INTRODUCTION
In tropical dry deciduous forest (TDF), soil moisture (McLaren & McDonald Reference McLAREN and McDONALD2003a), shade (Lieberman & Li Reference LIEBERMAN and LI1992) and light (Zimmerman et al. Reference ZIMMERMAN, WRIGHT, CALDERÓN, APONTE and PATON2007) play important regulatory roles in seed germination and seedling establishment and survival (Murphy & Lugo Reference MURPHY and LUGO1986), and some studies have suggested that the limited availability of water in TDF is a critical factor in these processes (Khurana & Singh Reference KHURANA and SINGH2004). Other studies of TDF have found two main responses to light resources: (1) high light in the open (unshaded areas) tends to inhibit germination, early establishment and survival, even for light-demanding species (González-Rivas et al. Reference GONZÁLEZ-RIVAS, TIGABU, CASTRO-MARÍN and ODÉN2009, Huante & Rincón Reference HUANTE and RINCÓN1998); (2) higher-than-average light in the understorey (shaded areas) stimulates these processes (Gerhardt Reference GERHARDT1996a, Williams-Linera et al. Reference WILLIAMS-LINERA, ALVAREZ-AQUINO, HERNÁNDEZ-ASCENCIÓN and TOLEDO2011), even for shade-dependent species (Marod et al. Reference MAROD, KUTINTARA, TANAKA and NAKASHIZUKA2004). Additionally, recent studies have demonstrated plant selectivity for shade during the early stages of some TDF species (Álvarez-Aquino & Williams-Linera Reference ÁLVAREZ-AQUINO and WILLIAMS-LINERA2012, Miranda-Jácome et al. Reference MIRANDA-JÁCOME, MONTAÑA and FORNONI2013, Sánchez-Velásquez et al. Reference SÁNCHEZ-VELÁSQUEZ, QUINTERO-GRADILLAB, ARAGÓN-CRUZ and PINEDA-LÓPEZ2004).
The TDF of the semi-arid region of Mexico is the driest of the northern Neotropics (Álvarez-Yépiz et al. Reference ÁLVAREZ-YÉPIZ, MARTÍNEZ-YRÍZAR, BÚRQUEZ and LINDQUIST2008). In Central Mexico, semi-arid TDF occurs in the Bajio region (Rzedowski & Calderón Reference RZEDOWSKI and CALDERÓN1987), where differences in soil water conditions and radiation levels have been observed among contrasting topographic aspects (Labat Reference LABAT1987). During TDF succession, this physical variation could be relevant in the processes of germination, seedling establishment and survival of trees (Labat Reference LABAT1995), such as Lysiloma microphyllum, a keystone legume species in the structure (Arriaga & León Reference ARRIAGA and LEÓN1989, León de la Luz et al. Reference LEÓN DE LA LUZ, DOMÍNGUEZ-CADENA and MEDEL-NARVÁEZ2012) and functioning (Cárdenas & Campo Reference CÁRDENAS and CAMPO2007, González-Ruiz et al. Reference GONZÁLEZ-RUIZ, JARAMILLO, PEÑA and FLORES2008) of Mexican semi-arid TDF. However, for many leguminous species, which are diverse in TDF, the specific impacts of these factors on their early demographic stages remain unclear (Vargas et al. Reference VARGAS, WERDEN and POWERS2015).
Lysiloma microphyllum establishes in a shrub/tree phase in secondary and mature TDF in shallow and rocky soils on moderate to steep slopes. Forest succession, which is still a poorly understood process, is limited by the scarce recruitment of L. microphyllum saplings, and their occurrence may be fundamental to the recovery of TDF in the Bajio region of Mexico. Even though the physical conditions across contrasting aspects (north, south, east and west) may represent important environmental differences in this semi-arid TDF, no single study has addressed the effects of aspect, radiation (understorey light) and seed predation on the recruitment of L. microphyllum, from seed germination to seedling establishment and growth. Therefore, the main question of this study was as follows: What are the roles played by these factors during the early development of this species in secondary TDF communities? Experiments were conducted to evaluate the responses of L. micropyllum in the early stages of its life cycle to two physical factors (aspect and understorey light) and one biotic factor (seed predation) over 2 y. We hypothesized that: (1) contrasting aspects represent different habitats in terms of shading (radiation) and predation, so we should observe differences in seed germination, establishment and survival among them; (2) assuming that L. microphyllum is shade-dependent, we should find higher seed germination and establishment percentages in shaded sites than in open spaces; and (3) we should find a greater number of living seeds and seedlings in seed predatory exclusion sites than in non-exclusion sites since early plant stages are directly influenced by seed predation.
METHODS
Study site
The study was carried out in the Bajio Queretano (20°28′–20°29′N, 100°21′–100°22′W) within El Cimatario National Park (2300 ha) in the southern part of Queretaro State (Figure 1a). The climate is semi-arid and highly seasonal, with a dry season of 8 mo (García Reference GARCÍA1988). The mean annual temperature is 19°C (the coolest of the neotropical TDF) and precipitation is 549 mm y−1, of which 86% falls between June and October. The study area is located at the upper altitudinal limit of the seasonally dry tropical forests of the Neotropics; so it is primarily a montane tropical dry forest, the altitude of which ranges between 1900 m and 2350 m asl. The natural vegetation is secondary TDF, which is also referred to locally as ‘bosque tropical caducifolio’ (sensu Rzedowski Reference RZEDOWSKI1978) or ‘selva baja caducifolia’ (sensu Miranda & Hernández-X Reference MIRANDA and HERNÁNDEZ-X1963) or globally as seasonally dry tropical forest (Bullock et al. Reference BULLOCK, MOONEY and MEDINA1995). It develops on shallow soils derived from a geological substrate dominated by Tertiary–Quaternary rocks, mainly basalt and volcanic breccia units (INEGI 1986) and the dominant plant families include Fabaceae, Burseraceae, Cactaceae and Euphorbiaceae.

Figure 1. Location of the study site in El Cimatario National Park, Southern Queretaro, Mexico (a) and a diagram of the basic experimental set-up by treatment factors (b).
Study species
Lysiloma microphyllum Benth. (Fabaceae–Mimosoideae) is a deciduous tree that grows up to 15 m and is associated with N nodulation-fixation (González-Ruiz et al. Reference GONZÁLEZ-RUIZ, JARAMILLO, PEÑA and FLORES2008). The flowering and fruiting seasons occur from July until the end of November; the tree forms dehiscent legumes with barochoric seeds (mean 0.04 ± 0.001 g per seed) that are dispersed in the dry season. This species is monoecious and wind-pollinated with slow to moderate growth (Andrade et al. Reference ANDRADE, CALDERÓN DE RZEDOWSKI, CAMARGO-RICALDE, GRETHER, HERNÁNDEZ, MARTÍNEZ-BERNAL, RICO, RZEDOWSKI and SOUSA2007), and it has nitrogen-rich compound leaves (Cárdenas & Campo Reference CÁRDENAS and CAMPO2007). Most of its seeds are predated by rodents, birds and ants.
Experimental design
Previous studies have recognized the importance of habitat conditions (e.g. light, moisture and biotic interactions) for the establishment and growth of TDF tree species (Marod et al. Reference MAROD, KUTINTARA, TANAKA and NAKASHIZUKA2004, McLaren & McDonald Reference McLAREN and McDONALD2003b), so, we conducted field experiments to elucidate two essential stages in the regeneration biology of L. microphyllum: (1) the transition from seed to seedling and establishment, growth and survival and (2) seedling growth and survival (hereafter, experiments 1 and 2, respectively). The experimental units were 40 seeds in experiment 1 and 12 seedlings in experiment 2. The experimental treatments were applied in a factorial design, and each unit was examined under (1) two levels of radiation (understorey light) in the habitat (shaded and open) of which shaded sites were considered canopy sites and represented by secondary TDF trees and shrubs including L. microphyllum; (2) two levels of predation (exclusion and non-exclusion of predators without distinguishing between vertebrates and invertebrates); and (3) four aspects (north, south, east and west) (Figure 1b). Each experiment unit was replicated three times (for a total, of 48 units for each experiment).
Site selection
Within El Cimatario National Park, we selected a hillside for each topographic aspect (north, south, east and west) to establish the experimental units for both experiments. The experiments were established in contiguous sites with the same soil conditions (lithosol), slope (~20°) and altitude (2100–2110 m asl) (Figure 1a).
General procedures
The seeds and seedlings in experiments 1 and 2, respectively were subjected to the study factors and their levels in an arrangement that produced 16 treatments (4 aspects × 2 radiation levels × 2 predation levels; 16 × 3 replicates = 48 experimental units per experiment). Treatments were organized to evaluate each of the cardinal aspects (Figure 1b), so the seeds and seedlings were placed in experimental plots on each slope to simultaneously test the effect of the two kinds of radiation in each habitat (shade and open). In turn, both radiation levels were examined under conditions that excluded and included predators. The L. microphyllum seedlings in the experimental plots lost their leaves at the same time in the dry season, and this was consistent across all experimental treatments.
Experiment 1: Seed germination, seedling establishment and seedling survival
Lysiloma microphyllum seeds were previously collected during fruiting in the dry season prior to the experiment, and for each treatment 40 seeds were sown in the soil surface in a 50 × 50-cm plot (48 units in total) at the beginning of the rainy season (June). The experimental plots were cleaned of other seeds before the seeds of the study species seeds were sown, taking care to minimize soil disturbance. Predators (birds and rodents) were excluded by a 50 × 50 × 50-cm metallic structure covered with 1.5-mm wire mesh buried to a soil depth of 10–15 cm, and ants were excluded by applying an insecticidal powder (formamide) applied every 3 d during the first 4 wk and every 7 d during the next 6 wk, until the seedlings were established with independent roots. The exclosures were large enough to permit seedling growth during the experiment. For all treatments, germination success was measured as the number of seeds with visible radicles within the first 4 wk, and initial seedling establishment was quantified as the number of live seedlings present in all plots after the first month. The survival of the seedlings was monitored for the next 24 mo.
Experiment 2: Survival of transplanted seedlings
Three-mo-old seedlings were cultivated in 25 × 10-cm individual bags with a mixed substrate (peat moss-agrolita-soil habitat), and each treatment (48 units in total) consisted of 12 seedlings transplanted to 50 × 50-cm plots (we ensured 100% survival of planted seedlings in all plots at the beginning of the experiment) under different conditions, as in the experimental design described above. The procedures and specifications for the predator exclusion plots and the controls of predators were conducted as in experiment 1, and seedling survival was checked monthly and measured as the number of live seedlings present in each plot over the 2-y period.
Photosynthetically active radiation (PAR)
We determined the percentage of PAR (400–700 nm) received by each plot (mean ± SD) in experiments 1 and 2 (open and shaded sites) using a reference measurement taken in an open area (full sunlight conditions). PAR levels were recorded with a quantum sensor (BQM, Apogee Instruments Inc. UT, USA).
Statistical analysis
To evaluate the maximum germination reached in situ 21 d after sowing (before the seeds became seedlings), one-way ANOVA was used to determine significant differences among treatments, and post hoc differences between the treatments were estimated with a Tukey's test (α = 0.05). The germination percentage was arcsine transformed accordingly to meet normality assumptions (Zar Reference ZAR1999).
We used general linear models (GLM) to explore the effects of the aspect, understorey light (radiation) and predation on the early life phases of L. microphyllum. A log-linear model with a Poisson distribution and a log link function was used to compare survivorship data (counts) among treatments at different time intervals for (1) the total number of germinated seeds over 3 wk, and (2) the total number of individuals alive at 1, 6, 12, 18 and 24 mo in experiment 1 (the transition from germinated seeds to established seedlings) and experiment 2 (transplanted seedlings), as described above. In cases in which the residual deviance suggested overdispersed/underdispersed data, we used a quasi-Poisson distribution and log link. The range of deviance (D 2) values for the goodness of fit of models was estimated as follows: deviance (D 2) = null deviance−residual deviance/null deviance × 100. We tested the null hypothesis of equal germination and number of living seedlings counts (frequencies) in all treatments, and the effects and interactions among all model factors were examined for statistical significance. All calculations were performed using the software R ver. 3.0.1. (The R Foundation for Statistical Computing Platform, Vienna, Austria), except for the ANOVA and survival analysis, for which Statistica 7.0 (StatSoft Inc., Tulsa, OK, USA) was used.
Survival analysis
The survival function S(t) = Pr{T>1}=1−F(t) was applied because it follows a cumulative mortality distribution F(t), it estimates the probability of seedling survival over any time interval as a function of the mortality probability f(t) (density function) in the same interval. Both functions were obtained using the survival time function procedure (Lee Reference LEE1992), and the survivorship curves of the seedlings in all treatments were elaborated and compared in pairs with a log-rank test (LR) (Pyke & Thompson Reference PYKE and THOMPSON1986) to detect statistically significant differences. The null hypothesis of equal survival functions between pairs of curves was tested by this method.
RESULTS
Photosynthetically active radiation (PAR)
The averaged PAR intensity in open plots was 1034 ± 87 μmol m−2 s−1 (92 ± 3% full sunlight), whereas it was 521 ± 64 μmol m−2 s−1 (43 ± 0.9% full sunlight) in shaded plots. This PAR pattern was not stable over the 24 mo (Figure 2), due to the loss of canopy foliage (including adult trees of L. microphyllum) in the dry season and canopy closure due to leaf production in the wet season. Therefore, the rainfall received at our study site was closely related to the PAR pattern (Figure 3), so the PAR received on shaded and open plots was significantly different (t = −3.9, P < 0.001). The PAR intensity under the 1.5-mm wire mesh (predator exclusion plots) was 45 ± 2% greater than the mean intensity beneath the canopies of shrubs and adult trees at noon.

Figure 2. Seasonal variation in photosynthetically active radiation (PAR) (mean ± SD) in shaded (black circles) and open (white circles) areas during a 2-y study in El Cimatario National Park, Queretaro, Mexico.

Figure 3. Annual rainfall distribution in the study site over a 2-y period in El Cimatario National Park area.
Experiment 1: in situ seed germination
The maximum seed germination under natural conditions occurred during the first 15 d after sowing, and it reached the highest percentage on plots exposed to shade and protected from predators for all aspects (north: F3,20 = 251, P < 0.00001, mean = 97.7 ± 1.2%; south: F3,20, = 557, P < 0.00001, mean = 95.2 ± 2.1%; east: F3,20 =107, P < 0.00001, mean = 95.4 ± 2.6%; west: F3,20 = 189, P < 0.00001, mean = 95.7 ± 1.6%). Although the observed germination percentage on shaded plots (under canopies) was high, there were no significant differences among aspects (F3,92 = 0.07, P = 0.9). In contrast, the lowest germination percentage occurred on unshaded and non-exclusion plots (mean 14.9 ± 3.6%), because predation occurred before germination, so seeds in open spaces had little probability of producing seedlings. On average, 96% of non-predated seeds (excluded) on shaded plots germinated in the field, and seed predation proportions in non-exclusion plots were similar in the open and in shade (t = −0.82, P = 0.4).
The topographic aspect of the plots did not significantly affect seed germination, at least during the first 21 d (Table 1). In contrast, predation (high germination of predator excluded seeds and low germination of non-exclusion seeds), understorey light (scarce seed germination in open space and high seed germination in shade) and their interaction, were highly significant explanatory variables for successful seed germination (Table 1), and this pattern of significance was similar over 3 wk of natural seed germination. The deviance of the models was 10.4%, 10.7% and 10.9% for the germination analyses applied at 7, 14 and 21 d, respectively; after 21 d, the first seedlings were established.
Table 1. Log-linear analysis of Lysiloma microphyllum germination success under experimental conditions in a secondary TDF in Bajio Queretano, within El Cimatario National Park. ** P < 0.0000001, * P < 0.00001, ns = not significant.

Experiment 1: seedling establishment and seedling survival
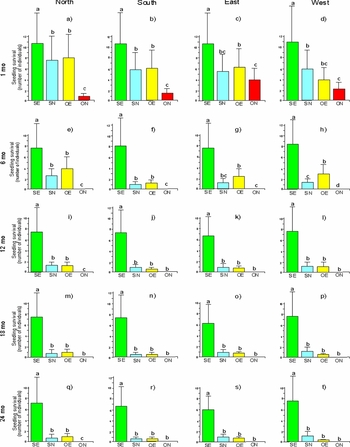
Similar to seed germination, early seedling establishment was influenced by predation, understorey light and their interaction, but the aspect was not significant. However, after the first month, aspect significantly influenced seedling survival (Table 2). After the first month, shaded and predator-exclusion treatments had significantly higher seedling establishment and survival (Figure 4a–d).
Table 2. Log-linear analysis of Lysiloma microphyllum seedling survival under experimental conditions in a secondary TDF in El Cimatario National Park. All factors included in the model are presented. * P < 0.00001, * * P < 0.0000001, ns = not significant.


Figure 4. Mean ± SE number of living Lysiloma microphyllum seedlings in experiment 1 (seed-to-seedling transition) in El Cimatario National Park, exposed to the following treatments: SE, shade-exclusion; SN, shade-non-exclusion; OE, open-exclusion; and ON, open-non-exclusion. Seedling survival was calculated by combining aspect and time at 1 mo (a, b, c, d), 6 mo (e, f, g, h), 12 mo (i, j, k, l), 18 mo (m, n, o, p) and 24 mo (q, r, s, t). Different letters above the bars indicate significant differences (Tukey's test, P < 0.05).
Throughout this experiment, predation and understorey light as independent factors were highly significant for explaining seedling survival; similarly, the predation–understorey light interaction was also highly significant (Table 2).Therefore, the treatments that were shaded and excluded predators had higher seedling survival, and this pattern remained significant throughout the 2-y study for seedlings germinated in situ (Figure 4e–t). Deviance values for the goodness of fit of models were 11.2, 12.9, 12.8, 10.5 and 11.4 for 1, 6, 12, 18 and 24 mo, respectively.
Survival function S(t) and density function f(t) in experiment 1
The estimated survival function, S(t), for the predator exclusion and shaded treatments showed a significant increase in seedling survival (Figure 5a–d; LR = 19.6, df = 3, P < 0.0001), and large differences were found between exclusion-shaded and non-exclusion-open plots (LR = 10.8, df =1, P < 0.001). Smaller differences were found between exclusion-open and non-exclusion-shaded (LR = 3.6, df =1, P < 0.05), and no seedlings survived in non-exclusion-open plots. The probability density function, f(t), for all topographic aspects (Figure 5e–h) predicted a high failure rate at the beginning of the study (months 3–4), which was especially high in the non-exclusion plots. Additionally, the function predicted a decreasing failure rate over time and two episodes of greater failure in the middle of the study (months 12–13, higher in predator exclusion-shaded plots), while at the end of the study, it predicted a slight decrease in failure (months 23–24, for treatments with final survivors, such as the exclusion-shaded plots). Non-exclusion-open plots had the highest failure rates (no survivors), which was not significantly different from that of the non-exclusion-shaded plots (t = −0.78, P = 0.3).

Figure 5. Lysiloma microphyllum seedling survival probabilities in experiment 1 in El Cimatario National Park, in response to four microhabitat conditions: SE, shade-exclusion; SN, shade-non-exclusion; OE, open-exclusion; and ON, open-non-exclusion. Survival probability is shown for each aspect (north, south, east and west), according to the distribution function (a, b, c, d) and the density function (e, f, g, h). Survival distribution functions were calculated using the life-tables procedure.
Experiment 2: transplanted seedling survival
Topographic aspect did not significantly affect transplanted seedlings at any time during the study, which was a remarkably different outcome to that of experiment 1; but, similar to experiment 1, the predation–understorey light interaction was significant only during the first 6 mo (Table 2). The independent effects of the understorey light and predation factors during the 2-y study were clear and significantly high; the best survivorship of transplanted seedlings was observed in the shaded and predator-exclusion plots, while the mortality of transplanted seedlings increased in open plots (Figure 6a–t).

Figure 6. Mean ± SE number of living (transplanted) Lysiloma microphyllum seedlings in experiment 2 in El Cimatario National Park, exposed to the following treatments: SE, shade-exclusion; SN, shade-non-exclusion; OE, open-exclusion; and ON, open-non-exclusion. Seedling survival was calculated by combining aspect and time at 1 mo (a, b, c, d), 6 mo (e, f, g, h), 12 mo (i, j, k, l), 18 mo (m, n, o, p) and 24 mo (q, r, s, t). Different letters above the bars indicate significant differences (Tukey's test, P < 0.05).
The response variable (survival) was well explained by predation and understorey light based on the goodness of fit of the models (deviance: 13.4, 15.8, 14.5, 14.3 and 13.0 for 1, 6, 12, 18 and 24 mo, respectively).
Survival function, S(t), and density function f(t) in experiment 2
The survival of transplanted seedlings was higher than that of individuals germinated in situ (experiment 1), and the estimated survival functions, S(t), for the four aspects were significantly different (Figure 7a–d; LR = 24.2, df = 3, P < 0.0001). Seedling survival differed significantly between exclusion-shaded plots and non-exclusion plots in the open (LR = 12.5, df = 1, P < 0.0001), but there were no significant differences between the other treatments (LR = 0.33, df = 1, P = 0.56).

Figure 7. Survival probabilities of transplanted Lysiloma microphyllum seedlings in experiment 2 in El Cimatario National Park, in response to the following treatments: SE, shade-exclusion; SN, shade-non-exclusion; OE, open-exclusion; and ON, open-non-exclusion. Survival probabilities are shown for each aspect (north, south, east and west), according to the distribution function (a, b, c, d) and the density function (e, f, g, h).
The density function curve, f(t), followed a pattern of high mortality at the beginning of the study, particularly in the non-exclusion plots (Figure 7e–h). Additionally, f(t) indicated low failure in months 11–13, mainly in the predator exclusion-open plots, and relatively low mortality at the end of the experiment. Seedling survival was higher on predator exclusion-shaded plots.
Seedling height distribution
The mean height of L. microphyllum seedlings at the age of transplantation in experiment 2 (3 mo) was 7.4 ± 1.6 cm, and at the end of the experiments (24 mo) it was 13.7 ± 3.4 cm. There were relatively large ranges among the final surviving seedlings of experiments 1 and 2; 60% exceeded the mean height (7.2 ± 2.5 cm) by 1SD.
DISCUSSION
Germination in natural conditions
The high level of seed germination displayed by L. microphyllum in shaded and predator-excluded microsites, compared with unshaded and non-exclusion sites, suggests that this process is influenced by the combined effect of two physical factors, soil moisture and light, as previously observed for other TDF species (González-Rivas et al. Reference GONZÁLEZ-RIVAS, TIGABU, CASTRO-MARÍN and ODÉN2009, Khurana & Singh Reference KHURANA and SINGH2001, Vargas et al. Reference VARGAS, WERDEN and POWERS2015). Lysiloma microphyllum seeds reached the maximum germination percentage (up to 97%) in shaded microsites, where the soil moisture content and water availability are retained for a longer period (Khurana & Singh Reference KHURANA and SINGH2004), so the probability of seed hydration is higher. In contrast, the soil on open sites tends to dry out more quickly. Spatially, the shaded microsites that favour seed germination could be scarce in TDF secondary communities, because most are largely dependent on seasonality; the wet season provides greater moisture availability for germination (Bullock et al. Reference BULLOCK, MOONEY and MEDINA1995). However, the rainy season is temporally very short (4.5 mo, with a dry period of more than 40 d, locally called ‘canícula’); and rainfall is highly variable among months. The intraseasonal variation in rainfall is closely related to soil water conditions and canopy shade in tropical dry-forest ecosystems, which can be affected by either light intensity or plant growth. For example, during the rainy season in the neotropical dry forest of Chamela, Mexico, above-ground biomass develops at different daily PAR values, which range between 35 mol m−2 d−1 at the top of the canopy and 3 mol m−2 d−1 at 20 cm above the soil; during the dry season, these values increase to 58 mol m−2 d−1 and 30 mol m−2 d−1, respectively (Barradas Reference BARRADAS1991).
The levels of photosynthetically active radiation (PAR) recorded in the open and shaded spaces in this study are comparable to the light levels in the treatments. However, the lack of a significant effect of topographic aspect on seed germination suggests that this factor is of secondary importance in the process, and that its main influence occurs due to the different levels of incident radiation in the shaded and open plots.
We observed a strong interaction between the factors of predation and radiation (understorey light). The germination of seeds excluded from predators increased in comparison to non-excluded seeds under canopy, because there were consistently more seeds in the exclusion treatments. In turn, understorey light in open spaces significantly decreased germination in open plots (predation × understorey light interaction in Table 1). This was probably due to (1) the negative effect of sunlight on soil water conditions, because direct radiation is the main factor driving soil moisture evaporation (Huante & Rincón Reference HUANTE and RINCÓN1998), which lowered seed germination in high light; and (2) seeds being removed before germination, which indicates that seeds were affected by predation rather than germination failure. Lysiloma microphyllum seeds are primarily taken by rodents, harvester ants, other arthropods and birds (pers. obs.), so the removal of L. microphyllum seeds is a greater cause of propagule loss before germination than the failure of germinated seeds (mortality after germination). Non-exclusion plots had lower survival; the percentages of predated seedlings were higher in the open (100% in experiments 1 and 2) than under the canopy (96% and 89% for experiments 1 and 2, respectively). Similar patterns have been observed in other species in secondary TDF in Mexico, which have been explained by the tree canopies or the understorey vegetation (nurse-plant phenomenon) protecting seedlings against predation. This factor plays an important role in the population demographics of TDF species (Vieira & Scariot Reference VIEIRA and SCARIOT2006).
Other studies of TDF species have found germination percentages above 80% under watering treatments (Blain & Kellman Reference BLAIN and KELLMAN1991, in Veracruz, Mexico) or 10% with shade treatments (Ray & Brown Reference RAY and BROWN1995, in the US Virgin Islands). These percentages increase due to shading in secondary tropical forests in Costa Rica (Gerhardt Reference GERHARDT1996b) and significantly increase with water supplementation and shading (McLaren & McDonald Reference McLAREN and McDONALD2003b, in Jamaica).
Seedling survival
The pattern of seedling establishment and survival in experiment 1 suggests that at the beginning of the study (months 1–5), the transition from seed to seedling was influenced by the interaction between predation and understorey light but was independent of aspect. Later (months 6–24), this factor had a positive influence, but predation and light were the factors that largely determined seedling survival.
The exclusion of predators had a large and positive impact on survival; in all cases this treatment increased seedling survival. It is likely that predation is key to the success of initial establishment; and it seems to be independent of microhabitat, because seed removal by granivory in non-exclusion plots caused high mortality (98.5% in open spaces and 87% in shaded spaces) during the first few days. It should be noted that the exclosures covered by the 1-mm wire mesh used to protect seeds and seedlings could have reduced evaporation rates, due to shading, but this shade was is not equivalent (almost 50% higher PAR) to the shade provided by canopies. Likewise, radiation (understorey light and full sunlight) played an important role throughout the experiment, the survival curves in the exclusion-shaded treatments reached the maximum final survival (26% and 61% for experiments 1 and 2, respectively), which suggests that the initial establishment of seedlings is shade-dependent (light-intolerant). The intensity of PAR received by shaded plots (in this study: mean 521 ± 64 μmol m−2 s−1) is within the levels of a low-light environment, according to Rincón & Huante (Reference RINCÓN and HUANTE1993). In experiment 1, the mortality peaks (density function) are apparent in the beginning, middle and end phases of the study (months 1–6, 12–14 and 23–24, respectively), so these conformed to a type-III pattern, in which the initial mortality is high and tends to decrease with time, mainly on exclusion-open, non-exclusion-shaded and non-exclusion-open plots. However high mortality was observed in exclusion-shaded plots, in all cases. These three peaks of seedling mortality (a pattern observed in all experimental treatments) can be attributed to seasonal drought, because they indicate the probability of maximum survival for the remaining individuals in a time interval f(t). For example, from June to July (months 12 and 13 in the figures, when the wet season starts), this interval is affected by the accumulated mortality of individuals that previously failed (died) during the dry-season drought (November–May). Therefore, these periods of time accumulate the greatest mortality of individuals. Signs of water stress were common in individuals established in open spaces, so seedling desiccation could be a likely cause of death, and is, in fact, considered an important cause of mortality in TDF (Vieira et al. Reference VIEIRA, LIMA, SEVILHA and SCARIOT2008). The causes of death were probably the same in all experimental treatments, as other possible causes of mortality, such as fungal diseases, were not observed, but seedlings under natural conditions are eaten or damaged by a great variety of insects (pers. obs.). Only the exclusion treatments under the canopy in experiments 1 and 2 resulted in individuals surviving for at least 5 y after the end of the field experiment (pers. obs.).
The survival of transplanted seedlings (experiment 2) was consistently higher than those in experiment 1, suggesting that the early acquisition of sapling or adult traits, which is typical of a drought-tolerant tree (Poorter & Markesteijn Reference POORTER and MARKESTEIJN2008), helps the plants cope with damaging biotic and abiotic factors. The apparent major physiological independence and drought-tolerance of transplanted seedlings were also exhibited in their lower mortality during the dry season in the first year.
The role of shade and water – or their combined effect – in increasing the survival of seedlings in TDF has been reported previously. By comparing the effects of supplemental water and rainfall in shaded plots, Blain & Kellman (Reference BLAIN and KELLMAN1991) and Gerhardt (Reference GERHARDT1996a, Reference GERHARDT1998) found that environments with high moisture availability and low light levels allowed the successful establishment of seedlings, and Ray & Brown (Reference RAY and BROWN1995) observed, in addition to the significant effects of shade on seedling survival, that seedling mortality increased as rainfall decreased. In a successional chronosequence of TDF in Southern Mexico, Hammond (Reference HAMMOND1995) reported that seedling survival was related to the ability of the habitat to retain moisture. Rainfall seasonality is an additional factor that affects regeneration in terms of the survival of seeds and seedlings (McLaren & McDonald Reference McLAREN and McDONALD2003b), so inter- and intra-regional variation in rainfall in the TDF of semi-arid regions such as the Mexican Bajio could have particular implications for seed ecology. For example, droughts during the wet season or a prolonged drought during the dry season are important causes of seedling mortality (Marod et al. Reference MAROD, KUTINTARA, TANAKA and NAKASHIZUKA2004) due to the gradual reduction in the photosynthesis and stomatal conductance of seedlings over time (Slot & Poorter Reference SLOT and POORTER2007). Other studies have demonstrated the significance of shade provided by nurse plants to the survival of the seedlings of late-successional trees in Mexican TDF (Sánchez-Velasquez et al. Reference SÁNCHEZ-VELÁSQUEZ, QUINTERO-GRADILLAB, ARAGÓN-CRUZ and PINEDA-LÓPEZ2004), and in TDF, the facilitative effect of nurse plants seems to be less intense if stress conditions are reduced (Miranda-Jácome et al. Reference MIRANDA-JÁCOME, MONTAÑA and FORNONI2013). The shading function of the canopy can supplement the composition and structure of shade-tolerant successional species (Álvarez-Aquino & Williams-Linera Reference ÁLVAREZ-AQUINO and WILLIAMS-LINERA2012, Griscom & Ashton Reference GRISCOM and ASHTON2011), because established canopies help to mitigate the environmental conditions facilitating regeneration by seed (de Souza Gomez & Scariot Reference DE SOUZA GOMES and SCARIOT2014, Lévesque et al. Reference LÉVESQUE, MCLAREN and MCDONALD2011), although differences between the understorey and overstorey seem to be species-specific (González-Rivas et al. Reference GONZÁLEZ-RIVAS, TIGABU, CASTRO-MARÍN and ODÉN2009). Lysiloma microphyllum reproduces from seeds, and it does not propagate vegetatively with root suckers. Therefore, our findings (shade, protection from predators) have predictive value for seed and establishment success.
According to the Janzen–Connell hypothesis, the high levels of seedling predation found in this study suggest that there could be an effect of planting density because density-dependent effects would be stronger in wet forests than in dry forests (Webb & Peart Reference WEBB and PEART1999). Furthermore, it is thought that negative density-dependence (e.g. seedling mortality or scarce recruitment of conspecific individuals at high densities due to herbivory or pathogens) mainly occurs in tropical wet forests (Comita et al. Reference COMITA, QUEENBOROUGH, MURPHY, ECK, XU, KRISHNADAS, BECKMAN and ZHU2014), while positive density-dependence (e.g. conspecific density facilitates seedling survival) has been associated with dry forests (Jia et al. Reference JIA, DAI, SHEN, ZHANG and WANG2011). The seedlings of tropical rain forests species in moist sites with low light suffer high mortality due to pathogens and predators; in contrast, in a dry forest, there is probably a positive density effect due to higher abiotic stress (water-limited environment) (Goodale et al. Reference GOODALE, BERLYN, GREGOIRE, TENNAKOON and ASHTON2014). Empirical studies have found that, contrary to predictions, adult conspecifics have stronger effects on seedling mortality in dry (Palamanui, Hawaii, 835 mm of annual rainfall) compared to wet forests (Inman-Narahari et al. Reference INMAN-NARAHARI, OSTERTAG, HUBBELL, GIARDINA, CORDELL and SACK2016), but in certain cases, damage (herbivory) is not correlated with density in tropical rain forests (Brenes-Arguedas Reference BRENES-ARGUEDAS2012). If negative density-dependence increases with rainfall (Comita et al. Reference COMITA, QUEENBOROUGH, MURPHY, ECK, XU, KRISHNADAS, BECKMAN and ZHU2014), then positive effects would be expected in dry forests, and we found two works addressing density-dependence in the seedling stage in this ecosystem. In Mudumalai, India (1230 mm of mean annual rainfall), negative density-dependence was observed in the recruitment of many species (John et al. Reference JOHN, DATTARAJA, SURESH and SUKUMAR2002), but it was also associated with the incidence of ground fires during the dry season. In Guanacaste, Costa Rica (2076 mm of mean annual rainfall), Sullivan (Reference SULLIVAN2003) found that herbivory by Cromarcha stroudagnesia (a sapling-specialist shoot-borer) increases with the number of conspecific Tabebuia ochracea saplings. Our Bajio Queretano site received 549 mm of annual rainfall (a drier and cooler site), but seedling predation by herbivores on conspecifics at low densities was very high. Therefore, these reports suggest that there are strong negative density-dependence effects in TDF. Seedling herbivory by arthropods, especially by leaf-cutting ants, is the principal cause of recruitment failure on predator-free forested islands in TDF from Lago Guri, Venezuela (López & Terborgh Reference LÓPEZ and TERBORGH2007), and excessive herbivory by grasshoppers has also been observed, even if the planting density of each experimental unit is a single individual (Hernández-Oria et al. unpubl. data).
In conclusion, our approach to the analysis of germination, establishment and survival of L. microphyllum seedlings allowed us to determine that (1) these processes are successful when they occur in shaded areas rather than open spaces; (2) topographic aspect plays a role after the transition from seed to seedling, a critical stage in early recruitment, although the seedlings tested in the field after this demographic transition were aspect-independent; (3) the roles of biotic (predation) and abiotic (radiation) factors during the process are highly relevant; and (4) shade conditions create a suitable microhabitat for establishment and early development. These findings may help establish principles for the regeneration of L. microphyllum in TDF secondary communities and provide the basis for its restoration and management.
ACKNOWLEDGEMENTS
The work is dedicated to the memory of Richard I. Yeaton Hawkins. The first author gratefully acknowledges the financial support (PhD scholarship No. 40284) of the Consejo Nacional de Ciencia y Tecnología (Mexico) as well as the facilities provided by the staff of El Cimatario National Park, Queretaro, Mexico. We are also grateful to two anonymous reviewers for their valuable comments on a draft version of the manuscript. This research was part of the PhD thesis of the first author within the Programa de Doctorado en Ciencias Biomédicas de la Universidad Nacional Autónoma de México.