal in the group to be considered is sub-adult. If this age class is not found in the group, another card with different number will be randomly drawn to select the category of the focal-animal from the group. Then we took focal observations of animals representing each category of sex and age.
We carried out observations for 10-min session for each focal-animal with the aid of binoculars (7 × 35). For each focal observation session, we noted the type of activity in which the focal-animal was engaged at the start of the observation period and recorded the length of time spent in different activities (i.e. vigilance, feeding, moving and/or resting) using a stopwatch. We also recorded the habitat type, transect number, date and season. Focal-animal observations were carried out early in the morning from 06h00 to 09h00 local time (ideal times to sample group-size and activity time-budget) when the mountain nyala are most active and easily available for focal-animal observations. In addition, humans and livestock are relatively absent at this time in Munessa so that the mountain nyala can safely use their habitats. Our main focus here is on vigilance, feeding and moving because these behavioural activities best reflect aspects of food and safety (Manor & Saltz Reference MANOR and SALTZ2003, Tadesse & Kotler Reference TADESSE and KOTLER2011). In addition, the mountain nyala rarely spend their time resting early in the morning (Brown Reference BROWN1969, Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Refera & Bekele Reference REFERA and BEKELE2004) when the focal observations were carried out. Thus, we note that our behavioural model does not model resting behaviour. So, we excluded time resting from the analyses and behavioural models built for the mountain nyala.
We took care to sample the mountain nyala on different days, locations and habitat types within the study site to reduce the chance of sampling the same individual twice. In addition, we collected focal observations from only a single individual of each age and sex class from a group. This spread out observations across habitats, time, location, sex and age class as much as was possible for this population. The interspersion of data collection over days, locations, sex-age class and habitats also increases our confidence in the independence of observations and helps minimize artifacts of behavioural time-budget interpretation.
Measurement of habitat variables
A systematic sampling design was employed to collect microhabitat and environmental data. The first plot within each habitat patch on each transect was randomly located; then successive plots were systematically added at 100-m intervals along each transect. The total number and distribution of sample plots for each habitat type varied with the total size of each habitat patch. A total of 109 plots were assessed (i.e. 31 plots in the cleared vegetation, 41 plots in the plantation and 37 plots in the natural forest). We first laid out a circular sample plot with a radius of 5 m on each line transect. All trees (a tree is arbitrarily defined as any woody plant with a height of ≥ 3 m) within the 5-m-radius circular plot were identified and counted, and crown diameter for each tree in the plot was measured with a metre tape. Then a circular nested plot with a radius of 2 m was laid out within the larger circular plot and all shrubs (a shrub is defined as any woody plant with a height of < 3 m) within this nested plot were identified and counted. Within each nested plot, another sub-nested plot with a radius of 0.5 m was laid out, and per cent plant cover (grass and herbs) and bare soil were estimated with a square grid (10 × 10 cm). The square grid was just used in order to precisely estimate the per cent plant cover (grass and herbs) and bare soil in the sub-nested circular plot. In addition, at the centre of each circular sample plot, slope (using declinometer) and altitude (using altimeter) were measured.
Data analyses
Group-size of mountain nyala was analysed as functions of habitat type and season. We logarithmically transformed the time-budget data to ensure normality. We analysed the effect of habitat type and sex-age class on the behavioural responses of the mountain nyala as a function of season. Furthermore, to analyse the overall activity time-budget of the mountain nyala, we pooled together data for time allocation of each activity type from focal-animal observations for all the four age-sex categories, i.e. adult males, adult females, sub-adults and juveniles. We used a post hoc Tukey's HSD test for the multiple comparisons across activity types. We analysed the data using ANOVA.
To test the effects of season, habitat type, group size, sex-age class and environmental variables on the activity time-budget of the mountain nyala, Generalized Linear Model (GLM) with multivariate tests were used. In the same analysis, univariate tests followed the multivariate ones for better interpretation of the multivariate results. Since the per cent cover of herbs and grass and the per cent cover of bare soil in each plot are not independent (i.e. they share a degree of freedom), this might have consequences for the multivariate analysis. Herbs and grass are valuable sources of forage for the mountain nyala (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007), thus we excluded the per cent cover of bare soil from the multivariate analysis. So, the independent variables entered into the GLM included the followings: season + habitat type + abundance of trees (number of trees per plot) + crown diameter of trees (total crown diameters of trees in a plot) + abundance of shrubs (number of shrubs per plot) + per cent cover of grass and herbs + altitude + slope + group-size + sex-age class of the focal animal. For the vector of dependent variables, we used proportion of time vigilant, feeding and moving.
Behavioural models were developed in accordance with the habitat variables, group-size and sex-age class. We used linear regression to generate the response curves that defined the relationship between each independent variable (i.e. abundance of trees, crown diameter of trees, abundance of shrubs, per cent cover of herbs and grass, altitude, slope, group-size and sex-age class) on each dependent variable (i.e. proportion of time vigilant, feeding and moving). For the categorical independent variable (i.e. sex-age class), we used a dummy variable to correlate the categorical independent variable with each dependent variable in the linear regression to determine the correlation coefficients. In our final models, we included only those independent variables that showed significant effect on the dependent variables as function of season. For all analyses, we defined the alpha value to be 0.05. The data analyses were performed with STATISTICA version 10.
RESULTS
To quantify the time-budget of the mountain nyala, we assessed 119 and 116 focal individuals in the wet and dry season respectively. In the wet season, the greatest maximum group-size was observed in the cleared vegetation habitat (max = 22 individuals). However, in the dry season, the greatest maximum group-size was recorded in the natural forest habitat (max = 13 individuals). In both seasons, the minimum group-size was one individual in each habitat type. Season significantly affected group-size (F (1, 84) = 8.90; P = 0.004), where the mountain nyala had higher group-size in the wet season (7.35 ± 0.16 individuals) than in the dry season (6.29 ± 0.15 individuals). Habitat type also affected group-size (F (2, 84) = 4.00, P = 0.022), where natural forest had the highest overall group-size (7.5 ± 0.92 individuals). Season interacted with habitat type (F (2, 84) = 10.8, P < 0.001) to affect the group-size of the mountain nyala.
In the wet season, the general time-budget of mountain nyala differed among activity type (F (2, 354) = 25.3; P < 0.001). Mountain nyala spent most of their time vigilant (Figure 1). A post hoc Tukey's HSD test for multiple comparisons across activity type showed a significant difference in time-budget between vigilance versus feeding, vigilance versus moving and moving versus feeding. The mountain nyala were most vigilant (Figure 2a) in the plantation; however, they allocated most of their time to feeding in the cleared vegetation habitat (Figure 2b). The mountain nyala spent the largest proportion of their time on moving in the plantation habitat (Figure 2c).
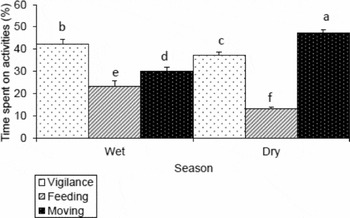
Figure 1. General activity time-budget of the mountain nyala (Tragelaphus buxtoni) during the wet and dry season pooled over four categories: adult males, adult females, sub-adults and juveniles. The number of focal individuals (N) included in this analysis was 119 and 116 for the wet and dry season respectively. The error bars represent +1 SE. Bars labelled with different letters differed significantly.
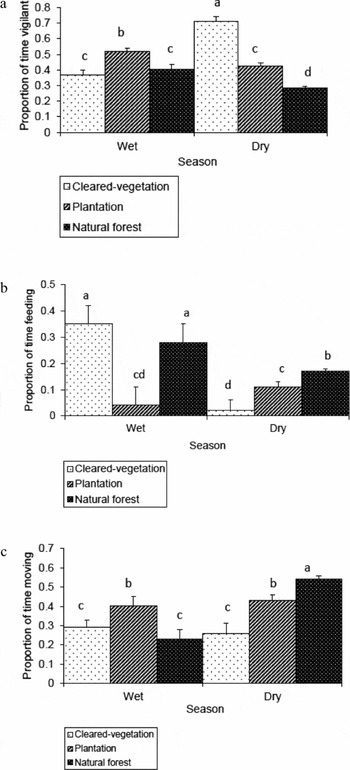
Figure 2. Effect of habitat type on vigilance (a), feeding (b) and moving (c) behaviour of the mountain nyala (Tragelaphus buxtoni) during the wet and dry season pooled over four categories: adult males, adult females, sub-adults and juveniles. For the wet season analysis, 45, 36 and 38 focal individuals were considered for cleared vegetation, plantation and natural forest respectively. However, for the dry season analysis, 14, 29 and 73 focal individuals were included from cleared vegetation, plantation and natural forest respectively. The error bars represent +1 SE. Bars labelled with different letters differed significantly.
In the dry season, time-budget of the mountain nyala also differed among activity type (F (2,345) = 21.8; P < 0.001), where they spent most of their time moving (Figure 1). A post hoc Tukey's HSD test for multiple comparisons across activity type showed a significant difference in time-budget between moving versus vigilance, moving versus feeding and vigilance versus feeding. In contrast to the wet season, the mountain nyala were most vigilant in the cleared vegetation (Figure 2a). However, they significantly allocated most of their time-budget to feeding (Figure 2b) and moving (Figure 2c) in the natural forest habitat.
In the wet season, sex-age class of the focal animal did not affect the time-budget that the mountain nyala allocated to vigilance (Figure 3a), feeding (Figure 3b) and moving (Figure 3c). In contrast, during the dry season, sex-age category of the focal animal affected the proportion of time vigilance (Figure 3a) and feeding (Figure 3b). Compared with the other sex-age classes, adult males spent the highest proportion of their time-budget on vigilance (Figure 3a). In contrast, adult males allocated the lowest proportion of their time to feeding (Figure 3b). As in the wet season, sex-age class of the focal animals did not affect the proportion of time spent on moving (Figure 3c).
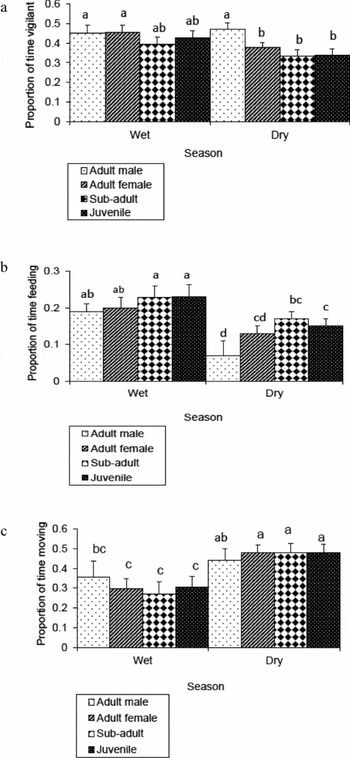
Figure 3. Effect of sex-age class on vigilance (a), feeding (b) and moving (c) behaviour of the mountain nyala (Tragelaphus buxtoni) during the wet and dry season pooled over three habitats (i.e. cleared vegetation, plantation and natural forest). The number of focal individuals considered for the wet season analysis was 17 adult males, 41 adult females, 27 sub-adults and 31 juveniles. However, 17 adult males, 38 adult females, 28 sub-adults and 36 juveniles were considered for the dry season analysis. The error bars represent +1 SE. Bars labelled with different letters differed significantly.
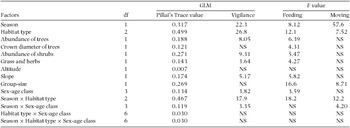
The multivariate results showed that abundance of trees, per cent cover of grass and herbs, abundance of shrubs, slope and sex-age class of the focal-animal affected vigilance and feeding (Table 1). Proportion of time feeding and moving significantly increased with the increase in group-size. Crown diameter of trees affected only feeding. Season, habitat type and their interactions affected the proportion of time that the mountain nyala allocated to vigilance, feeding and moving. Season and sex-age class of the focal animal interacted to affect vigilance and moving (Table 1). Altitude of the landscape did not affect any behaviour of the mountain nyala. The direction of the significant relationships between each independent and dependent variables were shown in the wet and dry season behavioural models (Table 2).
Table 1. Effects of habitat variables, group-size, season, habitat type and sex-age class on the activity time-budget of the mountain nyala (Tragelaphus buxtoni). Different statistical values are shown. NS = not significant at P ≤ 0.05.
Wet-season mountain nyala behavioural models
We developed the behavioural models by correlating each habitat variable, sex-age category and group-size measured with the proportion of time quantified for each activity type of the focal mountain nyala. Proportion of time vigilant significantly increased with the abundance of trees and the per cent cover of grass and herbs, but decreased with slope. Proportion of time feeding significantly increased with slope and per cent cover of grass and herbs, but decreased with abundance of trees (Table 2).
Table 2. Behavioural models for the mountain nyala (Tragelaphus buxtoni) in the wet and dry season. Independent variables whose coefficients significantly differ at 0.05 alpha value are only included in the table. All the habitat variables did not significantly affect the proportion of time moving in the wet season so that it is not included in the table. ↑ implies positive effect on the dependent variable whereas ↓ shows negative effect.
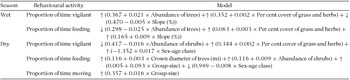
Dry-season mountain nyala behavioural models
Proportion of time vigilant significantly decreased with abundance of shrubs, but increased with per cent cover of grass and herbs and sex-age class of the focal-animal, where adult males spent the largest proportion of their time-budget in vigilance. Proportion of time feeding significantly increased with crown diameter of trees, abundance of shrubs and group-size, but decreased with sex-age class of the focal-animal where adult males allocated the least proportion of their time to feeding. Proportion of time moving significantly increased with group-size only (Table 2).
DISCUSSION
We have shown how measuring time-budget through focal-animal observations yield behavioural indicators for the mountain nyala (Morris et al. Reference MORRIS, KOTLER, BROWN, SUNDARARAJ and ALE2009). Season, habitat type, environmental variables, group-size and sex-age class all affected the time-budget that the mountain nyala allocated to different activities. We also developed behavioural models by correlating time-budget of the focal mountain nyala with environmental variables, group-size and sex-age class as function of season. Our results showed that patterns of time-budget in the mountain nyala reflect a complex interplay between environmental variables, group size and sex-age class.
Mountain nyala allocated the greatest proportion of their time to vigilance in the wet season, suggesting that there is a difference in risk of predation in Munessa across season. Peak calving period for the mountain nyala is during the wet season (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Hillman Reference HILLMAN1985). Calving activity may increase the predation cost to the mountain nyala and thereby force them to allocate most of their time-budget to safety-related behaviours, such as increased level of vigilance at the expense of foraging and resting activities (Brown Reference BROWN1999, Gill et al. Reference GILL, SUTHERLAND and WATKINSON1996, Stankowich Reference STANKOWICH2008). Childress & Lung (2003) also reported that Rocky Mountain elk (Cervus elaphus) allocate more time to vigilance and less to feeding during peak calving season. Unlike the wet season, the mountain nyala allocated the greatest portion of their time to moving in the dry season. The scarcity of palatable forage in the dry season may urge the mountain nyala to allocate their greatest time to moving in search of sufficient food. In a recent study, Tadesse & Kotler (2011) also noted that Nubian ibex (Capra nubiana) in Israel allocate most of their time-budget to moving in the dry season when there is a scarcity of forage in the surrounding environment.
Habitat characteristics affect the trade-offs between food acquisition and safety (Lima & Dill Reference LIMA and DILL1990, Stephens & Krebs Reference STEPHENS and KREBS1986). Habitat type affected the time-budget that the mountain nyala allocated to different activities. For example, mountain nyala devoted more time to vigilance and less to feeding in the cleared vegetation habitat during the dry season, possibly because the perceived risk of predation is greater in the cleared vegetation due to the absence of cover for concealment or high probability of encounter risk (Brown Reference BROWN1999, Brown et al. Reference BROWN, KOTLER and BOUSKILA2001). As there is both legal and illegal hunting practice on the mountain nyala in Munessa (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007), the other possible reason is that it is easier for hunters to target and shoot the mountain nyala in the cleared vegetation than in the plantation or the natural forest habitat. In contrast, the mountain nyala allocated greater proportion of their time to feeding and less to vigilance in the natural forest, suggesting that the perceived risk of predation is low there due to the availability of protective cover or low probability of encounter risk. Previous studies on the mountain nyala noted that dense vegetation provides with good cover from risk of predation including human- and livestock-induced disturbances (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Mamo Reference MAMO2007). Studies on other taxa noted that vegetation cover can be important prey refugia where the potential to encounter predators greatly decreases or the ability to escape encounters greatly increases (Arenz & Leger Reference ARENZ and LEGER1999, Mech Reference MECH1977, Ripple & Beschta Reference RIPPLE and BESCHTA2003, Reference RIPPLE and BESCHTA2004).
The behavioural responses of animals may be affected by environmental variables because animals optimize their activity time-budget where benefits are maximized and risks are minimized (Brown Reference BROWN1999, Brown & Kotler Reference BROWN and KOTLER2004). Several environmental variables affected the time-budget that the mountain nyala allocated to different behaviours. For example, per cent cover of grass and herbs, slope and abundance of shrubs affected the vigilance and the feeding behaviour of the mountain nyala. In the wet season, the mountain nyala devoted a considerable proportion of their time to feeding where there is high per cent cover of grass and herbs. Furthermore, the mountain nyala allocated more time to feeding and less to vigilance in steep terrain, because slope provides the mountain nyala with safety (Evangelista et al. Reference EVANGELISTA, NORMAN, LAKEW, KUMAR and ALLEY2008), and hence they can allocate more time to feeding. Other studies also supported our findings. For example, the Nubian ibex usually allocate more time to feeding and less to vigilance on a steep slope, because they can easily escape dangerous predators on such terrain (Hochman & Kotler Reference HOCHMAN and KOTLER2006, Kotler et al. Reference KOTLER, GROSS and MITCHELL1994, Muller et al. Reference MULLER, KOHLMANN and ALKON1995, Tadesse & Kotler Reference TADESSE and KOTLER2011).
In the dry season, the feeding behaviour of mountain nyala increased with the abundance of shrubs. Previous studies also noted that the mountain nyala exclusively depend on shrubs as their typical sources of food during the dry season when grass and herbs are less abundant and poor in nutritional quality and digestibility (Hillman Reference HILLMAN1985, Hillman & Hillman Reference HILLMAN and HILLMAN1987, Refera & Bekele Reference REFERA and BEKELE2004). Furthermore, the mountain nyala devoted less time to vigilance when the abundance of shrubs increases, suggesting that shrubs may also provide good cover for concealment from risk of predation including human disturbances (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Mamo Reference MAMO2007). In other studies, Tchabovsky et al. (Reference TCHABOVSKY, KRASNOV, KHOKHLOVA and SHENBROT2001) found that the fat sand rat (Psammomys obesus) was less vigilant under dense shrub cover. However, we should be conscious that shrub cover may either be beneficial to a prey species if it prevents a predator from detecting prey, or it may be harmful, if it merely obstructs the prey's view and provides little or no protection from predator attack (Arenz & Leger Reference ARENZ and LEGER1999).
Several behavioural studies have shown that sex-age class is an important factor affecting the vigilance level of animals (Childress & Lung Reference CHILDRESS and LUNG2003, Frid Reference FRID1997, Hunter & Skinner Reference HUNTER and SKINNER1998, Laundré et al. Reference LAUNDRÉ, HERNANDEZ and ALTENDORF2001, Rieucau & Martin Reference RIEUCAU and MARTIN2008, Ruckstuhl Reference RUCKSTUHL1998). Sex-age class affected the vigilance level of the mountain nyala in Munessa, but only seasonally. In the wet season, sex-age class of the mountain nyala did not affect the time spent on vigilance. However, this is contrary to the classical predation risk hypothesis which predicts that adult females should spend much more time to vigilance and safety-related activities than do adult males (Main et al. Reference MAIN, WECKERLY and BLEICH1996, Muller et al. Reference MULLER, KOHLMANN and ALKON1995, Prins Reference PRINS1996, Ruckstuhl & Kokko Reference RUCKSTUHL and KOKKO2002, Sukumar & Gadgil Reference SUKUMAR and GADGIL1988, Young & Isbell Reference YOUNG and ISBELL1991). The mountain nyala exhibit a defined calving peak during the wet season (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Hillman Reference HILLMAN1985). Although adult females with calves have additional costs arising from the risk of predation on the young (Childress & Lung Reference CHILDRESS and LUNG2003, Ruckstuhl Reference RUCKSTUHL1998, Reference RUCKSTUHL2007), adult male mountain nyala in the Munessa hunting block have additional costs due to legal trophy hunting that solely targets them in the wet season (Ethiopian Wildlife Conservation Authority, unpubl. field reports). Thus, both adult males and females allocated almost equal proportion of their time to vigilance in the wet season. Stankowich (Reference STANKOWICH2008) also noted that vigilance rate increases in male ungulates as long as they are subjected to hunting.
Similar departure from the classical predation risk hypothesis occurred in the dry season. At this time, adult males were even more vigilant than adult females or young sub-adults. While in the wet season, it was legal trophy hunting that targeted adult males. In the dry season, adult males are also illegally targeted by local people for their spectacular horns because they are used for ritual ceremonies, local medicines and nipples for traditional milk bottles (Evangelista et al. Reference EVANGELISTA, SWARTZINSKI and WALTERMIRE2007, Mamo Reference MAMO2007). Another possible explanation is that adult males are more concerned with mating (also known as the male investment hypothesis) than feeding. They may be more vigilant toward other bulls for female guarding during courtship. In contrast, adult males allocated significantly the least time to feeding, suggesting that adult male behaviour is a reflection of a trade-off between food acquisition and safety (Brown & Kotler Reference BROWN and KOTLER2004, Stephens & Krebs Reference STEPHENS and KREBS1986). Burger & Gochfeld (1994) also noted that adult male zebra, wildebeest and waterbuck were all more vigilant than their conspecific adult females.
Conclusion
Behavioural indicators based on time-budget provide leading indicators of habitat quality. In the present study, the application of behavioural indicators allowed us to track changes in behaviours of the mountain nyala from season to season rather than on the longer time scale that demographic methods would require. More importantly, behavioural indicators provide answers from the animal's perspective rather than ours, and they have the potential to provide leading indicators of change. Rather than having little to offer for conservation and management, behavioural ecologists have valuable concepts and tools that can obtain just the information most needed by conservation managers and do so quickly, cheaply and accurately. In this way, adaptive behavioural ecology can help conservation and management of wildlife.
The behavioural models based on time-budget help to predict how the mountain nyala perceive their environment and trade-off between food acquisition and safety. The study improves our understanding of the adaptive behavioural ecology of the endangered mountain nyala. Such knowledge can help wildlife managers to develop proper conservation strategies and management plans. For example, to conserve the mountain nyala, wildlife managers need to promote behaviours that enhance survivorship in response to hazards. From a management perspective, searching for the fitness consequences of behavioural responses may provide insights into the impact of certain human activities (e.g. the prevalence of hunting, human-induced disturbances, etc. in Munessa) on the mountain nyala. Future wildlife management should gear toward solving those problems confronted with the survival of the endangered mountain nyala.
ACKNOWLEDGEMENTS
We are pleased to thank E. Ersedo and T. Ayalew for their many hours of assistance in the fieldwork. We extend our gratitude to the Wondo Genet College of Forestry and Natural Resources (WGCF-NR) for the vehicle and equipment support during our fieldwork. We also thank two anonymous reviewers whose comments helped much to improve the manuscript. We express our heartfelt thanks to the staff of the Arsi Forest Branch and the Munessa Forest District in particular and the Ethiopian Wildlife Conservation Authority (EWCA) and the Oromiya Forest and Wildlife Enterprise (OFWE) in general for their cooperation in permitting us to work on the endangered mountain nyala and their habitats. During this research, the State of Israel and the Rufford Small Grant Foundation (RSGF) provided financial assistance.







