INTRODUCTION
The disturbances created by tree falls are major drivers of tropical forest composition and structure (Bongers et al. Reference BONGERS, POORTER, HAWTHORNE and SHEIL2009, Denslow Reference DENSLOW, Pickett and White1985, Uriarte et al. Reference URIARTE, CANHAM, THOMPSON, ZIMMERMAN, MURPHY, SABAT, FETCHER and HAINES2009). Regularly, mature-forest species cannot germinate and establish in the understorey conditions under a closed canopy (Canham et al. Reference CANHAM, DENSLOW, PLATT, RUNKLE, SPIES and WHITE1990, Chazdon & Fetcher Reference CHAZDON and FETCHER1984, Chazdon et al. Reference CHAZDON, PEARCY, LEE, FETCHER, Mulkey, Chazdon and Smith1996) and thus they depend on canopy gaps for their regeneration (Kellner et al. Reference KELLNER, CLARK and HUBBELL2009, Schnitzer & Carson Reference SCHNITZER and CARSON2001, White Reference WHITE1979). Our knowledge on the role of gaps in tropical forest regeneration is strongly biased towards tropical humid forests (THF) (Gravel et al. Reference GRAVEL, CANHAM, BEAUDET and MESSIER2010, Popma et al. Reference POPMA, BONGERS, MARTÍNEZ-RAMOS and VENEKLAAS1988, Schnitzer & Carson Reference SCHNITZER and CARSON2001, van der Meer et al. Reference VAN DER MEER, BONGERS, CHATROU and RIÉRA1994).
Various cause-effect relationships are involved in gap dynamics occurring in THF. Among the most remarkable is the relationship between gap frequency and size and average tree size (Jansen et al. Reference JANSEN, VAN DER MEER and BONGERS2008), the prominent role of physical factors (water, topography, wind) as causal factors of tree falls with a lesser role of pathogens (Franklin et al. Reference FRANKLIN, SHUGART and HARMON1987, Peterson & Pickett Reference PETERSON and PICKETT1995, Zhu et al. Reference ZHU, LU and ZHANG2014), and the decreasing susceptibility to gap formation with increasing disturbance rates due to the resulting scarcity of large trees (Whigham et al. Reference WHIGHAM, DICKINSON, BROKAW and Walker1999). Also, gap size influences the physical conditions within it (Brokaw Reference BROKAW, Pickett and White1985a, Muscolo et al. Reference MUSCOLO, BAGNATO, SIDARI and MERCURIO2014), and the growth of the newly established individuals depends mostly on their functional traits (Rüger et al. Reference RÜGER, HUTH, HUBBELL and CONDIT2009, Reference RÜGER, WIRTH, WRIGHT and CONDIT2012).
Tropical dry forests (TDFs) occur in regions with high rainfall seasonality (500–2000 mm falling in a 4–7-mo period). Apparently for this reason, regeneration in TDFs differs substantially from THF; for example, sprouting is thought to be relatively more important due to germination constraints and high seedling mortality (Vieira & Scariot Reference VIEIRA and SCARIOT2006). The effects of canopy gaps in TDFs has seldom been analysed despite their large extent and their ecological and social relevance (Maass et al. Reference MAASS, BALVANERA, CASTILLO, DAILY, MOONEY, EHRLICH, QUESADA, MIRANDA, JARAMILLO, GARCÍA-OLIVA, MARTÍNEZ-YRIZAR, COTLER, LÓPEZ-BLANCO, PÉREZ-JIMÉNEZ, BÚRQUEZ, TINOCO, CEBALLOS, BARRAZA, AYALA and SARUKHÁN2005, Murphy & Lugo Reference MURPHY and LUGO1986). The available studies suggest a minor role for gaps in TDF regeneration. For one, there is evidence that gap formation rate in TDF is lower than for the average THF (Durán Reference DURÁN2004). Also, gap size in these forests tends to be smaller than in THF (Whigham et al. Reference WHIGHAM, DICKINSON, BROKAW and Walker1999). Given the harsh dry-season conditions for plant life in TDFs, the benefits offered by gaps in the rainy season can be counterbalanced by the conditions prevailing during the yearly drought. Consequently, Swaine et al. (Reference SWAINE, LIEBERMAN and HALL1990) contended that the concept of gap-phase dynamics is not applicable to TDFs.
Support for the idea that canopy gap formation is infrequent in TDFs stems from the following factors: (1) trees are small and thus wind is less likely to throw (Durán Reference DURÁN2004) or snap them (Putz et al. Reference PUTZ, COLEY, LU, MONTALVO and AIELLO1983), (2) trees have relatively large root/shoot ratios (Richards Reference RICHARDS1996), (3) trees tend to die standing, which results in the formation of small or ill-defined gaps (Durán Reference DURÁN2004), and (4) TDFs experience a short wet season and therefore are less frequently subjected to storms capable of causing structural damage (Dickinson et al. Reference DICKINSON, HERMANN and WHIGHAM2001, but see Dunphy et al. Reference DUNPHY, MURPHY and LUGO2000 for a counterexample).
In contradiction of these arguments, there is strong evidence that TDFs, most of which have an extra-equatorial occurrence, largely coincide with the areas of high cyclone incidence (Schreck et al. Reference SCHRECK, KNAPP and KOSSIN2014), and it is undeniable that at least in some TDFs canopy trees do fall and create canopy gaps frequently (Baker et al. Reference BAKER, BUNYAVEJCHEWIN, OLIVER and ASHTON2005, Lewis & Bannar-Martin Reference LEWIS and BANNAR-MARTIN2012, Lugo Reference LUGO2008). Therefore, the question as to what the role of canopy gaps is in the regeneration of TDF is still warranted. Here we used a comparative approach to examine structural and compositional patterns in the forest understorey of a TDF in southern Mexico in order to assess the effects of canopy gaps in this ecosystem. We hypothesized that canopy gap formation is an important process in TDF regeneration. More specifically, we anticipated that the understorey vegetation in canopy gaps would have a higher density of individuals (derived from a more successful establishment), a richer and different species composition (resulting from a selective effect on different species), and a distinct growth-form distribution due to potential differences in their establishment probabilities (but with a good representation of mature-forest tree species), than its counterpart beyond the canopy gaps.
METHODS
Study area
The study was conducted in Nizanda (16°39′30″ N; 95°00′40″ W), Oaxaca State, Mexico (Gallardo-Cruz et al. Reference GALLARDO-CRUZ, MEAVE, PÉREZ-GARCÍA and HERNÁNDEZ-STEFANONI2010, Pérez-García et al. Reference PÉREZ-GARCÍA, MEAVE, VILLASEÑOR, GALLARDO-CRUZ and LEBRIJA-TREJOS2010). Annual rainfall in the area is highly seasonal and totals 878 mm. Due to its position in the southern portion of the Isthmus of Tehuantepec, where topography creates a funnel effect of the prevailing trade winds coming from the north-east, this region is regularly exposed to strong winds, many equivalent to category I cyclonic events (Brennan et al. Reference BRENNAN, COBB and KNABB2010, Jaramillo & Borja Reference JARAMILLO and BORJA2004).
Gap description
During a severe windstorm event in January 2008, numerous gaps formed, 75 of which were inventoried by exploring the main (north-east- and south-west-facing) slopes of Mt. Cerro Verde, an area where TDF is well preserved (Gallardo-Cruz et al. Reference GALLARDO-CRUZ, MEAVE and PÉREZ-GARCÍA2005). Gaps were defined following Brokaw (Reference BROKAW1985b) as continuous vertical openings in the canopy reaching down to 2 m above the ground. Every gap was characterized by its total area (from two perpendicular diameters), geographic position (Garmin 60 csx GPS receptor), terrain slope, elevation, and the bearing of the fall of the tree that formed the gap. A Rayleigh test was performed to assess deviations from randomness of tree-fall direction (Zar Reference ZAR1999). Fallen trees were carefully inspected for bark characteristics and the overall tree and crown shape to determine as precisely as possible their taxonomic identities.
Vegetation sampling
Understorey vegetation was sampled in 50 randomly selected gaps during the rainy season (July and August) of 2009 using four 1 × 3-m plots for each gap. Two plots, referred to as gap plots, were located parallel to, and 1 m apart from, the fallen stem of the largest tree within the gap. The other two plots, referred to as non-gap plots, were placed outside the gap, i.e. under the closed canopy, 10 m away from the gap edge and in opposing directions. For all plants ≥ 1 m but < 3 m high and rooted inside the plots, the taxonomic identity was recorded (or vouchers were collected for determination in the laboratory), and total height and two perpendicular crown diameters were measured. In a randomly selected 1 × 1-m subplot within each plot all plants ≥ 0.3 m but < 1 m in height were also measured for total height and crown diameters. Data by plant size category from the two equal plots (i.e. the two gap plots and two non-gap plots) for each gap were combined for all analyses. The rationale for separating these groups based on plant size is that smaller plants were the most likely to have established after the creation of the gap, or to have been exposed to its effects at an earlier stage of their life cycle.
Data analysis
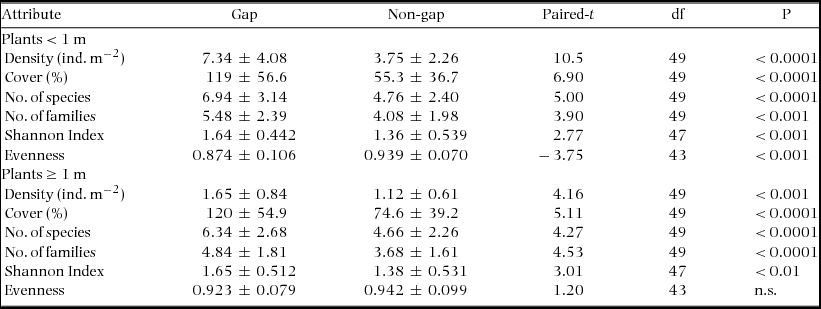
We used chi-squared (χ2) tests to compare the frequency distributions of individual plant attributes (height and crown cover) between gap and non-gap plots. At the community level, paired Student's t-tests were conducted to compare the density of individuals, community cover, species and family richness, Shannon Diversity index (H') and Shannon evenness (Magurran Reference MAGURRAN2004) between gap and non-gap plots. These community-level comparisons were made for each plant size category separately.
To identify whether there were species subsets preferentially distributed in the gap vs. the non-gap condition, we applied a multinomial model that classifies species by habitat affinity (Chazdon et al. Reference CHAZDON, CHAO, COLWELL, LIN, NORDEN, LETCHER, CLARK, FINEGAN and ARROYO2011) based on species abundances in the two habitats. The model classified species into one of the following four groups: (1) gap specialists, (2) non-gap specialists, (3) generalists, or (4) too rare to classify with confidence. The settings used for the model were a simple majority threshold (K = 1/2) and P = 0.05. Similarly, most (morpho-)species recorded in the sampling plots were classified into seven growth forms (trees, shrubs, graminoids, forbs, creepers, climbers and rosettes sensu Pérez-García et al. Reference PÉREZ-GARCÍA, MEAVE, VILLASEÑOR, GALLARDO-CRUZ and LEBRIJA-TREJOS2010) in order to test whether any of these groups was favoured by the formation of gaps.
To further examine a potential floristic differentiation between gap and non-gap conditions we used non-metric multidimensional scaling (NMDS; Kent & Coker Reference KENT and COKER1994) to ordinate the vegetation samples from the two habitats based on a species × abundance matrix; a dissimilarity matrix using binomial distances was employed as the input matrix. All analyses were performed on R, mostly with the vegan package.
RESULTS
Gap characterization
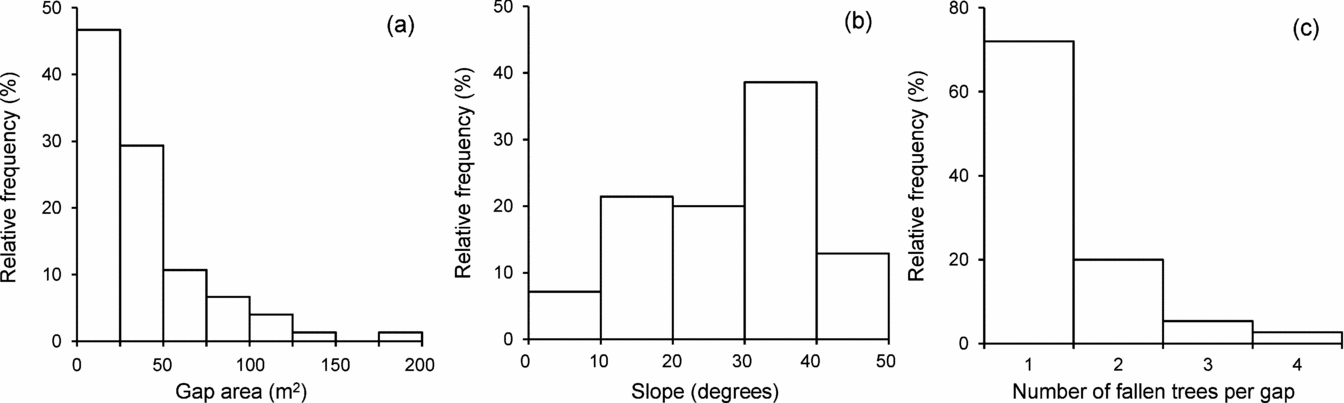
Almost 50% of the 75 surveyed gaps had very small sizes (< 25 m2), while only a few (6.7%) were > 100 m2 in area (mean ± SD = 38.1 ± 33.6 m2; Figure 1a). Similarly, although the sites where these gaps formed were quite variable, 51.4% of them occurred on relatively steep terrain (> 30°; Figure 1b). Most gaps (72%) formed by the fall of a single tree; a further 20% formed by the fall of two trees, and the remaining 8% involved the fall of three or four adult trees (Figure 1c).

Figure 1. Characteristics of treefall gaps in the tropical dry forest of Nizanda, Oaxaca, southern Mexico. Frequency distribution of gap size (area) (a). Frequency distribution of terrain slope where the gaps were created (b). Frequency distribution of the number of trees fallen in the gaps surveyed (c). N = 75 in all cases.
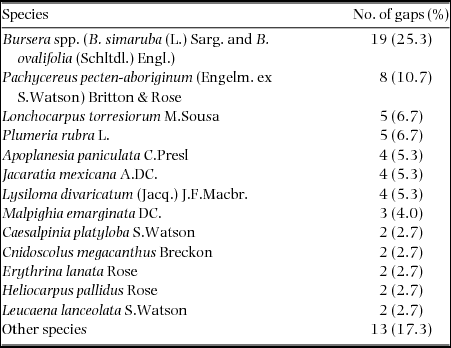
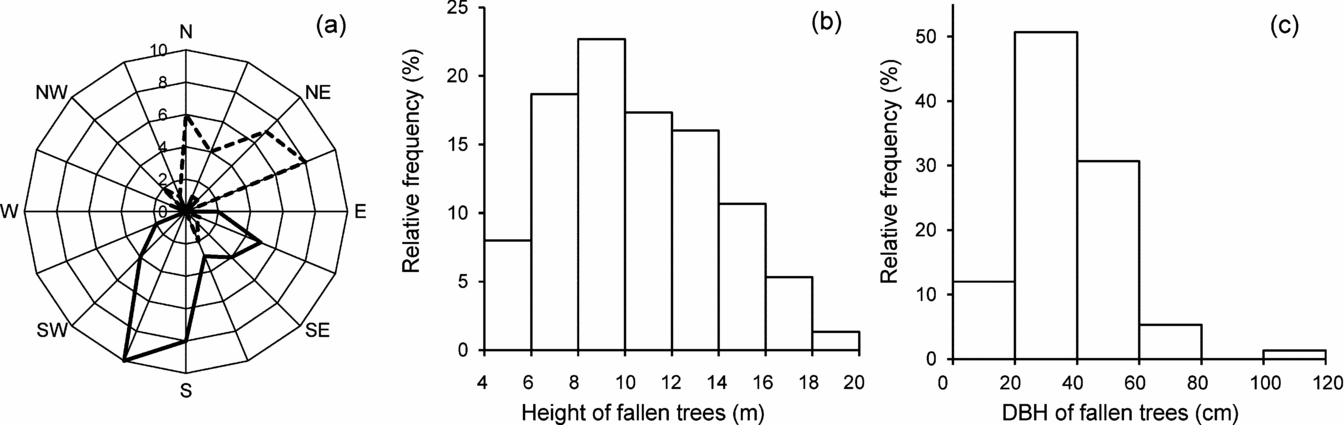
A large proportion of gaps were created by the fall of Bursera spp. trees (Bursera simaruba and B. ovalifolia, which could not be told apart in dead, leafless individuals) (19 gaps; 25%); this group was followed by the columnar cactus Pachycereus pecten-aboriginum (8 gaps, 11%), with decreasing frequencies of other important 11 species listed in Table 1. These 14 species together accounted for 82.7% of all gap-forming trees. The direction of tree fall was unrelated to the direction of the prevailing winds but to slope orientation (Rayleigh test; P < 0.0001; Figure 2a). Former height of most fallen trees varied between 6 and 14 m (mean ± SD = 10.4 ± 3.4 m; range = 4.2–19.3 m: Figure 2b). Their dbh peaked between 20 and 40 cm (Figure 2c). The tallest fallen tree belonged to either one of the two above-mentioned Bursera species, while the largest diameter (58.6 cm) belonged to an individual of P. pecten-aboriginum.
Table 1. Canopy-gap forming tree species recorded in 75 gaps surveyed in the tropical dry forest of the Nizanda region, southern Mexico, along with the number of gaps created by each species and the corresponding percentage. In the case of trees belonging to the genus Bursera it was not possible to distinguish between the two main tree species occurring in the region (Bursera simaruba and B. ovalifolia), thus it is labelled as Bursera spp.


Figure 2. Characteristics of the trees fallen in the studied treefall gaps in the tropical dry forest of Nizanda, southern Mexico. Frequency distribution of the orientation of the fall of the largest tree fallen in the gaps, separated by gaps located in north-east-facing (broken line) and south-west-facing (solid line) slopes (a). Frequency distribution of the height of the largest fallen tree in each gap (b). Frequency distribution of the diameter at breast height (dbh) of the fallen trees (c).
Comparison of individual plants between gap and non-gap plots
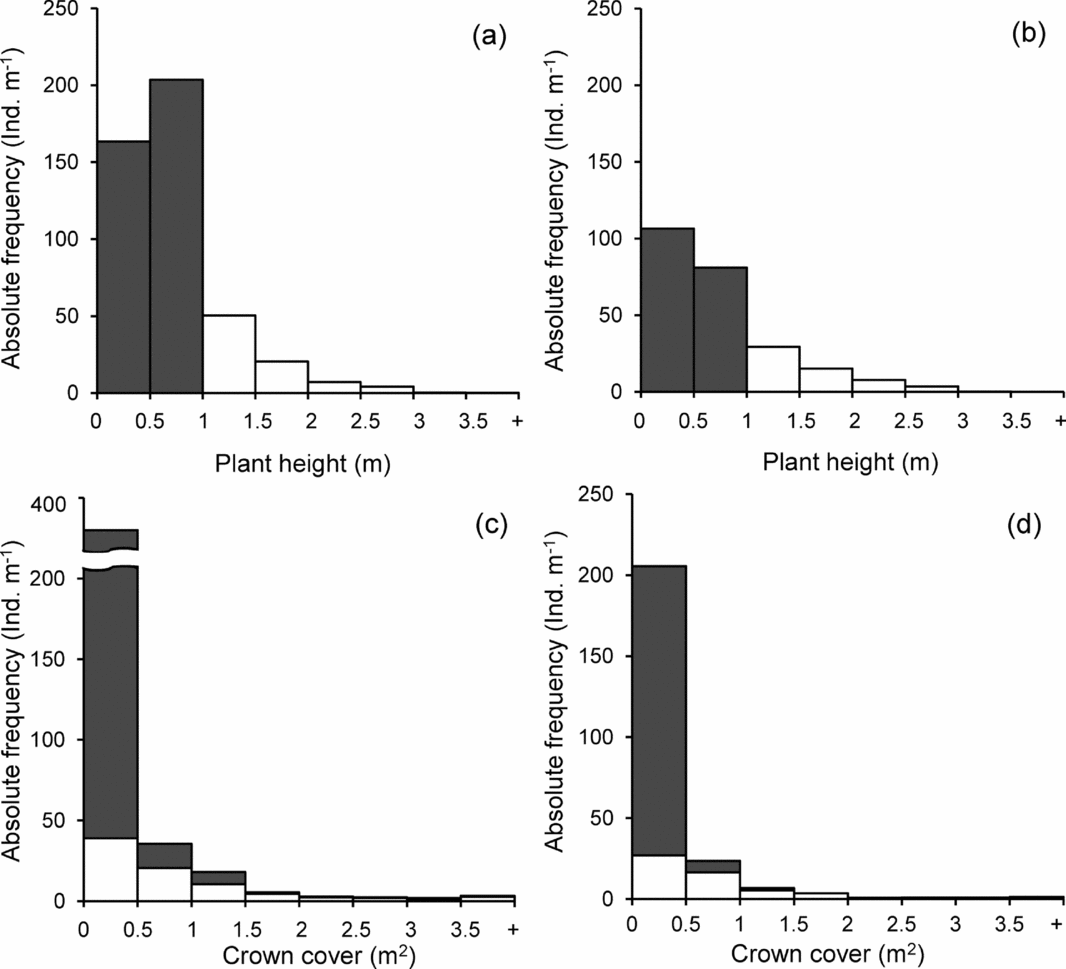
A total of 1940 individual plants (for the two size categories combined) were recorded in the aggregate area of 600 m2 sampled; of these individuals, 1230 (63.4%) occurred within gaps and 710 (36.6%) in non-gap plots (Appendix 1). Frequency distributions of understorey plant height and crown cover were markedly right-skewed, regardless of the gap vs. non-gap condition (Figure 3a–d). Computation of densities from these numbers revealed that the percentages of individuals with heights < 1 m were about 82% in gap and 77.4% in non-gap plots. Regarding crown cover (Figure 3c, d), the proportion of individuals with crown covers < 0.5 m2 were similar in gaps (31.0%) and non-gaps (28.9%). Height frequency distributions of all plants differed between the two habitats (χ2 = 11.6, df = 4; P = 0.021). However, the corresponding frequency distributions for crown cover did not differ between them (χ2 = 1.22, df = 3; P = 0.749; note that some classes of the frequency distributions corresponding to large height and crown cover values were merged because of small numbers, which reduced the number of degrees of freedom).

Figure 3. Characteristics of the understorey plants sampled in gap and non-gap conditions in the tropical dry forest of Nizanda, southern Mexico. Frequency distribution of individual plant height in gap (a) and non-gap (b) plots. Frequency distribution of individual crown cover in gap (c) and non-gap (d) plots. Grey bars (or grey sections of bars) correspond to plants < 1 m in height; white bars (or white sections of bars) correspond to plants ≥ 1 m in height. Because these two groups were sampled in different areas, all data were transformed into densities (m−2). Note the break in the y-axis of (c).
Vegetation structure and diversity in gap and non-gap plots
Table 2 shows the results of the comparison of structural and diversity community attributes between gap and non-gap conditions. In the case of structural attributes (density and cover), all variables were significantly larger for gaps than for non-gaps, both for small and large plants. Remarkably, differences in community structural attributes were larger for plants < 1 m than for plants ≥ 1 m.
Table 2. Mean (± 1 SD) community attributes in gap and non-gap condition in the tropical dry forest of Nizanda, Mexico, and results of the paired t-test conducted to compare each attribute between the two conditions. df = degrees of freedom. N = 50 for most variables, with the exceptions indicated in the df column.

Individuals recorded in all plots belonged to 187 morphospecies, 132 of which (70.6%) were identified to species, 18 (9.6%) to genus, 23 (12.3%) to family, and 14 (7.5%) were left unclassified. The richest family was Euphorbiaceae (20 species), followed by Asteraceae (13), and Acanthaceae and Malvaceae (10 each). Total observed richness in gap plots was 163 species, a figure 35% larger than the observed richness in non-gap plots (121 species). Because this difference could be caused by the disparity in total density between habitats, we rarefied richness in gaps to the number of individuals in non-gap plots (710) using Hurlbert's formula in the vegan package implemented in R; this procedure reduced the former 163 species to 134.4 in gaps, but this figure still represents a 10% excess over the observed richness in non-gap plots. At the plot level, the statistical comparison of means between gaps vs. non-gaps revealed a larger diversity in gaps in terms of both species and family richness, and of Shannon index, but this was not the case of Shannon evenness, as this variable was larger in non-gaps for small plants, and not significantly different for the larger plants (Table 1).
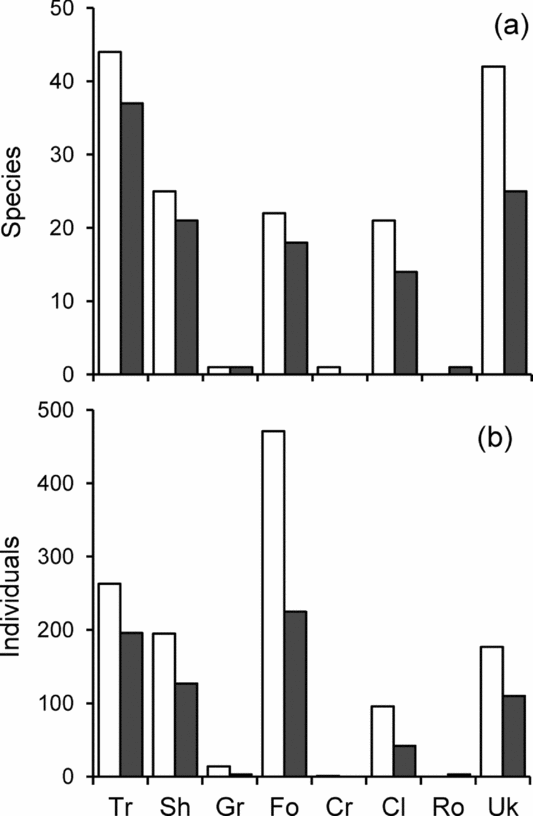
In terms of species richness, the ranking of growth forms was the same in gap and non-gap plots, although their absolute frequencies were larger for gaps (trees, 44 vs. 37 species; shrubs, 25 vs. 21 species; forbs, 22 vs. 18 species for the most frequent growth forms; Figure 4a). A similar pattern was observed when considering individuals instead of species, but in this case the differences for all growth forms were much larger and forbs dominated (forbs, 471 vs. 225 individuals; trees, 263 vs. 196, and shrubs, 195 vs. 127 for the most abundant growth forms; Figure 4b). Despite the greater abundance of both species and individuals in gaps, the χ2 test to compare the distributions of species and individuals among growth forms was significant in the case of individuals (χ2 = 16.8, df = 4, P = 0.0021) but not in the case of species (χ2 = 0.263, df = 3, P = 0.967).

Figure 4. Distribution of understorey plants among growth form categories in gaps (white bars) and non-gaps (black bars) in the tropical dry forest of Nizanda, southern Mexico. Distribution of species (a) and distribution of individuals (b). Growth form abbreviations: Tr, tree; Sh, shrub; Gr, graminoid; Fo. forb; Cr, creeper; Cl, climber; Ro, rosette; Uk, unknown (sensu Pérez-García et al. Reference PÉREZ-GARCÍA, MEAVE, VILLASEÑOR, GALLARDO-CRUZ and LEBRIJA-TREJOS2010).
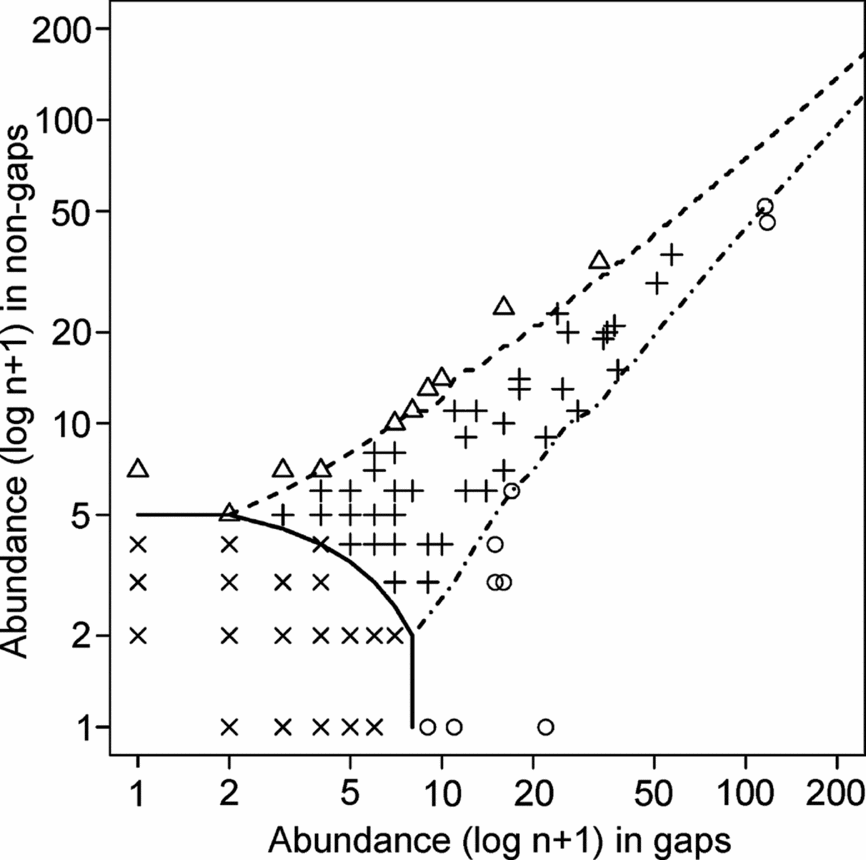
The classification of 187 species in groups of habitat specialization produced by the CLAM model (Figure 5) revealed that most species (119, 63.6%) were too rare to be classified with confidence. Interestingly, 48 more species (25.6%) had relatively similar abundances in both plot types so that they were classified as habitat generalists. These two groups together represented almost 90% of all species included in the analysis. Eleven species (5.9%) had a significant preference for the non-gap habitat, whereas nine species (4.8%) showed a significant preference for gaps. The gap-specialist group included mostly opportunistic forbs, and no single important shrub or tree in the mature forest was included in this group. In turn, although the non-gap specialists included some typical mature-forest species like Coursetia oaxacensis (Fabaceae) and Pilocarpus racemosus (Rutaceae), these species also had various individuals in the gap plots, and the differences in abundance between the two habitats were rather modest, albeit significant.

Figure 5. Multinomial classification of 187 species in four groups based on habitat specialization according to the CLAM analysis (see text for details). Group 1, generalists (crosses, 48 species); Group 2, non-gap specialists (triangles, 11 species); Group 3, gap specialists (circles, 9 species); group 4, too rare to classify (multiplication symbols, 119 species). Species included in the non-gap specialist group are: Coursetia oaxacensis, Pilocarpus racemosus, Asteraceae sp. 6, Jacobinia candicans, Aphelandra schiedeana, Portulaca oleraceae, Thouinia acuminata, Plumeria rubra, Thevetia plumeriaefolia, Bunchosia strigosa and Unknown undetermined no. 12. Species included in the gap specialist group are: Justicia caudata, Ruellia inundata, Ruellia pringlei, Amphilophium paniculatum, Arrabidea floribunda, Celastraceae sp. 1, Tradescantia andrieuxii, Malpighiaceae sp. 9 and Panicum trichoides.
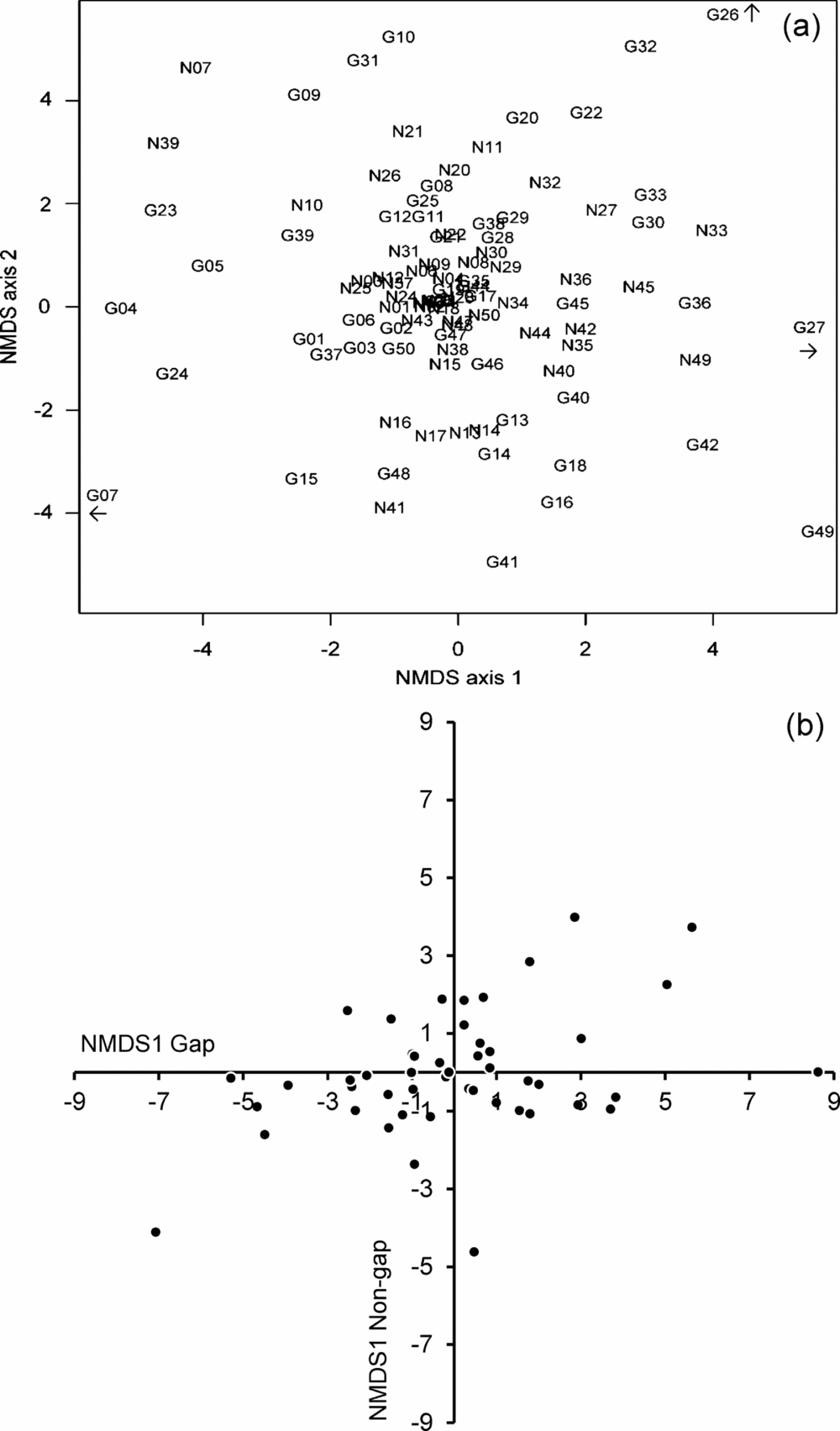
The NMDS analysis did not result in any segregation of gap and non-gap plots on the ordination space (Figure 6a). In fact, it showed a tendency for the two kinds of plots from the same site (i.e. associated to the same canopy gap) to be closely located on the graph, with most distances for each pair being between 1 and 2 ordination units with respect to one or both axes (see for example the locations of the gap and non-gap plots for canopy gaps no. 10, 33, 39 and 41 on the ordination graph). Moreover, axis 1 scores of the NMDS ordination for all pairs of gap and non-gap plots showed a significant correlation (r = 0.397, P < 0.005; Figure 6b). That is, there is a stronger site effect on the floristic composition of the plots rather than a habitat effect (gap vs. non gap).

Figure 6. NMDS ordination of gap (G) and non-gap (N) plots, for plants < 1 m in height, based on a species abundances × sites matrix and Euclidean distances as dissimilarity measure (a). Correlation of the scores on the first axis of the NMDS ordination for gap and non-gap plots (b). Numbers in (a) indicate the particular canopy gap to which either gap or non-gap plots are associated.
DISCUSSION
Risk of fall and the potential to create canopy gaps among TDF trees
The average height (10.4 m) of the fallen trees was substantially larger than the average forest height in the region (7 m, Pérez-García et al. Reference PÉREZ-GARCÍA, MEAVE and GALLARDO2001), and it is also above the mean height for the top 10% of canopy trees in this forest (9.1 m; Gallardo-Cruz et al. Reference GALLARDO-CRUZ, MEAVE and PÉREZ-GARCÍA2005). This implies a higher susceptibility of bigger trees to the mechanical effect of wind. This conclusion is further supported by the under-representation in the gap-forming group of many smaller, subcanopy species that are very abundant in this forest, such as Capparis verrucosa, Casearia nitida, Euphorbia schlechtendalii and Pilocarpus racemosus, and agrees with other studies showing that bigger trees (as well as bigger stems in multi-stemmed trees) are more susceptible to stem damage and uprooting (Lewis & Bannar-Martin Reference LEWIS and BANNAR-MARTIN2012, Reilly Reference REILLY1991, Walker Reference WALKER1991, Zimmerman et al. Reference ZIMMERMAN, EVERHAM, WAIDE, LODGE, TAYLOR and BROKAW1994). In fact, the disproportionately high probability of bigger tree to fall has been suggested to be the main cause of the overall shorter size of trees in the TDF (Van Bloem et al. Reference VAN BLOEM, MURPHY and LUGO2007).
A large percentage (76%) of all surveyed gaps were small (< 50 m2). For comparison, Popma et al. (Reference POPMA, BONGERS, MARTÍNEZ-RAMOS and VENEKLAAS1988) reported that only five out of 12 gaps were < 40 m2 in the nearby rain forest of Los Tuxtlas. In Nizanda, the few gaps exceeding such size were almost always formed by the fall of Bursera spp. trees or the cactus Pachycereus pecten-aboriginum; their ability to create bigger gaps, and to do so more frequently, suggests a particularly important role of these species in the dynamics of the local TDF. In addition to their bigger sizes, these species may be more susceptible to falling because of their shallow root systems, a common trait among TDF species able to accumulate water in stem tissues (Paz et al. Reference PAZ, PINEDA-GARCÍA and PINZÓN-PÉREZ2015). Interestingly, these species tend to fall by uprooting rather than by snapping, despite their low-density wood (Chave et al. Reference CHAVE, COOMES, JANSEN, LEWIS, SWENSON and ZANNE2009). Among the other species often responsible for the formation of canopy gaps, many also have low wood density (Erythrina lanata, 0.32 g cm−3; Heliocarpus pallidus, 0.52 g cm−3; Jacaratia mexicana, 0.26 g cm−3; Plumeria rubra, 0.50 g cm−3). However, this was not always the case, as the remaining species included in this group (all of them legumes) have high wood densities (Apoplanesia paniculata, 0.87 g cm−3; Lonchocarpus torresiorum, 0.85 g cm−3; Lysiloma divaricatum, 0.77 g cm−3). Therefore, the risk of falling seems to depend on a combination of tree height, as well as rooting depth, wood density and abundance of the species in the forest.
The effect of canopy gaps on TDF understorey vegetation
An initial expectation of this study was that the largest differences in community attributes between gap and non-gap conditions would be observed for the subset of smaller plants (< 1 m in height), as these had established most probably after the creation of the canopy gaps. The results showed that this was the case, and it was particularly evident for structural community attributes, as density and community cover were almost two-fold in gaps than in the non-gap counterparts. The result for Shannon evenness was opposite to this pattern, as this variable was larger in non-gap plots, which probably results from the large abundance of three Acanthaceae in gap plots (Justicia caudata, Ruellia inundata and R. pringlei). Interestingly, the paired t-tests actually showed significant, albeit smaller, differences for the subset of bigger plants as well, both for all structural and most diversity variables. Despite this finding, we did not observe any evidence for an equivalent effect on the performance of individuals plants, as these were neither taller nor had wider crowns in gaps. The significant differences in the number of individuals, species and families between gap and non-gap plots lead to the conclusion that gaps are potentially better sites for the successful natural regeneration of the forest. Overall, the increase in density is consistent with findings of studies on gap dynamics, in which it has been proposed to be a driving force for regeneration of woody species in forests (Zhu et al. Reference ZHU, LU and ZHANG2014).
Initially, we expected that the effect of canopy gaps would also be apparent in the frequency distribution of growth forms in the vegetation understorey, as canopy gaps were expected to promote the establishment of mature-forest trees. The comparison of plant growth-form spectra between gaps and non-gaps failed to reveal differences in the respective proportions when the analysis was based on species richness. However, the frequency distributions of growth-form categories did differ in the case of individuals, and although this may be related to a particularly high abundance of forbs (mostly of Acanthaceae) in gaps, it is also clear that more trees and shrubs establish and grow in gaps than in the forest understorey.
A central question of this study was whether there were floristic arrays associated to the gaps and the closed understorey, clearly differentiated from each other. The existence of such arrays could be indicative of differential environmental conditions in gaps that could favour certain species (these species could be labelled as gap-specialists; Brown Reference BROWN1993, Denslow Reference DENSLOW1987), or that could have potential negative effects of gaps on particularly sensitive species (species intolerant of direct sun light). We examined these possibilities through the CLAM model and the NMDS ordination, as these analyses could reveal a floristic segregation related to the gap vs. non-gap dichotomy. Although most species turned out to be too rare to be classified by the CLAM model, many of those sufficiently abundant species were classified as habitat generalists. Thus, only 20 species showed a significant preference for one habitat, whereas a mere four species were exclusive of either habitat. In other words, virtually all species were recorded in both habitats, in clear agreement with the findings of Swaine et al. (Reference SWAINE, LIEBERMAN and HALL1990) for Ghanaian TDF, and of Lieberman et al. (Reference LIEBERMAN, LIEBERMAN, PERALTA and HARTSHORN1995) for the THF from La Selva, Costa Rica. Lastly, but not least importantly, none of the gap specialist species was an important woody component of the mature forest, as this group comprised small-sized woody or herbaceous species, including some climbers, which appear to display an opportunistic strategy in colonizing canopy gaps.
The results of the NMDS ordination were consistent with the CLAM-based species classification; the NMDS made evident that pairs of gap and non-gap plots associated to any given canopy gap tend to be more similar to each other than to other plots from the same habitat, thus discarding a floristic segregation between gap and non-gap conditions. This implies some degree of spatial autocorrelation (Gallardo-Cruz et al. Reference GALLARDO-CRUZ, MEAVE, PÉREZ-GARCÍA and HERNÁNDEZ-STEFANONI2010) and may be due at least partly to limited seed dispersal (Pennington et al. Reference PENNINGTON, LAVIN and OLIVEIRA-FILHO2009), preventing floristic homogenization between different forest sectors, and consequently between their gaps.
Potential mechanisms involved in gap-phase regeneration
As discussed so far, the results of this study suggest that gaps do represent windows of increased opportunity for TDF regeneration. This conclusion brings about the question as to what mechanisms are involved in this process. Unfortunately, it is difficult to examine this issue based on our results due to the lack of understorey environmental information, but published literature may help to attempt an initial examination of potential factors in order to provide some guidance for future research.
For tropical humid forests the role of increased light in gaps in the germination of seeds of gap specialists or light-demanding species is well established (Pearson et al. Reference PEARSON, BURSLEM, MULLINS and DALLING2002, Vázquez-Yanes & Orozco-Segovia Reference VÁZQUEZ-YANES and OROZCO-SEGOVIA1993), and generally light is recognized as a major limiting factor for successful forest regeneration (Montgomery & Chazdon Reference MONTGOMERY and CHAZDON2002, Nicotra et al. Reference NICOTRA, CHAZDON and IRIARTE1999, Rüger et al. Reference RÜGER, BERGER, HUBBELL, VIEILLEDENT and CONDIT2011). In contrast, there is also important evidence suggesting that gaps enhance seedling establishment and sapling growth of whatever mix of tree species that is locally present in the gap, disregarding clear differences between shade-tolerant and light-demanding species (Hubbell et al. Reference HUBBELL, FOSTER, O'BRIEN, HARMS, CONDIT, WECHSLER, WRIGHT and LOO DE LAO1999, Wright et al. Reference WRIGHT, MULLER-LANDAU, CONDIT and HUBBELL2003). Our results agree more with this latter scenario. Although less clear, there is also evidence that in TDF increased radiation may also have a positive effect, according to studies showing that an improved light regime may promote the establishment and growth of trees (Lee Reference LEE1989, Rincón & Huante Reference RINCÓN and HUANTE1993). However, in a water-limited system such as the TDF of Nizanda it is likely that water also critically affects germination and establishment (Lebrija-Trejos et al. Reference LEBRIJA-TREJOS, PÉREZ-GARCÍA, MEAVE, POORTER and BONGERS2011, McLaren & McDonald Reference MCLAREN and MCDONALD2003, Vieira & Scariot Reference VIEIRA and SCARIOT2006). In fact, there is at least one study that attributes more diversity to more shaded environments in a TDF because of a reduction of the adverse effects of high radiation (Sagar et al. Reference SAGAR, PANDEY and SINGH2012). The role of gaps related to water availability remains uncertain as they could have a positive effect by decreasing canopy interception of rain (Jetten Reference JETTEN1994), but this effect could be offset by faster soil drying in the gaps (Brouwer Reference BROUWER1996). Research on the prevailing environmental conditions in TDF gaps is needed to clarify this issue.
Contrast between gap-phase dynamics and secondary succession in TDF
An interesting feature of the floristic composition in the studied gaps is that it greatly differs from the composition found in secondary successional stands of the same community (Lebrija-Trejos et al. Reference LEBRIJA-TREJOS, BONGERS, PÉREZ-GARCÍA and MEAVE2008). In very young successional stands the dominant species is the shrub Waltheria indica L., which is shortly after replaced by trees of either Mimosa acantholoba (Humb. & Bonpl. ex Willd.) Poir. or M. tenuiflora (Willd.) Poir. None of these three species was recorded in any of the 100 gap plots used in this study. This finding is not only consistent with the lack of floristic segregation revealed by the CLAM and NMDS analyses, but also very relevant as it indicates that gaps in TDF represent sites for the natural regeneration of mature forest species but not of secondary successional species.
Despite the lack of data for understorey environmental conditions for gaps and shaded understorey in our forest, one may reasonably suspect that differences in species composition between gaps and successional forests are related to differences in environmental conditions, with temperature being a major factor influencing early secondary succession (Lebrija-Trejos et al. Reference LEBRIJA-TREJOS, PÉREZ-GARCIA, MEAVE, BONGERS and POORTER2010). Increased temperatures are associated with the occurrence of stress-tolerant species in early succession stages, such as legumes, while more advanced successional stages are dominated by plants having other traits such as simple leaves, which are less stress tolerant (Romero-Duque et al. Reference ROMERO-DUQUE, JARAMILLO and PÉREZ-JIMÉNEZ2007, Saynes et al. Reference SAYNES, HIDALGO, ETCHEVERS and CAMPO2005). Hence, in a successional process, species tolerant of high temperatures are crucial for succession to proceed, as only they are capable of allowing the subsequent establishment of less-tolerant species underneath them. Since drought episodes are a severe ecological filter, the importance of stress-tolerant pioneers is higher for the TDF succession, and sites occupying larger areas and completely rid of vegetation have a successional pattern that necessarily involves such plants at the beginning, which results in a slower recovery of the original vegetation. Conversely, the understorey environment in gaps appears to be less stressful so that the successional array of stress-tolerant species may not be adapted to it. This situation is further complicated by the fact that agricultural activities leading to land abandonment and the initiation of secondary succession generally involve the use of fire, a very selective factor in tropical systems (Mueller-Dombois & Goldammer Reference MUELLER-DOMBOIS, GOLDAMMER and Goldammer1990, Murphy & Lugo Reference MURPHY and LUGO1986). Hence, gaps could be responsible for a more direct regeneration of the forest, skipping the phases dominated by more stress-tolerant species. In this scenario, plants with relatively higher requirements of light but intolerant to very high temperatures would be the ones most favoured by creation of gaps in tropical dry-forest ecosystems.
Concluding remarks
In partial disagreement with earlier views that gap-phase dynamics is inconsequential for TDF dynamics (Swaine et al. Reference SWAINE, LIEBERMAN and HALL1990), the results of this study suggest a more active, albeit modest, role of treefall gaps in the regeneration of mature-forest species. Our study confirms the role of windthrow as the main cause of treefall gaps in the TDF of Nizanda, in agreement with other studies (Schliemann & Bockheim Reference SCHLIEMANN and BOCKHEIM2011). This finding underscores the importance of high-energy wind events (hurricanes, strong wind gusts or gales) in driving forest dynamics in areas prone to these phenomena (Baker et al. Reference BAKER, BUNYAVEJCHEWIN, OLIVER and ASHTON2005, Bullock Reference BULLOCK1986, Dittus Reference DITTUS1985, Imbert et al. Reference IMBERT, LABBE and ROUSTEAU1996, Lewis & Bannar-Martin Reference LEWIS and BANNAR-MARTIN2012, Lin et al. Reference LIN, HAMBURG, LIN, WANG, CHANG, HSIA, VADEBONCOEUR, MCMULLEN and LIU2011, Lugo Reference LUGO2008, Quigley & Platt Reference QUIGLEY and PLATT2003, Whigham et al. Reference WHIGHAM, OLMSTED, CABRERA-CANO and HARMON1991), and establishes a direct link between such large-scale disturbing events with the regeneration of many TDF communities. Since the worldwide distribution of TDF largely coincides with regions that are the most frequently impacted by hurricanes and similar events, the dynamics of these systems appear to be intimately linked to these meteorological events and thus such dynamics should be temporally scaled to them.
ACKNOWLEDGEMENTS
We are grateful to the people of Nizanda for their hospitality and support. Rodrigo Muñoz assisted in data analysis. This research was supported by the Mexican Council of Science and Technology (CONACYT, grant SEP-CONACYT- 2009–128136). This paper benefited from very constructive comments from Janet Franklin and an anonymous reviewer.
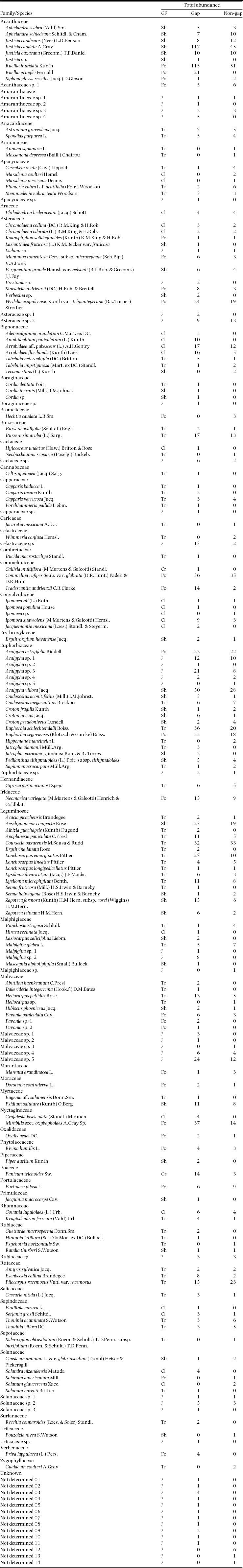
Appendix 1. List of species recorded in gap and non-gap plots from the tropical dry forest of Nizanda, Oaxaca, Mexico, along with their growth form and total abundance in gap and non-gap plots. Growth forms: Cl, climber; Cr, creeper; Fo, forb; Gr, graminoid; Ro, rosette; Sh, shrub; Tr, tree (sensu Pérez-García et al. Reference PÉREZ-GARCÍA, MEAVE, VILLASEÑOR, GALLARDO-CRUZ and LEBRIJA-TREJOS2010). For each species summed abundance for two size categories (small plants, ≥ 0.3 m and < 1 m in height; and large plants, ≥ 1 m but < 3 m in height) is given.