INTRODUCTION
The elephant (Loxodonta africana Blumenbach) and giraffe (Giraffa camelopardalis Linnaeus) are two important large browsers of African savanna woodlands. Elephants break branches and stems, strip bark and uproot mature trees, at times causing considerable damage (Beuchner & Dawkins Reference BEUCHNER and DAWKINS1961, Croze Reference CROZE1974a, Lamprey et al. Reference LAMPREY, GLOVER, TURNER and BELL1967). In contrast, giraffes strip leaves and unlignified shoots from stems, the main impact of which is to retard plant growth (Birkett Reference BIRKETT2002, Birkett & Stevens-Wood Reference BIRKETT and STEVENS-WOOD2005, Norton-Griffiths Reference NORTON-GRIFFITHS, Sinclair and Norton-Griffiths1979, Pellew Reference PELLEW1983a, Ruess & Halter Reference RUESS and HALTER1990). Pellew's seminal studies in Serengeti National Park, Tanzania, in the 1970s contributed greatly to our understanding of giraffe browsing behaviour and the potential impacts of elephant, giraffe and fire on the Serengeti woodlands (Pellew Reference PELLEW1981, Reference PELLEW1983a, Reference PELLEW and Owen-Smithb, Reference PELLEWc, Reference PELLEWd, Reference PELLEW1984a, Reference PELLEWb). In the current study, we revisit the locations of Pellew's studies to determine the extent of woodland change in Seronera, central Serengeti, and whether or not it is qualitatively consistent with his predictions.
In particular, we explore and develop the hypothesis that giraffe browsing has contributed to the suppression of palatable woody plant species, such that the giraffe may have reduced the quality of its own food supply. Previous work suggests that large-herbivore browsing activity can increase the relative dominance of chemically defended, unpalatable species relative to palatable species (Augustine & McNaughton Reference AUGUSTINE and MCNAUGHTON1998). For example, by browsing on more palatable competitors, moose (Alces alces) on Isle Royale, Michigan, may have led to increases in the relative abundance of white spruce (Picea glauca) (McInnes et al. Reference MCINNES, NAIMAN, PASTOR and COHEN1992), and, in Burkina Faso, heavy elephant browsing suppressed palatable shrub species to the advantage of unpalatable species (Jachmann & Croes Reference JACHMANN and CROES1991).
First, we evaluate changes in the structure and composition of the Seronera woodlands and consider the possible impact of elephant and giraffe browsing. We test the hypothesis that unpalatable species have increased in relative dominance while preferred species have decreased. We also consider whether selective giraffe browsing could drive an increase in the dominance of the unpalatable species Acacia robusta Burch. (synonym: Acacia clavigera E. Mey) relative to the more palatable Acacia tortilis (Forssk.) Hayne (Pellew Reference PELLEW1981). Second, we test the hypothesis that the quantity and quality of the giraffe's food supply have recently changed, by comparing data from 2009 with 1978 (Pellew Reference PELLEW1983c). Finally, we qualitatively assess the predictive power of Pellew's woodland dynamics model (Pellew Reference PELLEW1983a), which predicts the number of mature A. tortilis trees in the Seronera woodlands as a function of elephant and giraffe impact and fire frequency.
STUDY SITE
The Serengeti ecosystem covers 25000 km2 of northern Tanzania and southern Kenya, loosely bounding the path of the migrating wildebeest (Connochaetes taurinus Burchell) (Sinclair Reference SINCLAIR, Sinclair and Norton-Griffiths1979a). The area is subject to seasonal rains with a June–October dry season. Annual rainfall is highest in the north-west (>1000 mm y−1) and decreases toward the south-eastern plains (500 mm y−1) (Sinclair Reference SINCLAIR, Sinclair and Norton-Griffiths1979b).
Fire and herbivores are critical drivers of woodland-grassland dynamics in the Serengeti (Dublin Reference DUBLIN, Sinclair and Arcese1995, Dublin et al. Reference DUBLIN, SINCLAIR and MCGLADE1990, Norton-Griffiths Reference NORTON-GRIFFITHS, Sinclair and Norton-Griffiths1979, Pellew Reference PELLEW1983a, Shaw et al. Reference SHAW, SINCLAIR, METZGER, NKWABI, MDUMA and BAKER2010, Sinclair et al. Reference SINCLAIR, HOPCRAFT, OLFF, MDUMA, GALVIN, SHARAM, Sinclair, Packer, Mduma and Fryxell2008). For example, following the elimination of the rinderpest virus in the early 1960s, wildebeest numbers climbed from <300000 to over one million in just over a decade (Sinclair & Norton-Griffiths Reference SINCLAIR and NORTON-GRIFFITHS1982). Resultant heavy grazing reduced fires, in turn fostering the regeneration of Acacia woodland in the 1970s and 1980s (Sinclair et al. Reference SINCLAIR, HOPCRAFT, OLFF, MDUMA, GALVIN, SHARAM, Sinclair, Packer, Mduma and Fryxell2008).
This study focuses on an area of c. 120 km2 around Seronera, central Serengeti, roughly bounded by the Seronera and Sangere Rivers to the west and east and the Wandamu River to the south (Croze Reference CROZE1974a, Pellew Reference PELLEW1983c, Ruess & Halter Reference RUESS and HALTER1990). Rainfall averages ~800 mm y−1 (Pellew Reference PELLEW1983c). We surveyed the woodlands in 2009 and compare the results with prior surveys completed in 1971 (Croze Reference CROZE1974a), 1978 (Pellew Reference PELLEW1981, Reference PELLEW1983a, Reference PELLEW and Owen-Smithb, Reference PELLEWc) and in 1982 (Ruess & Halter Reference RUESS and HALTER1990). To draw parallels with these earlier studies, we focus on the possible roles of the elephant and giraffe in driving local woodland change.
Elephants and giraffes utilize most of the principal tree species of Seronera, such as Acacia tortilis, roughly in proportion to their availability (Croze Reference CROZE1974a, Pellew Reference PELLEW1984b, Ruess & Halter Reference RUESS and HALTER1990). Notably, however, they avoid two relatively common woody species: (1) Acacia robusta, a medium-sized tree with lush foliage and relatively short stipular spines and (2) Commiphora trothae Engl., a small tree with pungent foliage. The giraffe avoids A. robusta year-round and consumes C. trothae only during its short foliated phase (Pellew Reference PELLEW1981, Reference PELLEW1984b). The elephant consumes these plants infrequently, stripping the bark of A. robusta and consuming the bark and roots of C. trothae (Croze Reference CROZE1974a, Reference CROZEb; Lamprey et al. Reference LAMPREY, GLOVER, TURNER and BELL1967, Ruess & Halter Reference RUESS and HALTER1990). In contrast, preferred species, particularly Acacia senegal Willd. and Acacia xanthophloea Benth. have been subject to heavy browsing pressure, especially from the elephant (Croze Reference CROZE1974a, Reference CROZEb, Pellew Reference PELLEW1984b, Ruess & Halter Reference RUESS and HALTER1990).
Elephant and giraffe abundance in Serengeti have fluctuated substantially over the last several decades. The earliest description of the elephant in the Serengeti is from 1955 (Lamprey et al. Reference LAMPREY, GLOVER, TURNER and BELL1967) but thereafter density rapidly increased, reaching ~0.2 elephants km−2 in the early 1970s (Norton-Griffiths Reference NORTON-GRIFFITHS, Sinclair and Norton-Griffiths1979). In central Serengeti, bull elephants were first reported in 1963–1964 (Croze Reference CROZE1974b, Lamprey et al. Reference LAMPREY, GLOVER, TURNER and BELL1967) and cow-calf groups appeared several years later (A.R.E. Sinclair, pers. comm.). From 1975–1977, elephant density (mean ± SD) in the Seronera woodlands was 0.2 ± 0.16 elephants km−2 (Pellew Reference PELLEW1983a). From 1977–1986, poaching drastically reduced the elephant population inside the Park to <0.04 elephants km−2 (Dublin Reference DUBLIN, Sinclair and Arcese1995). However, the population subsequently recovered, approaching 1970s densities (Sinclair et al. Reference SINCLAIR, HOPCRAFT, OLFF, MDUMA, GALVIN, SHARAM, Sinclair, Packer, Mduma and Fryxell2008). Early studies of elephant-tree interactions in Seronera focused on the rapid destruction of mature trees by bulls (Croze Reference CROZE1974b, Lamprey et al. Reference LAMPREY, GLOVER, TURNER and BELL1967).
Giraffe density has likewise fluctuated. In the mid-1970s, there were 1.47–2.64 giraffes km−2, with the population increasing at a rate of 5–6% y−1 in response to the concurrent increase of young Acacia trees (Pellew Reference PELLEW1983d). However, giraffe numbers have since declined, possibly beginning in the 1980s. In Seronera, giraffe density (mean ± SE) fell from 1.47 ± 0.27 giraffes km−2 in 1975–1976 (Pellew Reference PELLEW1983d) to 0.28 ± 0.03 giraffes km−2 by 2008–2010 (Strauss et al., unpubl. data).
Small and medium-sized mammals also feed on woody vegetation in Seronera, including dikdik (Rynchotragus (Madoqua) kirkii Günther), eland (Taurotragus oryx Pallas), impala (Aepyceros melampus Lichtenstein), Grant's gazelle (Gazella granti Brooke) and Thomson's gazelle (G. thomsoni Günther), however, we do not consider their browsing impacts.
METHODS
Measurements of woodland structure and composition
To enable direct comparison with previous studies, we sampled the Seronera woodlands using the point-centred quarter method (Croze Reference CROZE1974a, Cottam & Curtis Reference COTTAM and CURTIS1956, Heyting Reference HEYTING1968), the suitability of which has been described elsewhere (Croze Reference CROZE1974a, Pellew Reference PELLEW1981, Reference PELLEW1983c; Ruess & Halter Reference RUESS and HALTER1990). Following Pellew (Reference PELLEW1983c), we divided the woodlands into four types based on vegetation and position in the drainage catena: (1) ridge-top and upper-slope woodland, (2) mid-slope woodland, (3) seasonal drainage woodland (‘korongo woodland’ in Pellew Reference PELLEW1983c) (includes stream-beds and banks) and (4) riverine woodland (includes perennial watercourses and banks). Areas of open grassland (<1% canopy cover) were excluded. Within each woodland type, we sited line transects of fixed direction to overlap areas surveyed in 1978 (Pellew Reference PELLEW1983c, R. Pellew, pers. comm.) and close to areas sampled in 1971 by Croze (Reference CROZE1974a) and in 1982 by Ruess & Halter (Reference RUESS and HALTER1990).
Along each transect, sample points were placed at even intervals of 40–50 m for ridge-top and mid-slope transects and 25 m for the more species-rich riverine and seasonal drainage areas. At each sample point, we placed a cross on the ground, dividing the area into four quarters, and located the tree or shrub in each quarter closest to the centre of the cross using a Haglöf DME measuring device. We included all woody perennials >0.5 m tall. Thus, four plants were evaluated at each sample point, recording species name, distance from the centre of the cross, total plant height, canopy diameter (the mean of two measurements taken at right angles) and canopy depth. The canopy diameter and depth measurements excluded canopy above 5.75 m, which is outside the maximum reach of an adult male giraffe.
Distance measurements were used to estimate woody plant density (plants ha−1) for each woodland type and the relative density of each species, using statistics defined in Croze (Reference CROZE1974a). We compared plant density, canopy cover (%) and volume (m3 ha−1), and species composition with Pellew's 1978 survey (Pellew Reference PELLEW1983c). We compared tree population structures with the 1971, 1978, and 1982 surveys (Croze Reference CROZE1974a, Pellew Reference PELLEW1983c, Ruess & Halter Reference RUESS and HALTER1990).
Elephant and giraffe usage of Acacia robusta
To estimate current elephant and giraffe usage of common Acacia species, we assessed elephant and giraffe browsing/damage to A. robusta, A. tortilis and A. drepanolobium Harms ex Sjöstedt on trees with a canopy bottom >1.5 m from the ground, which excludes browse damage from smaller species such as impala. Browsing assessments were performed simultaneously with other routine tree measurements during the point-centred quarter survey. In addition, we visited nine even-aged stands of A. robusta and five even-aged stands of A. drepanolobium in several locations across the Serengeti. At each location we set up circular plots with a 10-m radius and recorded browsing/damage. Surveys were completed in the dry season when the majority of trees were in a deciduous phase or producing few shoots. Thus, we recorded evidence of cumulative browsing over several seasons. We sampled only living trees, which underestimates the impact of elephants. Even-aged stands of A. robusta are extremely dense, often covering large areas. We established plots both on the inside of stands (n = 4) and on stand edges (n = 5). We calculated the proportion of trees of each species showing evidence of elephant or giraffe browsing but did not assess the severity of browsing. Elephant usage includes browsing of leafy material, bark stripping and branch breaking.
RESULTS
Summary of changes in Seronera woodland structure and composition
Mostly, the same species were encountered in the 1978 and 2009 surveys, although Hibiscus spp. and Aspilia mossambicensis (Oliv.) Wild – small woody perennials – were encountered frequently in the riverine and seasonal drainage areas in 2009 but were unreported in 1978, despite being present elsewhere in Serengeti in the 1970s.
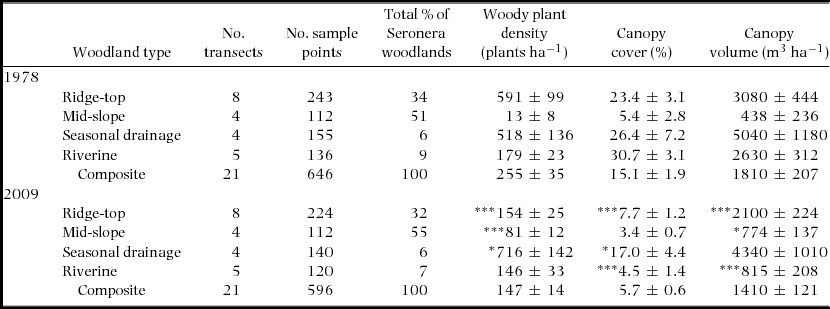
Woody plant density (mean ± SD) in the Seronera woodlands has decreased from 255 ± 35 plants ha−1 to 147 ± 14 plants ha−1 (Table 1), largely due to a significant decrease in plant density in the ridge-top woodland type, which supported extremely dense thickets of regenerating Acacia seedlings in 1978. Across all woodland habitats, significant increases in plant density in the seasonal drainage and mid-slope woodlands and an increase in the extent of mid-slope woodland have been insufficient to replace the loss of density in ridge-top areas.
Table 1. A comparison of the mean structure of the Seronera woodlands, Serengeti in 1978 (Pellew Reference PELLEW1983c) and 2009, with 95% confidence limits. Woody plants <0.5 m in height were excluded. Canopy measurements include only foliage/stems below 5.75 m, the maximum reach of an adult male giraffe. To estimate per cent total area of each woodland type in 2009, we compared Pellew's woodland map to recent aerial photographs and high-resolution satellite imagery (Google Maps for 2013), using ArcMap10. P-values test the null hypothesis that woodland structure in 2009 is equivalent to 1978. *P < 0.05, **P < 0.01, ***P < 0.001.

Canopy volume has remained comparable to 1978 values, with the exception of the riverine sample area, which has experienced especially heavy elephant impact (Table 1). The significant decrease in canopy volume in this area reflects a loss of regenerating and mature A. xanthophloea trees and an increase in the relative density of smaller shrub species.
Species composition has shifted throughout the woodlands (Figure 1, Appendix 1). For example, in the seasonal drainage woodland type, smaller woody shrubs are now dominant to species of Acacia. In the riverine sample area, located near Downey's Dam on the Seronera River, elephants have decimated the A. xanthophloea population, the previous riverine dominant.
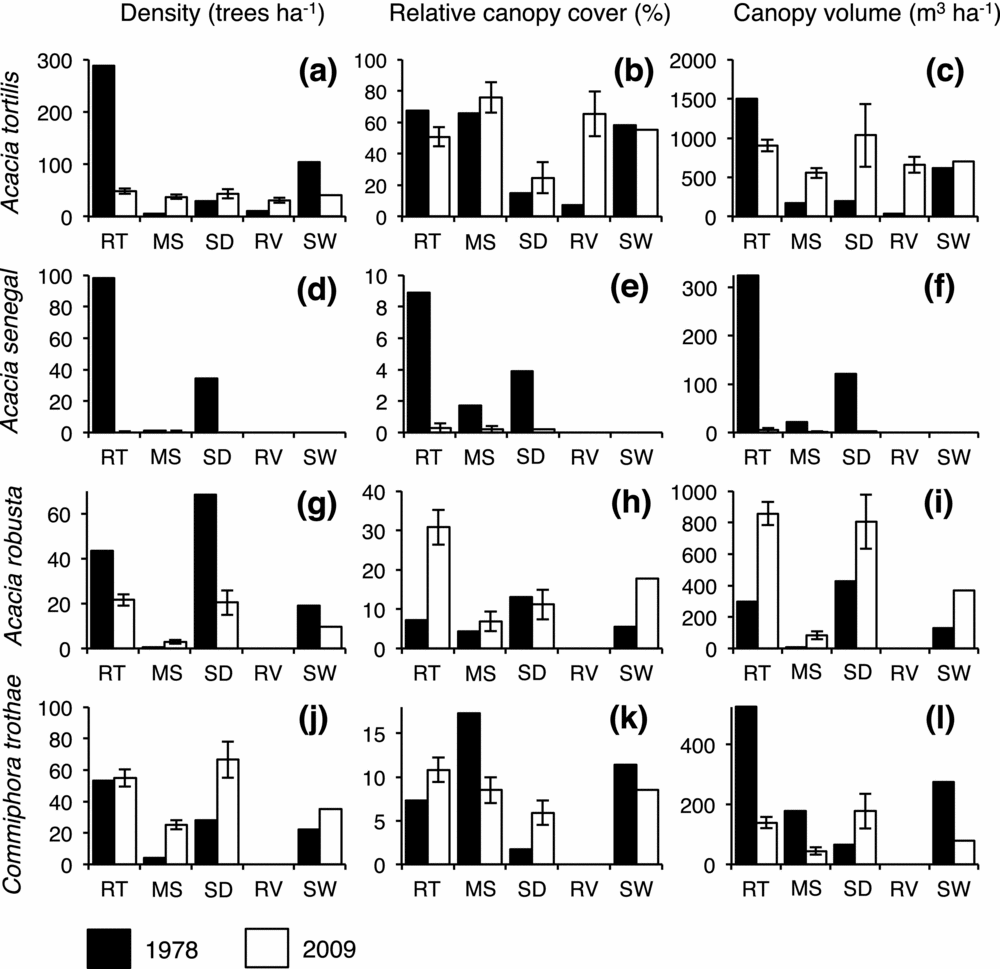
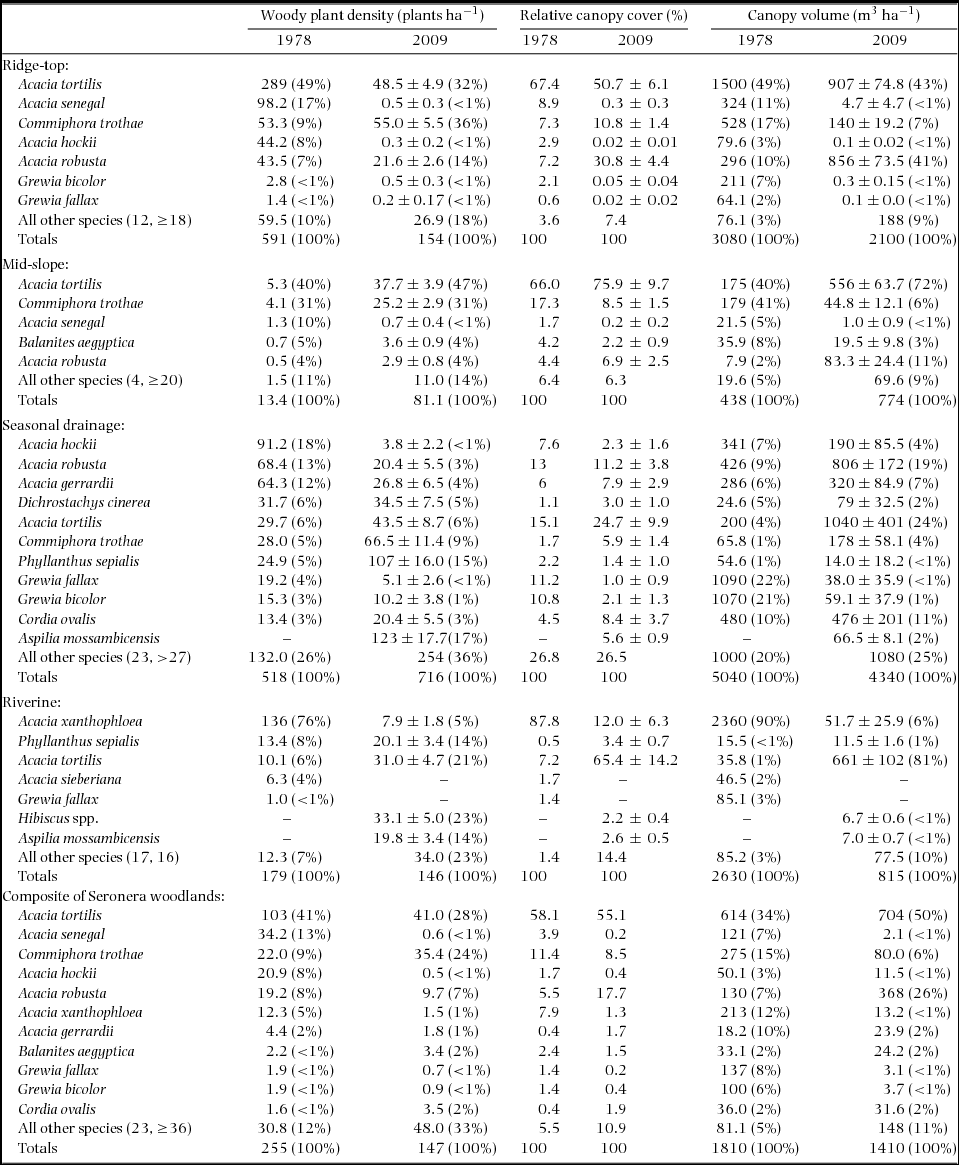
Figure 1. Changes in tree density (trees ha−1, includes plants >0.5 m tall), relative canopy cover (%) and available canopy volume (m3 ha−1) between 1978 (black bars, Pellew Reference PELLEW1983c) and 2009 (white bars; error bars are ± SD) for: Acacia tortilis, Acacia senegal, Acacia robusta and Commiphora trothae. RT = ridge-top woodland, MS = mid-slope woodland, SD = seasonal drainage woodland, RV = riverine woodland and SW = Seronera woodlands, weighted by the % area of each woodland type. Although Acacia tortilis has decreased in density (a), its overall contribution to canopy cover (b) and volume (c) is similar to 1978. Acacia senegal has been decimated in all measures, (d), (e) and (f). Acacia robusta has decreased in density (g), but increased in relative canopy cover (h) and volume (i). Commiphora trothae has increased in density (j), although its relative canopy cover (k) and volume (l) have decreased.
Changes observed in the relatively sparsely treed mid-slope woodland, which accounts for 55% of woodland area, contrast with those observed in the other woodland types (Table 1, Appendix 1). Woody plant density in the mid-slope woodland increased significantly (z = 9.2, P < 0.001, Table 1). The relative densities of the principal woody species remained almost identical to the 1978 values, with the notable exception of A. senegal, the preferred browse species. All principal species (as defined in 1978) aside from A. senegal have increased in absolute density by a factor of 5–7. Additionally, the total number of species encountered in the mid-slope woodland has almost tripled (Appendix 1).
Changes in key woody species
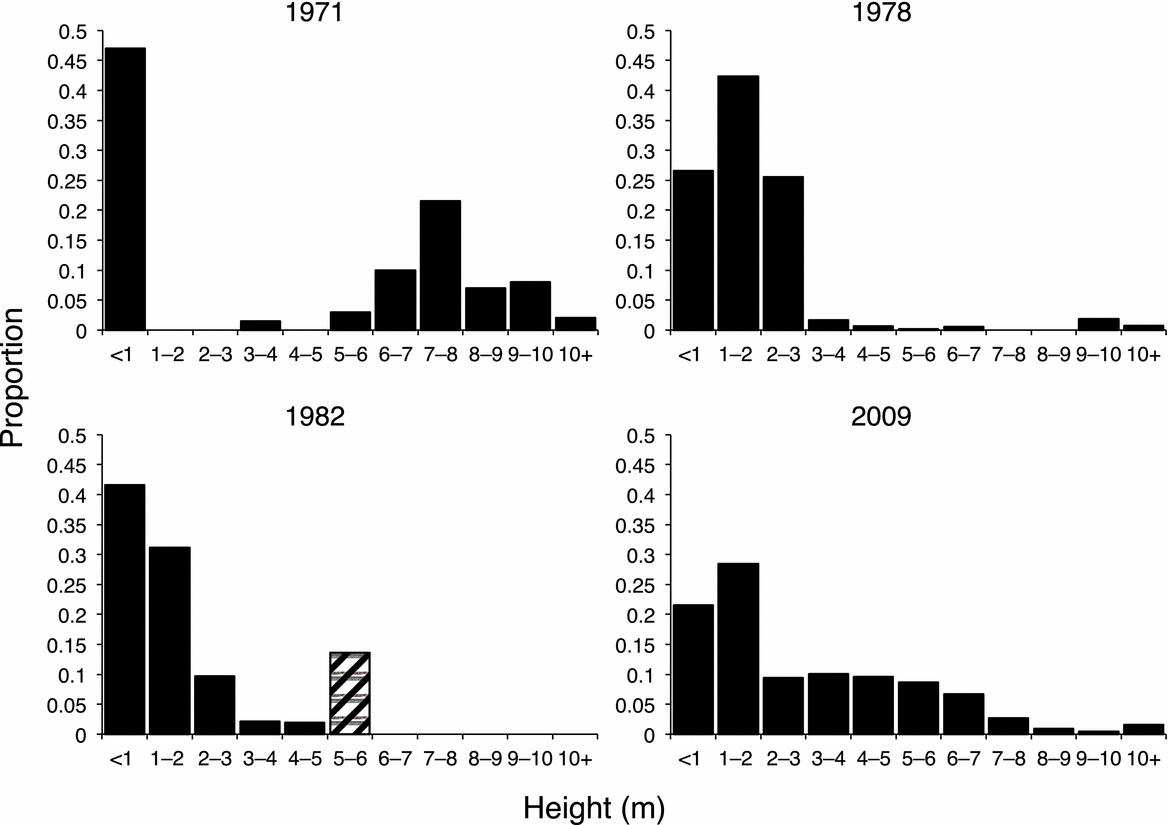
Across the woodlands, preferred and heavily utilized species have been reduced and unpalatable species have thrived. The unpalatable species A. robusta has increased in dominance relative to A. tortilis (Figure 1, Appendix 1) and the relative canopy volume of A. robusta has increased by a factor of 3.6 (Appendix 1). Figure 2 indicates that the increase in A. robusta canopy volume is due to a dramatic shift in the population from mostly small trees to mostly mature, large-canopied trees. The fluctuation of the A. robusta population between a phase with a high proportion of mature trees and a phase dominated by regeneration is consistent with pulsed growth (Figure 2d). Similar changes in structure have been observed in the A. tortilis and A. senegal populations (Figure 2a, b).

Figure 2. Temporal patterns (1971–2009) in the population structure of Acacia senegal (a), Acacia tortilis (b), Acacia xanthophloea (c), Acacia robusta (d) and Commiphora trothae (e) in the Seronera woodlands. Data were not available from 1971 for (d) and (e), and the sample size for (a) in 2009 was trivial. Mature trees >5 m tall are lumped into a single height class. Height classes in orange/yellow are most vulnerable to fire, although A. senegal is more tolerant than A. tortilis at small sizes and A. xanthophloea can remain fire vulnerable up to a height of 7 m (Herlocker Reference HERLOCKER1976). Populations of A. senegal, A. tortilis and A. robusta show pulsed growth: periods with a high proportion of either mature or young trees. Data are from Croze (Reference CROZE1974a), Pellew (Reference PELLEW1983c), Ruess & Halter (Reference RUESS and HALTER1990) and the current study.
In 1978, the unpalatable species, C. trothae, and the preferred species, A. senegal, occurred at densities of 22 trees ha−1 and 34 trees ha−1 respectively, and in each species, >90% of the population was vulnerable to fire (<3 m tall) (Figure 2a, e, Appendix 1). Strikingly, C. trothae has not only persisted, but has increased in density and become co-dominant with A. tortilis: C. trothae has more than tripled in relative dominance in the ridge-top woodland, whereas densities of all other principal species have decreased. In contrast, A. senegal, once a principal species in the ridge-top woodland, has been reduced to a handful of specimens in the sample areas (Figure 1, Appendix 1). Another preferred species, A. hockii De Wild., has also become rare. A dominant in the seasonal drainage woodland type in 1978, A. hockii has decreased in density by 96% (Appendix 1).
As noted, elephants have severely reduced the population of A. xanthophloea trees along the upper Seronera River. In 1978, A. xanthophloea constituted ~90% of total canopy volume in the riverine woodland. In 2009, its contribution had shrunk to a mere 6% (Appendix 1). Many of the small A. xanthophloea specimens in the area today are the coppicing remnants of once larger trees. Interestingly, despite the dramatic reduction in the density of A. xanthophloea trees along the Seronera River, the height structure of the A. xanthophloea population has remained relatively stable (Figure 2c).
The absolute density of A. tortilis has been halved due in part to thinning and maturation of the once-dense stands of A. tortilis regeneration observed in the 1970s (Figure 1). However, A. tortilis remains the woodland dominant. The density of mature A. tortilis (>6 m) in the Seronera woodlands has increased by a factor of 1.6 (Appendix 1). Figure 3 shows that the population structure of A. tortilis is now more evenly distributed among height classes in contrast to a bimodal structure observed in 1971.

Figure 3. Temporal changes in the Acacia tortilis population of Seronera from 1971–2009. For 1982, trees >5 m tall are lumped (hatched bar). The mature canopy suffered heavy elephant damage after 1963 (Lamprey et al. Reference LAMPREY, GLOVER, TURNER and BELL1967), and the persistence of mature trees >10 m, was a management priority in the 1970s (Croze Reference CROZE1974a, Pellew Reference PELLEW1983a, Reference PELLEW and Owen-Smithb). By 2009, the population of A. tortilis trees had become more evenly distributed among height classes.
Elephant and giraffe usage of Acacia robusta vs. control species
In the point-centred quarter sample of A. robusta plants (n = 74), 23% of plants had evidence of giraffe browsing and 86% had evidence of elephant browsing/damage. This is similar to results from even-aged stands of A. robusta, where 32% of plants (n = 330) had evidence of giraffe browsing and 95% had evidence of elephant use. Elephant damage was occasionally severe. There was no difference in the proportion of A. robusta trees browsed at edge versus inside plots for the giraffe (z = 0.72, P = 0.47) or the elephant (z = 0.82, P = 0.41). In the A. tortilis sample (n = 179), 65% and 57% had evidence of giraffe and elephant use, respectively. In the A. drepanolobium sample (n = 114), 84% and 73% respectively had evidence of giraffe and elephant use.
Changes in the giraffe's food resource
Overall canopy volume (mean ± SD) in the Seronera woodlands decreased from 1800 ± 207 to 1400 ± 121 m3 ha−1 between 1978 and 2009 (Table 1). This decrease has been particularly marked in the ridge-top and riverine areas. Canopy volume available to giraffes has dropped by two-thirds in the riverine woodland, mostly due to a >97% reduction in A. xanthophloea. Another important change in the food resource is the dramatic reduction, throughout the woodlands, in the absolute density and volume of preferred species such as A. senegal, A. hockii, and principal browse species such as the broad-leaved Grewia species. However, in the seasonal drainage woodland, canopy volume has not significantly declined due to an increase in A. tortilis, though A. tortilis produces few shoots (i.e. low edible biomass) in the dry season.
The total canopy volume of A. tortilis, the principal wet-season food, has remained stable. However, the preferred species A. senegal and A. hockii previously contributed 172 m3 ha−1 but now contribute only 14 m3ha−1. Moreover, a significant proportion of the available biomass is unpalatable. Acacia robusta has tripled in absolute volume and now contributes 26% of total canopy volume, up from 7% in 1978. Large, dense, monospecific stands of A. robusta were a notable feature at the periphery of some sample areas. Thus, A. robusta may contribute even more to the canopy volume of the woodlands. The C. trothae population is made up of mostly small trees. Thus, despite an increase in density, C. trothae actually contributes less to canopy volume now than in 1978. Together, the unpalatable species A. robusta and C. trothae contribute 31% of canopy volume in 2009 vs. 22% in 1978 (Appendix 1).
DISCUSSION
Woodland change in Seronera
The Seronera woodlands have undergone substantial change over the last 30–40 y: we observed substantial declines in woody plant density in three of four woodland types (Table 1) and major shifts in species composition (Appendix 1). Although woodland dynamics are invariably complex, some of the changes we observed may be linked to the activities of elephant and giraffe. The elephant, for example, has clearly caused major damage to A. xanthophloea trees along the Seronera River, consistent with findings of the 1982 survey (Ruess & Halter Reference RUESS and HALTER1990), which reported a 30% decline in the density of A. xanthophloea along the river from 1971–1982. The giraffe may also have contributed to observed changes in plant density. In the midslope woodland type, where giraffe browsing pressure was historically low (Pellew Reference PELLEW1981, Reference PELLEW1984b), woodland plant density increased significantly. In contrast, woody plant density declined in the ridge-top and seasonal drainage/riverine woodland types, favoured by giraffes in the wet and dry seasons respectively (Pellew Reference PELLEW1981, Reference PELLEW1984b).
Through selective browsing, we suggest that the elephant and giraffe may also have played an important role shaping species composition, not unlike changes observed in other savanna woodlands. For example, in north-western Zimbabwe, elephant browsing likely drove a switch in the dominant woodland species from Brachystegia boehmii to Pseudolachnostylis maprouneifolia (Mapaure & Moe Reference MAPAURE and MOE2009). The interaction of heavy giraffe browsing with species tolerance also led to shifts in species composition in South African woodland savanna (Bond & Loffell Reference BOND and LOFFELL2001).
Consistent with Pellew's predictions, there has been a clear shift toward species that are unpalatable to the elephant or giraffe and a corresponding decrease in species that have been preferred or heavily utilized. Pellew (Reference PELLEW1981) predicted that giraffe avoidance of A. robusta would cause an increase in the dominance of A. robusta relative to A. tortilis. In the ridge-top woodland, where the vast majority (80%) of A. robusta trees were encountered, the ratio of A. robusta to A. tortilis trees has increased threefold over the past 30 y. We observed a similar pattern in a second species, C. trothae, which is unpalatable to the elephant and giraffe. The ratio of C. trothae to A. tortilis trees has increased almost fourfold in the Seronera woodlands and C. trothae has become co-dominant with A. tortilis (Appendix 1).
In the early 1970s, A. senegal was considered a principal tree species in parts of Seronera (Croze Reference CROZE1974a, Herlocker Reference HERLOCKER1976). Pellew (Reference PELLEW1981), however, predicted that the combined activity of the elephant and giraffe would rapidly reduce the dominance of this preferred species and indeed our data show that the density of A. senegal has been reduced from 34.2 trees ha−1 in 1978 to a mere 0.6 trees ha−1 in 2009.
The broad-leaved Grewia spp., which dominated seasonal drainage woodland canopy volume and giraffe dry-season diets in the 1970s (Pellew Reference PELLEW1981, Reference PELLEW1983c, Reference PELLEW1984b), have more than halved in density and decreased in canopy volume by >96% from 1978 to 2009. In contrast. A. tortilis, which the elephant and giraffe browse roughly in proportion to its availability, remains a dominant woodland species.
Avoidance of Acacia robusta?
Although earlier studies suggested that the elephant infrequently utilizes A. robusta, Ruess & Halter (Reference RUESS and HALTER1990) observed that 63.9% of A. robusta trees had some herbivore damage, and we observed elephant damage (broken stems, stripped bark) on 86–95% of A. robusta trees (excluding uprooted/dead trees), suggesting that elephant impact on this species has increased as it has become more abundant.
Giraffes, however, still avoid A. robusta, despite its increased availability: only 23–32% of A. robusta trees exhibit evidence of giraffe browsing compared with 84% of the preferred food species, A. drepanolobium. During field observations of giraffes, foraging bouts on A. robusta trees were rare and typically lasted less than 1 min whereas foraging on the commonly browsed A. tortilis lasted 2–10 min (Appendix 2). Acacia robusta with evidence of browsing usually had only several browsed shoots, whereas giraffes may remove almost all new shoots from a 2-m-tall A. tortilis tree. Giraffe avoidance of A. robusta may be due to chemical defence (Pellew Reference PELLEW1984b), possibly the anti-nutritive effects of polyphenolic compounds (Brockman et al., unpubl. data).
Qualitative evaluation of Pellew's woodland dynamics model
Pellew's woodland dynamics model is often employed as a framework for other studies (Ben-Shahar Reference BEN-SHAHAR1996, Birkett Reference BIRKETT2002, Dublin et al. Reference DUBLIN, SINCLAIR and MCGLADE1990, Holdo et al. Reference HOLDO, HOLT and FRYXELL2009). The initial conditions, describing Seronera in the 1970s, are generally used as a starting point. The model has been used to describe, for example, the woodland component of woodland-grassland dynamics in the Serengeti (Holdo et al. Reference HOLDO, HOLT and FRYXELL2009). However, there has been little effort to test the validity of the model. While a precise quantitative statistical test is beyond our present scope, based on data from the 1980s and 2000s (Dempewolf et al. Reference DEMPEWOLF, TRIGG, DEFRIES and EBY2007, Strauss, pers. obs, Stronach Reference STRONACH1989), we estimate that the fire-return interval in the majority of Seronera sample areas was roughly 2–8 y between 1978–2009, and elephant and giraffe numbers have dropped by about 30–75%. Given these inputs, Pellew's model predicts a modest growth in the population of mature A. tortilis trees (Pellew Reference PELLEW1983a). For example, if elephant impact falls by 50% and the fire return interval is 8 y, then the population of mature A. tortilis trees increases by ~50%. This is in qualitative agreement with our observation of a ~60% increase in mature A. tortilis trees. Holdo et al. (Reference HOLDO, HOLT and FRYXELL2009) suggest incorporating a dynamic feedback between herbivores and vegetation, which seems appropriate given our results.
Proliferation of Acacia robusta in Serengeti National Park
Total woody cover in the Serengeti National Park has increased over the last 30 y (Packer et al. Reference PACKER, HILBORN, MOSSER, KISSUI, BORNER, HOPCRAFT, WILMSHURST, MDUMA and SINCLAIR2005, Sinclair et al. Reference SINCLAIR, MDUMA, HOPCRAFT, FRYXELL, HILBORN and THIRGOOD2007, Reference SINCLAIR, HOPCRAFT, OLFF, MDUMA, GALVIN, SHARAM, Sinclair, Packer, Mduma and Fryxell2008) in contrast to the local decrease in Seronera. Much of this landscape-level change can be attributed to a pulse of A. robusta regeneration, which began in the late 1970s and early 1980s (Sinclair et al. Reference SINCLAIR, HOPCRAFT, OLFF, MDUMA, GALVIN, SHARAM, Sinclair, Packer, Mduma and Fryxell2008), although other species of Acacia also increased in density over this period (Shaw et al. Reference SHAW, SINCLAIR, METZGER, NKWABI, MDUMA and BAKER2010). Acacia robusta is now dominant in the Serengeti, along with A. tortilis (Shaw et al. Reference SHAW, SINCLAIR, METZGER, NKWABI, MDUMA and BAKER2010, Sinclair et al. Reference SINCLAIR, HOPCRAFT, OLFF, MDUMA, GALVIN, SHARAM, Sinclair, Packer, Mduma and Fryxell2008). Acacia robusta appears to regenerate in pulses, leading to dense even-aged stands, with densities of up to 3000–5000 stems ha−1 (Sinclair Reference SINCLAIR, Sinclair and Arcese1995, Stronach Reference STRONACH1989).
The first known pulse of Acacia robusta regeneration in the Serengeti occurred around 1900–1920, and a subsequent pulse in the 1970s–1980s coincided with a period of low fire prevalence (Sinclair Reference SINCLAIR, Sinclair and Arcese1995). We hypothesize that heavy, selective giraffe browsing on competitor species combined with reduced elephant damage also contributed to the recent pulse of A. robusta regeneration across Serengeti National Park: the 1970s–1980s pulse of A. robusta coincided with a very high density of giraffe (1.47–2.64 km−2 in the mid-1970s, Pellew Reference PELLEW1983d), and with the decimation of the elephant population due to poaching (~80% reduction, Dublin Reference DUBLIN, Sinclair and Arcese1995). However, a more experimental approach would be required to test whether giraffe and elephant browsing actually cause the large-scale proliferation of A. robusta in savanna ecosystems like Serengeti.
Conclusions
We have documented substantial change in the structure and composition of the Seronera woodlands between 1971 and 2009 as well as a decline in the quantity and quality of the giraffe's food supply compared with 1978. We have focused on the possible roles of the elephant and giraffe in driving these changes. Our data provide initial support for the hypothesis that elephant and giraffe activity, in combination with fire, has mediated an increase in the relative dominance of unpalatable species, particularly A. robusta, over the last 30 y. This implies that the giraffe population may have played an important role in mediating the decline in the quality of its own food resource. In a future paper, we consider the link between the diminished food supply and the low density of giraffes observed in 2008–2010 (Strauss et al., unpubl. data).
Our interpretation has limitations. Principally, we have considered the effects of elephant and giraffe largely in isolation without controlling for additional drivers of woodland dynamics. For example, impala and other small herbivores affect seedling survival and growth (Belsky Reference BELSKY1984, Moe et al. Reference MOE, RUTINA, HYTTEBORN and DU TOIT2009, O’Kane et al. Reference O’KANE, DUFFY, PAGE and MACDONALD2014, Prins & van der Jeugd Reference PRINS and VAN DER JEUGD1993, Sharam et al. Reference SHARAM, SINCLAIR and TURKINGTON2006). Beetles and rodents are important seed predators, and can greatly reduce seedling survival (Goheen et al. Reference GOHEEN, KEESING, ALLAN, OGADA and OSTFELD2004, Pellew & Southgate Reference PELLEW and SOUTHGATE1984, Shaw et al. Reference SHAW, KEESING and OSTFELD2002). Wildebeest horning can severely damage up to 24% of small trees/shrubs inside woodland areas (Estes et al. Reference ESTES, RAGHUNATHAN and VAN VLECK2008). Woodland dynamics in savannas result from intricate interactions between such biotic drivers and abiotic factors, such as fire, rainfall, soils and topography (Norton-Griffiths Reference NORTON-GRIFFITHS, Sinclair and Norton-Griffiths1979, Pellew Reference PELLEW1983a, Reed et al. Reference REED, ANDERSON, DEMPEWOLF, METZGER and SERNEELS2009, Sankaran et al. Reference SANKARAN, HANAN, SCHOLES, RATNAM, AUGUSTINE, CADE, GIGNOUX, HIGGINS, ROUX, LUDWIG, ARDO, BANYIKWA, BRONN, BUCINI, CAYLOR, COUGHENOUR, DIOUF, EKAYA, FERAL, FEBRUARY, FROST, HIERNAUX, HRABAR, METZGER, PRINS, RINGROSE, SEA, TEWS, WORDEN and ZAMBATIS2005, Sinclair Reference SINCLAIR, Sinclair and Norton-Griffiths1979b). Although our study does not provide an irrefutable causal link between the elephant, giraffe and woodland composition, careful monitoring of the impact of elephant and giraffe will likely prove valuable in understanding woodland dynamics in African savannas.
ACKNOWLEDGEMENTS
We thank the Tanzania Commission for Science and Technology, Tanzania National Parks and the Tanzania Wildlife Research Institute for permission to carry out this study in Serengeti National Park. We are especially grateful to E. Kalumbwa Berere for assisting with the woodland survey and to R. Pellew for correspondence about the 1978 survey. We thank the Seronera Wildlife Research Centre for herbarium access and R. Gerreau for assistance with plant identification. We also thank the Serengeti–Mara data collaboration, particularly K. Metzger and S. Eby, for access to fire maps. MS is grateful for financial support from the National Science Foundation GRFP, the American Society of Mammalogists, Columbus Zoo, Riverbanks Zoo and Garden, the Bell Museum and the University of Minnesota Graduate School. Finally, we thank E. Thrane for feedback and statistical advice and A.R.E. Sinclair, C. O’Kane and an anonymous reviewer for comments on earlier drafts of the manuscript.
Appendix 1. Comparison of 1978 (Pellew Reference PELLEW1981, Reference PELLEW1983c) and 2009 structure and composition of the Seronera woodlands, Serengeti. Table entries are mean ± SD in 2009, for four woodland types (ridge-top, mid-slope, seasonal drainage and riverine). Percentage values in parentheses indicate relative density/canopy volume for each species. The composite section combines all habitat types, weighted by the % area of each. Data are organized in decreasing order of 1978 density. Woody plants <0.5 m in height were excluded. Canopy measurements include only foliage/stems below 5.75 m, which is the maximum reach of an adult male giraffe. - represents species not encountered/not reported in one of survey years.

Appendix 2. Length of giraffe feeding bouts on Acacia tortilis and Acacia robusta trees in the Seronera woodlands. A feeding bout was defined as the time from first to last bite on a single tree. Data taken from observations of adults (ages 5+ y) and subadults (ages 1–5 y) of both sexes.