INTRODUCTION
The question of why some alien species become invasive has attracted considerable research (Crawley Reference CRAWLEY, Gray, Crawley and Edwards1987, D'Antonio Reference D'ANTONIO1993, D'Antonio & Kark Reference D'ANTONIO and KARK2002, Hobbs & Humphries Reference HOBBS and HUMPHRIES1995, Kolar & Lodge Reference KOLAR and LODGE2001, Pyšek et al. Reference PYŠEK, RICHARDSON and WILLIAMSON2004), but it has proved difficult to generalize about which factors are important. One reason for this limited success is that ecological factors interact in complex ways, resulting in expected outcomes ranging from inability to establish, to naturalization (i.e. coexistence of native and introduced species), conditional invasion (dependent on quantity and spatial distribution of propagules), and unconditional takeover (i.e. replacement of native with introduced species) (Eppstein & Molofosky Reference EPPSTEIN and MOLOFSKY2007).
Many plant traits have been proposed as contributing to invasion success: these include short generation time, life form, long fruiting period, capacity for both sexual and asexual reproduction, large seed crops, small seed size, prolonged seed viability and pronounced phenotypic plasticity (Goodwin et al. Reference GOODWIN, MCALLISTER and FAHRIG1999, Kolar & Lodge Reference KOLAR and LODGE2001, Rejmánek & Richardson Reference REJMÁNEK and RICHARDSON1996, Williamson & Fitter Reference WILLIAMSON and FITTER1996). However, the functional significance of these traits has rarely been quantified (Kolar & Lodge Reference KOLAR and LODGE2001). Many factors influencing the invasibility of ecosystems have also been proposed, including climate, level of environmental stress, disturbance regime, abundance of natural enemies, propagule pressure, resource availability, community structure and ecosystem type (Alpert et al. Reference ALPERT, BONE and HOLZAPFEL2000, Crawley Reference CRAWLEY, Gray, Crawley and Edwards1987, Freckleton Reference FRECKLETON2004, Kolar & Lodge Reference KOLAR and LODGE2001, Levine Reference LEVINE2000, Smith & Knapp Reference SMITH and KNAPP1999). However, no single trait or factor can explain local variation in the performance of invasive species, which probably arises from the interaction between its traits and local conditions. To evaluate what makes particular communities invasible therefore requires both site-specific and species-specific studies (Hobbs & Humphries Reference HOBBS and HUMPHRIES1995, King & Grace Reference KING and GRACE2000, Pierson & Mack Reference PIERSON and MACK1990, Thébaud et al. Reference THÉBAUD, FINZI, AFFRE, DEBUSSCHE and ESCARRE1996).
Among the factors thought to influence ecosystem invasibility, many authors have stressed the importance of the disturbance regime (Davis et al. Reference DAVIS, PERGL, TRUSCOTT, KOLLMANN, BAKKER, DOMENECH, PRACH, PRIEUR-RICHARD, VEENEKLAAS, PYŠEK, DEL MORAL, HOBBS, COLLINS, PICKETT and REICH2005, Horvitz et al. Reference HORVITZ, PASCARELLA, MCMANN, FREEDMAN and HOFSETTER1998, Levine Reference LEVINE2000, Vitousek et al. Reference VITOUSEK, D'ANTONIO, LOOPE and WESTBROOKS1996). This is partly because disturbance can lead to considerable changes in plant community composition, though the mechanisms responsible have rarely been studied in detail (Suding & Goldberg Reference SUDING and GOLDBERG2001). In most forests, treefall represents the main endogenous form of disturbance, with patterns of forest regeneration being closely linked with the resulting gap dynamics (Hubbell & Foster Reference HUBBELL, FOSTER and Crawley1986). In addition, however, the endogenous patterns of disturbance may be strongly modified by external factors such as high winds, especially in regions prone to hurricanes or cyclones. Thus, mature tropical forests can be seen as a mosaic of patches representing episodes of past disturbance (Denslow Reference DENSLOW1987, Koike & Syahbuddin Reference KOIKE and SYAHBUDDIN1993, Whitmore Reference WHITMORE1989). Many aspects of the physical environment – such as light, humidity and temperature conditions – are different in openings and beneath trees (Lieberman et al. Reference LIEBERMAN, LIEBERMAN and PERALTA1989), providing a range of niches for species with differing life history strategies.
Exotic species are rarely found in undisturbed tropical forests, probably because the great majority of introduced species lack the necessary traits, especially shade tolerance, to invade these ecosystems. However, some species can readily invade disturbed forest, and in some cases may even dominate and irreparably change the ecosystem (Fine Reference FINE2002). Such effects tend to be particularly severe on oceanic islands, where they sometimes cause a significant loss of biodiversity (Fosberg Reference FOSBERG1983, Fritts & Rodda Reference FRITTS and RODDA1998, Kell Reference KELL1997, Mueller-Dombois & Loope Reference MUELLER-DOMBOIS and LOOPE1990, Vitousek Reference VITOUSEK and Wilson1988).
In this paper, we examine the influence of gap dynamics upon the success of alien plant species in lowland tropical rain forest on the tropical island of Réunion. We performed experiments to address the following research questions (1) What are the gap characteristics of the study site? (2) How are alien species distributed with respect to gaps? (3) How do cyclones affect light conditions in the forest? and (4) Is the germination of alien species affected by gap characteristics?
METHODS
Study area
The volcanic island of Réunion is one of the three tropical islands which form the Mascarene archipelago, the other two being Mauritius and Rodrigues (Figure 1a). It lies within the Malagasy region, which is recognized as a hotspot for biological diversity (Myers et al. Reference MYERS, MITTERMEIER, MITTERMEIER, DA FONSECA and KENT2000). Despite its small size, Réunion (2512 km2) has by far the largest area of relatively intact habitats in the Mascarene archipelago, with around 30% of the original vegetation remaining intact (Strasberg et al. Reference STRASBERG, ROUGET, RICHARDSON, BARET, DUPONT and COWLING2005), compared with less than 5% on Mauritius and none on Rodrigues (Lorence & Sussman Reference LORENCE and SUSSMAN1986, Reference LORENCE and SUSSMAN1988). However, Réunion faces many threats, including to the rapid spread of invasive alien species, especially plants (Baret et al. Reference BARET, MAURICE, LE BOURGEOIS and STRASBERG2004, Reference BARET, ROUGET, RICHARDSON, LAVERGNE, EGOH, DUPONT and STRASBERG2006; Lavergne et al. Reference LAVERGNE, RAMEAU and FIGIER1999, Macdonald et al. Reference MACDONALD, THÉBAUD, STRAHM and STRASBERG1991, Strasberg et al. Reference STRASBERG, ROUGET, RICHARDSON, BARET, DUPONT and COWLING2005). The spread of alien plants on Réunion has been well documented; since European colonization in the 17th century, more than 2000 plant species have been introduced, of which some 628 species are naturalized and 62 are highly invasive (Macdonald et al. Reference MACDONALD, THÉBAUD, STRAHM and STRASBERG1991; alien status defined according to Richardson et al. Reference RICHARDSON, PYŠEK, REJMÁNEK, BARBOUR, PANETTA and WEST2000).

Figure 1. Location of Réunion island (a) and of study site (b) where transects (black bands, total area per transect: 4000 m2) have been set up in Mare Longue reserve (c). Details of transects (d).
Our study was conducted in a remnant of tropical lowland rain forest in the Mare Longue natural reserve on the east coast of Réunion (Figure 1b; Strasberg et al. Reference STRASBERG, ROUGET, RICHARDSON, BARET, DUPONT and COWLING2005). The reserve is formed by 68 ha of forest, and lies between 150 and 700 m asl on the southern slopes of Piton de la Fournaise, one of the most active volcanoes in the world (Bachelery et al. Reference BACHELERY, BLUM, CHEMINEE, CHEVALLIER, GAULON, GIRARDIN, JUAPART, LALANNE, MOUEL, RUEGG and VINCENT1982). The average annual precipitation measured at the meteorological station of Saint-Philippe (2 km away from the reserve) is 3855 mm, and is regularly distributed throughout the year. The annual average temperature ranges from 19 to 20°C. The soils are derived mainly from basaltic lava flows aged at about 300 y (Bory de Saint-Vincent Reference BORY DE SAINT-VINCENT1804).
The most abundant exotic species in the study area is the bramble Rubus alceifolius Poiret (Rosaceae). This species was introduced into the Mascarene Islands from Asia around 1850 (Cadet Reference CADET1977, de Cordemoy Reference DE CORDEMOY1895) and has spread extensively into intact tropical forest (Baret & Strasberg Reference BARET and STRASBERG2005, Baret et al. Reference BARET, LE BOURGEOIS and STRASBERG2005a, Reference BARET, RADJASSEGARANE, LE BOURGEOIS and STRASBERGb). It is considered to be the most threatening species of undisturbed native ecosystems (Macdonald et al. Reference MACDONALD, THÉBAUD, STRAHM and STRASBERG1991). On Réunion, it is abundant on the eastern and south-eastern coasts from sea level to 1700 m asl (Baret et al. Reference BARET, MAURICE, LE BOURGEOIS and STRASBERG2004), while along the drier western coast it occurs mainly in gullies above 500 m asl.
Transect method and biological material studied
Three belt transects consisting of twenty 10 × 20-m2 contiguous plots (i.e. total area per transect 4000 m2) were randomly set out in January 2002 spanning different ranges of elevation (transect 1: 560–620 m; transect 2: 445–505 m; transect 3: 270 and 300 m; Figure 1c). Each plot was subdivided into eight 25-m2 quadrats (Figure 1d). In each quadrat, the vegetation classified as ‘gap’ or ‘understorey’, and the presence and abundance of all exotic plants were recorded. Nomenclature follows ‘Index commenté de la flore vasculaire de la Réunion version 2007.2’ (http://flore.cbnm.org). In addition, for Rubus alceifolius we recorded the relative abundance in each quadrat of plants at different development stages (seedlings, established vegetative plants and mature, i.e. fruiting, plants).
Gap identification and measurements
A gap was defined as an opening in the canopy of more than 1 m2 caused by the breaking or uprooting of a tree, or by the breaking of a branch. For each gap along the transects the following information was recorded: size, ground slope, orientation and mean height of surrounding vegetation. To characterize gap area, the length and width of gaps were measured at 2 m above ground level, with the edge being defined by the position of the canopy of the first tree with a dbh > 10 cm. This technique allowed us to estimate the gap area through an elliptic approximation (Ott & Juday Reference OTT and JUDAY2002, Runkle Reference RUNKLE1982), using the formula: π × (length/2) × (width/2). The height of the canopy was measured with a measuring pole. In determining the origin of the gaps, we distinguished two treefall types, trunk breaking and uprooting, while branchfalls were defined by the presence on the ground of large branches (diameter > 10 cm) not associated with fallen trunks.
LAI* measurements
To determine the relationship between LAI (Leaf Area Index) and gap structure, we made measurements of an LAI proxy at eight sites located on or close to transect 2. For this purpose we used a recently designed instrument that measures the transmitted light below the canopy but not the incident light above the canopy. Cournac et al. (Reference COURNAC, DUBOIS, CHAVE and RIÉRA2002) have shown that variation of incident light (due to weather conditions or presence of fog) can be estimated from visual records, without the need of a simultaneous second measurement above the canopy or in the open. Light extinction follows a law of the form I/Io = exp(−k × LAI), where I is the light below the canopy, Io is incident light and k is a shape coefficient which depends on cover type. It is then possible to derive k × LAI directly from these records. If an independent estimate of k can be provided (typically within the range 0.8–0.9 for broadleaved forest), LAI can be calculated. This method of determining LAI measurement gives similar results to the widely used LAI2000 (LICOR) device (Cournac et al. Reference COURNAC, DUBOIS, CHAVE and RIÉRA2002). Because we did not have an independent measure of k, we used the value of LAI* = kLAI for quantifying canopy density in the studied places.
The eight sites studied were selected to represent gaps of varying size (6 sites), closed canopy (1 site) and fully open conditions (1 site); the open site was located at 15 m from the forest border (Figure 1c). LAI* was measured at 17 points for each gap: one in the centre of the gap and two along each of eight radial transects, one at the edge of the gap and one 5 m within the forest. For the open site and the closed canopy site, nine measurements were made, with one at the centre and eight radially arranged at 2 m from the central point.
These measurements were repeated on four dates – in July 2000, July 2001, January 2002 and March 2002. On 23–24 January 2002, a severe cyclone crossed Réunion, with winds at the highest point of the island (Maïdo) reaching 277 km h−1. The last two series of measurements were made 1 and 6 wk after the passage of this cyclone.
Germination of Rubus alceifolius seeds
To investigate how canopy closure affects the germination of Rubus alceifolius seeds, we performed an experiment using seeds harvested in March–April 2001 from plants growing along transect 2. As the Rubus genus is known for strong in tegument dormancy (Marks Reference MARKS1983), the seeds were first scarified by placing them in 95% sulphuric acid for 45 min (Amsellem Reference AMSELLEM2000). In July 2001, four trays filled with forest soil containing 25 seeds were placed at each of the eight sites used for the LAI measurements (i.e. 100 seeds per site). A control measurement was made (five non-scarified seeds per tray, i.e. 20 per site): none germinated. The trays were examined every 2 d for 3 mo to record the appearance of seedlings.
RESULTS
Gap characteristics
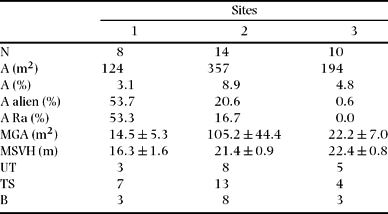
In the three belt transects (total area 12 000 m2), we recorded 32 gaps with an area of 674.6 m2. Thus, the mean density of gaps was 26.7 ha−1, and gaps represented 5.62% of the area surveyed. For the highest transect 1, the area occupied by gaps was 3.10% (8 gaps) while for transects 2 and 3 it was 8.92% (14 gaps) and 4.84% (10 gaps), respectively. The mean height of the surrounding canopy was lower in transect 1 (16.2 ± 1.57 m) than in transects 2 and 3 (respectively 21.4 ± 0.93 m and 22.4 ± 0.75 m; Table 1). Most gaps were formed by the fall of trees, due either to trunk-snapping (53 individuals) or to uprooting (35 individuals). However 15 gaps were attributed to branch fall. Most of the gaps were small (< 25 m2) and only three, all in transect 2, were >100 m2.
Table 1. Gap number (N) and gap characteristics within the three transects of 4000 m2 surveyed: cumulated areas (A) occupied by gaps, mean (± SE) of gap area (MGA), mean (± SE) of surrounding vegetation high (MSVH) and cause of the gap creation –frequency of uprooting trees (UT), trunk-standing (TS) and branches (B) observed. The percentages of areas within gaps occupied by all alien plants observed and by the widespread, Rubus alceifolius (Ra) are also indicated.
Gap evolution and cyclone impact
The changes in LAI* at the eight study sites on or near transect 2 were mainly small during the first two years (July 2000 and July 2001) except at the open site that was affected by the growth of a tree fern. The average LAI* during this period in the understorey was 4.77 ± 0.12 (n = 28), but it fell to 3.03 ± 0.11 (n = 28) immediately after the passage of a cyclone in January 2002. LAI* values also fell in the gaps, reaching levels similar to those recorded at the open site in 2001. However, LAI* recovered rapidly, and 6 wk after the cyclone the mean values were close to those recorded earlier (average LAI* understorey in March 2002: 4.51 ± 0.08, n = 28). Only in gap 4, did the LAI* in the centre of the gap remain significantly lower. The cyclone did not cause any further tree fall in the gaps studied, and subsequent changes in LAI* therefore reflect the balance between leaf fall and regrowth.
Distribution of invasive plants
We recorded 11 exotic plant species in the transects. These species were: Rubus alceifolius (recovering 2.43% within gaps vs. 0.32% understorey of the total transect area), Psidium cattleianum (0.08% within gaps vs. 0.13% understorey), Diospyros digyna (0.20% understorey), Rubus rosifolius (0.11% within gaps vs. < 0.01% understorey), Litsea glutinosa (0.01% within gaps vs. 0.03% understorey), Boehmeria macrophylla (0.01% within gaps vs. 0.01% understorey), Clidemia hirta (0.01% within gaps vs. 0.01% understorey), Artocarpus heterophyllus (0.01% understorey), Ardisia crenata (0.01% understorey), Citrus aurantium (< 0.01 understorey) and Begonia cucullata (< 0.01 within gaps). The overall percentage cover of alien plants was 3.35%, but they were strongly concentrated in gaps where they occupied 24.9% of the area, compared with only 0.8% beneath closed canopy.
Rubus alceifolius was overall the most abundant alien species, covering 2.75% of the total transect area, though it was not present in transect 3 (Table 1). In transects 1 and 2 R. alceifolius occupied total areas of 105 and 215 m2, respectively. In these transects, it was distributed throughout the forest, though it was most abundant in the larger gaps (Figure 2) and almost completely filled the six largest gaps (> 25 m2). This difference in abundance between gaps and understorey was statistically very significant (chi-square test: χ2 = 30.3, P < 0.0001), and there was also a positive correlation between gap area and R. alceifolius abundance (r = 0.982; P < 0.000). In most gaps, only mature individuals were found, though intermediate individuals (between seedlings and mature fruit stage) were present in one gap of 20-m2 and in various places in the understorey. A few seedlings were observed, all of them beneath the canopy in places close to gaps (chi-square test gave significant difference between developmental stages and environment: χ2 = 10.5, P < 0.005).

Figure 2. Gap area (white bars) and area colonized by Rubus alceifolius (black bars) within each gap (32) within the 12 000 m2 surveyed.
Germination experiment
The first seedlings to germinate were at the open site, where they emerged 14 d after trays were set out. These were followed by seedlings in the largest gaps, which emerged after 33 d, and later by seedlings in smaller gaps. No seedlings germinated in gaps of less than 5 m2 (corresponding to LAI* recorded in the centre of the gap of 4.35), and none at the understorey site. There was a significant negative correlation between LAI* values at the tray location and the number of seedlings to emerge (r = −0.944; P < 0.001; Figure 3).

Figure 3. Percentage germination success of Rubus alceifolius (±SE) among different gap sizes (evaluated by the LAI* values measured at the gap centre), displayed from the biggest to the smaller, understorey and in open environment. One hundred (25 × 4 trays) seeds were placed at each of the eight sites after having been scarified.
DISCUSSION
Frequency of gaps
The mean density of gaps in lowland rain forest on Réunion was twice that recorded by Hubbell & Foster (Reference HUBBELL, FOSTER and Crawley1986) in similar forest on Barro Colorado Island in Panama (26.7 vs. 12 gaps ha−1, respectively), and the area occupied by gaps was also approximately twice as great (5.62% vs. 2.99% of forest area, respectively). However, the distribution of gap sizes and the mean value were similar to those on Barro Colorado. Most gaps were apparently formed by trees breaking (50.5%) or uprooting (33.3%), though the proportion due to falling branches (14.3%) was probably underestimated because the branches soon disappeared. In many tropical forests gap creation is seasonal, with more being formed during the wet periods than in dry (Brokaw Reference BROKAW1982); this is because wet trees are heavier and less streamlined in wind, and wet or loosened soil provides less stable support for trees. Seasonality in treefall may influence the success of establishment of species whose dispersal and germination rely on gaps (Brokaw Reference BROKAW1982, Garwood Reference GARWOOD1983). On Réunion, most gaps were probably formed between December and March when high winds are most frequent. The higher density of gaps than on Barro Colorado can probably be explained by a combination of steeper topography (slopes were 0–35% on Réunion compared with 10–20% on Barro Colorado) and more intense storms.
Relation between gaps and exotic plants
Because of the variety of ways in which they may be formed, forest gaps vary in structure, which in turn affects local conditions for plant growth (Brokaw Reference BROKAW1985, Denslow Reference DENSLOW1980). Light intensities are much higher in gaps than in the understorey, and the spectral composition of light is also different. Temperatures regimes are also different in gaps, with higher soil and air temperatures during the day, and cooler temperatures at night. Humidity is often lower in gaps than in surrounding forest, but soil water content may actually be higher because of reduced root uptake and transpirational water loss (Lee Reference LEE1978). Patches of soil in the gaps are enriched in nutrients, particularly in areas adjacent to decaying fallen trees or in the disturbed soil of the uprooted area (Hubbell & Foster Reference HUBBELL, FOSTER and Crawley1986). All these characteristics of gaps are likely to favour the establishment of fast-growing alien plants requiring high levels of resources. In our survey, alien plants occupied 24.9% of the area of gaps but only 0.8% of the understorey area.
Rubus alceifolius, the commonest alien species, achieved its highest cover in large gaps (> 25 m2) in which it often formed extensive monospecific patches. Immature individuals of Rubus alceifolius were also present in the understorey and in smaller gaps (around 20 m2), but these grew much less vigorously. The species was especially abundant in the higher parts of the reserve (transect 3) where the surrounding vegetation was only 16.3 m tall compared with > 21 m at the other sites. Rubus alceifolius has a growth form intermediate between a shrub and a liana (Baret et al. Reference BARET, NICOLINI, LE BOURGEOIS and STRASBERG2003a), and once established can grow rapidly upwards into the canopy (Baret et al. Reference BARET, NICOLINI, LE BOURGEOIS and STRASBERG2003a); in addition, it colonises open areas by vigorous vegetative reproduction (successive terrestrial layering), forming dense monospecific patches that prevent indigenous plants from regenerating (Baret et al. Reference BARET, LE BOURGEOIS and STRASBERG2005a). Similar observations have made in other tropical rain forests, with the normal cycle of regeneration being interrupted in larger gaps by various species of invasive woody climbers (Schnitzer et al. Reference SCHNITZER, DALLING and CARSON2000, Whitmore Reference WHITMORE1989).
In the germination experiment, Rubus alceifolius only germinated in gaps of over 5 m2, and we conclude that the LAI* threshold allowing germination is around 4.35. Studies with other Rubus species (Amor Reference AMOR1974, Scott & Draper Reference SCOTT and DRAPER1967) have also shown that establishment was most successful in open conditions. However, once established, Rubus alceifolius can persist under shady conditions in a juvenile stage. If light conditions later become sufficient, it can then grow rapidly and reach maturity, as occurred in the larger gaps at Mare Longue.
Do cyclones favour the establishment of Rubus alceifolius?
Severe cyclones may cause considerable damage to rain-forest vegetation by defoliating trees, dislodging climbers and epiphytes, and breaking trunks and branches (Bellingham et al. Reference BELLINGHAM, TANNER and HEALEY1995, Boose et al. Reference BOOSE, FOSTER and FLUET1994, Brokaw & Walker Reference BROKAW and WALKER1991, Everham & Brokaw Reference EVERHAM and BROKAW1996, Lugo et al. Reference LUGO, APPLEFIELD, POOL and MCDONALD1983, Tanner et al. Reference TANNER, KAPOS and HEALEY1991). These impacts produce significant changes in light, temperature and humidity conditions within the forest (Bellingham et al. Reference BELLINGHAM, TANNER, RICH and GOODLAND1996, Fernandez & Fetcher Reference FERNANDEZ and FETCHER1991, Turton Reference TURTON1992), which in turn affect vegetation processes (Bellingham et al. Reference BELLINGHAM, TANNER and HEALEY1994, Harrington et al. Reference HARRINGTON, FOWNES, SCOWCROFT and VANN1997, Vandermeer et al. Reference VANDERMEER, MALLONA, BOUCHER, YIH and PERFECTO1995). In a region with frequent cyclones, the structure and species composition of forest vegetation reflects the differing responses of the native plants to such events. However, the structure and dynamics of the forest may be considerably altered by the introduction of alien species that benefit more than native species from pulses of resources. For example, when a new gap is formed, established juvenile plants of Rubus alceifolius can respond strongly to higher light conditions and rapidly develop to form a monospecific patch.
In contrast, a less intense cyclone such as the one that struck Réunion in January 2002 may cause some thinning of the canopy, but the effect is transient. In our study area, the mean LAI* was reduced from 4.77 to 3.03, but new leaves were soon produced and the period of higher light conditions on the forest floor only lasted about 6 wk. However, even this period may be sufficient to allow seedlings of species such as R. alceifolius to germinate or established plants to grow larger. While the changes in the canopy due to a single cyclone would probably not be sufficient for plants to reach maturity, they may be able to benefit from successive cyclones, eventually forming mature stems that reach the canopy (Baret et al. Reference BARET, NICOLINI, LE BOURGEOIS and STRASBERG2003a). In this way, high winds could promote the spread of an alien species such as R. alceifolius that is shade tolerant but responds strongly to light, even without any gaps being formed.
CONCLUSION
Our study in lowland rain forest on Réunion clearly indicates that gaps are important in allowing the rapid spread of invasive plant species. And because gaps are also essential for the regeneration of many native trees, there is a danger that the forest will be progressively degraded by alien plants (Strasberg et al. Reference STRASBERG, ROUGET, RICHARDSON, BARET, DUPONT and COWLING2005). Management to control alien species should be considered in a whole-ecosystem context, based upon an understanding of how forest dynamics and structure affects the spread of alien species. The effort to control species such as Rubus alceifolius should be focused mainly upon the larger gaps, where the species is most invasive. However, it may also be necessary to remove plants in the understorey that have reached the last juvenile stage (which can be distinguished morphologically; Baret et al. Reference BARET, NICOLINI, LE BOURGEOIS and STRASBERG2003a, Reference BARET, NICOLINI, HUMEAU, LE BOURGEOIS and STRASBERGb), since these may eventually reach reproductive maturity, benefiting from periodic thinning of the canopy due to cyclones. It is also probable that, by providing a large source of propagules, other large-scale natural disturbances such as landslides or lava flows play a role in the invasion of the forest (Strasberg Reference STRASBERG1995, Restrepo & Vitousek Reference RESTREPO and VITOUSEK2001). Interaction between these different types of disturbances would be worth investigating in future studies.
ACKNOWLEDGEMENTS
The study was conducted under, and financed by, the Région Réunion project, REG/97/0307. We thank two referees for their advice and Françoise Lebeau, Charlie Zongo, Esther Lobet and Julien Triolo for their field assistance.






