INTRODUCTION
Biomass and nutrients should be allocated within plants to optimize fitness in any given environment. Thus, natural selection acting on plants in infertile habitats should engender structural and physiological traits that maximize nutrient capture and nutrient-use efficiency (e.g. high root mass fraction, high specific root length, low tissue nutrient concentrations), but in fertile habitats should engender structural and physiological traits that maximize radiation capture and radiation-use efficiency (e.g. high leaf area fraction, high leaf nutrient concentrations, high C assimilation rates).
Nutrient-conserving traits often underlie habitat specialization, and resulting plant–soil associations, in mature rain-forest trees (Gleason et al. Reference GLEASON, READ, ARES and METCALFE2009, Paoli Reference PAOLI2006). These traits may be structural, such as increasing root mass fraction (RMF) in response to decreasing nutrient availability (Crick & Grime Reference CRICK and GRIME1987, Medina & Cuevas Reference MEDINA, CUEVAS and Proctor1989, but see van der Werf et al. Reference Van Der WERF, VAN NUENEN, VISSER and LAMBERS1993), or they may be physiological, such as decreasing dark respiration rate (Rd) in response to decreasing light availability (Valladares & Niinemets Reference VALLADARES and NIINEMETS2008, Wright et al. Reference WRIGHT, REICH, ATKIN, LUSK, TJOELKER and WESTOBY2006). Structural traits sometimes found in nutrient-limited plants include high RMF, high specific root length (SRL) (Eissenstat Reference EISSENSTAT2000), and high leaf mass per unit area (LMA) (Read et al. Reference READ, SANSON, DE GARINE-WICHATITSKY and JAFFRÉ2006, Reich et al. Reference REICH, WALTERS and ELLSWORTH1997, Turner Reference TURNER1994). Physiological traits sometimes found in nutrient-limited plants include low rates of maximum CO2 assimilation (Amax), Rd (Reich et al. Reference REICH, UHL, WALTERS and ELLSWORTH1991), and P uptake per unit root mass (Jackson et al. Reference JACKSON, MANWARING and CALDWELL1990, Nye Reference NYE1977).
These structural and physiological traits then manifest as nutrient-conserving outcomes, such as high phosphorus use efficiency (PUE) (Aerts Reference AERTS1999, Chapin Reference CHAPIN1980), low net assimilation rate (NAR) (Poorter & Remkes Reference POORTER and REMKES1990), and low relative growth rate (RGR) (Chapin Reference CHAPIN1980, Poorter & Remkes Reference POORTER and REMKES1990). Although these structural and physiological responses to low-nutrient conditions are common, they are by no means found among all species from low-nutrient sites. For example, tree species that grow on both nutrient-poor and nutrient-rich soils (i.e. habitat generalists) must optimize fitness in both these habitats, and therefore should demonstrate greater plasticity in nutrient-conserving traits (Grime Reference GRIME2002), but not necessarily have mean trait values similar to species specialized to nutrient-poor habitats.
In this experiment, physiological and structural traits known to confer high nutrient-use efficiency were compared among seedlings of soil generalists and soil specialists (species occurring only on P-infertile soils). We asked whether these traits are similar within functional groups (specialists vs. generalists) or whether each species has a different suite of traits, i.e. do species within functional groups have common or independent paths to success? Specifically, it was hypothesized that infertile soil specialists would allocate proportionately more biomass to roots than soil generalists (the structural equilibrium theory; Brouwer Reference BROUWER1983), whereas soil generalists would be more plastic in their biomass allocation patterns (Grime Reference GRIME2002). Similarly, it was hypothesized that infertile soil specialists would have higher LMA, SRL, PUE, and lower P uptake, Amax, Rd, NAR, and RGR than soil generalists, but that soil generalists would be more plastic in these traits.
METHODS
Study site
Seedlings were collected from evergreen rain forest within Wooroonooran National Park, Queensland, Australia (c. 17°22ʹS, 145°43ʹE, 700–800 m asl); 32 km from the coast and west of Mt. Bartle Frere. The forest is classified as complex mesophyll vine forest (Tracey Reference TRACEY1982) and is species-rich, with >1000 tree species within the regional area of north-east Queensland (Hyland et al. Reference HYLAND, WHIFFIN, CHRISTOPHEL, GRAY and ELICK2002). The study area receives c. 3.5 m of annual rainfall, of which c. 70% falls between November and April. Average maximum and minimum diurnal temperatures are c. 26 °C and 14 °C, respectively. The warmest months of the year and the coldest months of the year have mean diurnal maximum and minimum temperatures of 29 °C and 17 °C and 22 °C and 10 °C, respectively (Thompson et al. Reference THOMPSON, STOCKER and KRIEDEMANN1988).
Soils are derived from igneous basalt (Red Ferrosol – Maalan series) and metamorphic schist parent materials (Red Dermosol – Galmara series) (Malcolm et al. Reference MALCOLM, NAGEL, SINCLAIR and HEINER1999). Both soils have low pH (4.6 for basalt and 4.0 for schist soils), high organic C (4.3% and 2.7%), moderate effective cation exchange capacity (13.6 and 9.4 cmol + kg−1), and high aluminium saturation (79% and 72%) (Gleason et al. Reference GLEASON, WILLIAMS, READ, METCALFE and BAKER2008). Total N was higher for basalt soils than for schist soils (0.32% and 0.22%) (Malcolm et al. Reference MALCOLM, NAGEL, SINCLAIR and HEINER1999). Total P, as well as most P fractions (total inorganic, total organic, HCL-extractable, NaOH-extractable) were 2.5–7-fold greater for basalt soils than for schist soils. A more detailed description of soil P fractions and soil data can be found in Gleason et al. (Reference GLEASON, WILLIAMS, READ, METCALFE and BAKER2008). Growth experiments in a glasshouse (Kerridge et al. Reference KERRIDGE, ANDREW and MURTHA1972) and in planted timber plantations (Keenan et al. Reference KEENAN, ROBSON, WEBB, Schulte and Ruhiyat1998, Webb et al. Reference WEBB, REDDELL, HAMBLETON and ROBSON2000) have demonstrated P-limitation of plant growth on schist soils. Stoichiometry and the distribution of P-adapted species also support the hypothesis of P-limitation of soil generalists on schist soils (Gleason et al. Reference GLEASON, READ, ARES and METCALFE2009).
Seedling collection and treatment application
Species either occur on both soil types (soil generalists) or occur only on P-poor schist soils (schist specialists). All species examined in this study are overstorey climax species and presumably shade tolerant. Wild seedlings of Cryptocarya lividula B. Hyland, Cryptocarya mackinnoniana F. Muell. (Lauraceae), Ceratopetalum virchowii F. Muell. (Cunoniaceae), Franciscodendron laurifolium F. Muell. (Malvaceae), and Myristica insipida R. Br. (Myristicaceae) were collected from forest occurring on schist soil. All seedlings (50 of each species) were < 20 cm tall and judged to be less than 1 y old, based either on the presence of cotyledons (F. laurifolium, M. insipida, C. virchowii) or size (< 20 cm) and appearance (Cryptocarya). Cryptocarya lividula and C. virchowii are schist specialists and C. mackinnoniana, F. laurifolium and M. insipida are soil generalists. Whereas both schist specialists are true obligate specialists, the generalist species do differ somewhat in their soil fidelity; F. laurifolium and M. insipida display a stronger fidelity for schist and basalt, respectively (Gleason et al. Reference GLEASON, READ, ARES and METCALFE2010).
Seedlings were washed free of soil, sprayed with fungicide, and flown to Monash University, Melbourne within 3 d of collection (26 March 2006). Seedlings were transplanted into 2-litre pots filled with double-washed quartz sand and grown under these conditions for 4 mo to recover from damage that may have occurred during collection and potting, and to standardize growth conditions prior to the initial harvest on 5 September. After the initial harvest, Osmocote® (Scotts Australia Pty Ltd, Baulkham Hills, NSW, Australia) slow-release fertilizer was added (0.00 g, 1.88 g, 3.75 g or 7.50 g) to the pots. The slow-release fertilizer contained 16% N, 3.5% P, 10% K, 2.4% S, 1.2% Mg, 0.02% B, 0.05% Cu, 0.4% Fe, 0.06% Mn, 0.02% Mo and 0.015% Zn.
Four shade structures were made using 70% shade cloth and shade paint was applied to the glasshouse roof and windows to further reduce the photosynthetic flux density (PFD). Average maximum daily PFD values for the shade and sun treatments were 120 and 383 μmol m−2 s−1, respectively, and relative humidity (RH) never fell below 63%. Mean minimum and maximum diurnal temperatures ranged from 9.5 °C to 15.7 °C during the coldest month (July) and 15.3 °C to 28.1 °C during the hottest month (January). Humidifying misters were controlled via an evaporative switch and all plants were watered using drip irrigation twice a day for 2 min.
Six plants of each species were assigned to each treatment (6 plants × 2 radiation × 4 fertilization = 48 plants per species). Shaded (4) and non-shaded (4) benches were randomly arranged in the glasshouse. Plants were re-randomized on the glasshouse benches every month.
Plant harvest and analysis
Initial and final harvests were undertaken on 5 September 2006 and 9 August 2007, respectively. Surface area and root length was measured on freshly harvested leaves and roots from scanned images (236 pixels cm−1) using Mix image analysis software (Monash University, Melbourne, Australia). Specific root length (SRL) was calculated as total root length/total dry root mass. Specific root area (SRA) was calculated as total two-dimensional surface area/total dry root mass. Roots, stems and leaves were then dried at 65 °C to constant mass, weighed and then bulked to obtain one replicate per treatment (5 species × 2 radiation × 4 fertilization = 40 samples) for each tissue type (roots, stems or leaves). Dried samples were ground in a ball mill, and analysed for total P using the vanadomolybdophosphoric acid spectrophotometer method (APHA Reference Rand, Greenberg, Taras and Franson1976) following microwave-assisted digestion in nitric acid and hydrogen peroxide.
Biomass increment was calculated as: final harvest biomass (dry mass) – initial biomass (dry mass). Relative growth rates (RGR) were calculated as: (ln(final biomass) – ln(initial biomass))/time. Net assimilation rate (NAR) was calculated as: (final biomass – initial biomass)/time × (ln(final leaf area) – ln(initial leaf area))/(final leaf area – initial leaf area). This formula estimates net assimilation over the entire growth period (48 wk), but assumes a linear relationship between biomass and leaf area (Williams Reference WILLIAMS1946), which was met for all species and treatments.
Two months prior to harvesting, four plants from each treatment were randomly selected for measurement of gas exchange and modulated fluorescence (LI-6400–40, LI-COR Biosciences, Lincoln, Nebraska, USA). Plants were watered and brought into the laboratory 12 h before measurements. After an initial measurement at a PFD of 400 μmol m−2 s−1, PFD was decreased stepwise (seven steps) to 0 μmol m−2 s−1. Radiation was produced with a 6400–02B LED light source (LI-COR Biosciences). Plants were allowed to equilibrate for c. 20 min at each PFD step before taking measurements. Temperature, relative humidity and CO2 concentrations were kept relatively constant; 55%–65% RH, 23 °C–25 °C, and 399–401 μmol CO2 m−2 s−1. Radiation response curves were fitted using the Von Bertalanffy equation (Horton & Neufeld Reference HORTON and NEUFELD1998):
where Anet = net CO2 assimilation rate, Rd = dark respiration rate, Amax = maximum CO2 assimilation rate, and AQY = apparent quantum yield. This equation fit all species and treatments well, with adjusted r2 values generally higher than 0.98. LCP was calculated as Rd/AQY. A description of gas exchange and fluorescence measurements is given in Appendix 1.
P acquisition, use and conservation measurements
Two equations were used to estimate P uptake efficiency (UpE) during the growth period: (1) UpE1 = P uptake/time × (ln(final root mass) – ln(initial root mass))/(final root mass – initial root mass). (2) UpE2 = P uptake/time × (ln(final root area) – ln(initial root area))/(final root area – initial root area). We developed these equations from that used to estimate NAR over a growth period (Hunt Reference HUNT1978, Portsmuth & Niinemets Reference PORTSMUTH and NIINEMETS2007, Williams Reference WILLIAMS1946), but apply it to P uptake and root mass (or area). Although UpE1 is calculated as P uptake per unit root mass, P uptake is not affected by root mass per se, but by the surface area of that root mass and its physiology (P uptake per unit root surface area). UpE2 reflects the kinetics of P uptake (P uptake per unit root surface area).
Because the seedlings were treated with fungicide prior to shipment from Queensland (phytosanitation guidelines), native mycorrhizas associated with the seedlings may have been eliminated. Because mycorrhizal uptake is an important P conservation mechanism (Chuyong et al. Reference CHUYONG, NEWBERY and SONGWE2004, Newbery et al. Reference NEWBERY, ALEXANDER and ROTHER1997) UpE among the seedlings should be interpreted as an outcome of root functioning only.
Phosphorus productivity was calculated in two ways. The first method estimates how efficiently the total plant P pool is used to produce a unit of biomass throughout the growth period (Ingestad Reference INGESTAD1979, Ryser et al. Reference RYSER, VERDUYN and LAMBERS1997). We calculate this as Bio-P-prod = (final biomass – initial biomass)/time × (ln(final P content in biomass) – ln(initial P content in biomass))/(final P content in biomass – initial P content in biomass). Because CO2 assimilation takes place in leaves we calculated crown productivity (Crown-P-prod) (same equation as above), but consider only the P content in leaves rather than the entire plant (Harrington et al. Reference HARRINGTON, FOWNES and VITOUSEK2001). Crown-P-prod relates to how efficiently P in the crown is used to produce a unit of biomass.
Even though the glasshouse experiment ran for 11 mo, almost no litterfall occurred during this time. Therefore, leaf life span and P residence times were not calculated and P-use efficiency (PUE) calculations followed Shaver & Melillo (Reference SHAVER and MELILLO1984): PUE = biomass increment/P in biomass increment. This equation reflects both nutrient storage and use because luxury uptake was likely significant, especially in the high nutrient treatments.
Radiation-use efficiency (biomass increment/average daily maximum PFD) reflects the ability of a plant to acclimate structurally and physiologically to incident PFD.
Structural and morphological characteristics of biomass allocation
Biomass allocation was characterized by the fraction of total biomass contributed by either roots (RMF) or leaves (leaf mass fraction; LMF). The total amount of leaf surface area available to intercept radiation, relative to total plant mass (leaf area ratio; LAR) was calculated to characterize radiation capture strategy. Similarly, the total amount of root surface area available for nutrient uptake (estimated from flatbed scans), relative to total plant mass (root area ratio; RAR) was also calculated.
Data analysis
Biomass allocation, resource-use and gas exchange traits were compared among species, light treatments and fertilization treatments using ANOVA and ANCOVA (complete random design). Biomass was used as a covariate to control for plant size when required (Poorter & Nagel Reference POORTER and NAGEL2000, Weiner Reference WEINER2004) and data were transformed as necessary to meet the assumptions of all analyses. Natural logarithm transformations successfully normalized ratio traits (e.g. RMF, LMF), a requirement prior to comparing allocation ratios (Poorter & Nagel Reference POORTER and NAGEL2000). Principal components analysis (PCA) was performed on the normalized data set used for ANOVA described above. Trait coefficients and treatment scores associated with the first two PCA axes were generated for plant traits and treatments to better understand the data structure.
RESULTS
Structure and biomass allocation
Root mass fraction decreased in response to fertilization (F3, 158 = 12.0, P < 0.001), with schist specialists C. virchowii and C. lividula having the highest RMF in the lowest fertility treatment, but having similar RMF as the soil generalists at higher fertility (Figure 1a). Across all fertilization treatments, the highest RMFs were displayed by the schist specialists, but this result was only significant (F1, 57 = 6.31, P = 0.015) between schist specialist C. virchowii and soil generalist C. mackinnoniana. Cryptocarya mackinnoniana displayed the greatest plasticity in RMF between 0 and 1.88 g of fertilizer per pot, although specialist C. lividula displayed the greatest plasticity in RMF across all treatments (Figure 1a). Root mass fraction increased 10% in response to radiation, with schist specialist C. virchowii and soil generalist F. laurifolium showing little or no response, relative to the other species (Tukey Simultaneous Tests, P < 0.01).

Figure 1. Leaf and root physiology, structure, and biomass allocation characteristics for schist specialists and soil generalists in response to four fertilization treatments. Solid lines denote schist specialists Ceratopetalum virchowii (●) and Cryptocarya lividula (■), whereas broken lines denote soil generalists Cryptocarya mackinnoniana (○), Franciscodendron laurifolium (□) and Myristica insipida (△). RMF = root mass fraction (a). SRL = specific root length (b). LMF = leaf mass fraction (c). LMA = leaf mass area (d). UpE2 = P uptake efficiency (e). PUE = P-use efficiency (f). NAR = net assimilation rate (g). RGR = relative growth rate (h). All data are biomass-corrected (except for PUE and RGR) least square mean ± 1 SE (n = 8).
Specific root length (SRL) varied 20-fold across species, with M. insipida having the lowest and C. virchowii having the highest (Figure 1b). Despite the large spread of SRL values across species, schist specialist C. lividula and soil generalists F. laurifolium and C. mackinnoniana had similar SRL values (Figure 1b). Radiation had no effect on SRL and, although SRL decreased somewhat with increasing fertilization (F3, 111 = 2.92, P = 0.037), this effect was minor relative to the intrinsic differences between species (Table 1). Only C. virchowii supports the hypothesis that schist specialists would have higher RMF and SRL than soil generalists. Contrary to the hypothesis, only schist specialist C. virchowii demonstrated less plasticity in below-ground structural traits than soil generalists.
Table 1. Values for F and P (in parentheses) from ANOVA and ANCOVA for growth, allocation, nutrient-use and gas-exchange measures of seedlings grown under four fertilization levels and two radiation levels. Data were transformed as necessary to meet analyses assumptions and a biomass covariate was used when appropriate to control for size effects. LMF = leaf mass fraction. LAR = leaf area ratio. LMA = leaf mass area. RMF = root mass fraction. SRL = specific root length. SRA = specific root area. RAR = root area ratio. Root or leaf [P] = tissue P concentration. Amax = maximum CO2 assimilation rate. Rd = dark respiration rate. AQY = apparent quantum yield. LCP = light compensation point. ETR = electron transport rate. ETR/A = electron transport rate divided by CO2 assimilation rate. UpE1 = P uptake efficiency; P uptake per unit root mass. UpE2 = P uptake efficiency; P uptake per unit root area. NAR = net assimilation rate. Crown P prod = crown P productivity. Bio P prod = biomass P productivity. PUE = P-use efficiency. RUE = radiation use efficiency. RGR = relative growth rate. Gas exchange descriptions are given in Appendix 1.

Leaf mass fraction (LMF) increased in response to fertilization (F3, 158 = 16.2, P < 0.001), but this response was also species-specific (F12, 158 = 3.05, P = 0.001), with soil generalist C. mackinnoniana showing the largest (i.e. most plastic) response between 0 and 1.88 g fertilizer per pot (Figure 1c). Relative to plastic responses in LMF, intrinsic differences in LMF among species were small (Figure 1c). LMF decreased 10% with increasing radiation for all species (F1, 158 = 23.1, P < 0.001), except for soil generalist F. laurifolium which displayed little response, but this interaction was only significant between F. laurifolium and soil generalists C. mackinnoniana (F1, 59 = 16.0, P < 0.001) and M. insipida (F1, 64 = 7.86, P = 0.007).
Leaf mass per unit area (LMA), although markedly different among species (F4, 160 = 135, P < 0.001), did not respond to fertilization (F3, 160 = 1.81, P = 0.147) (Figure 1d). LMA increased by 8% in response to radiation in the two highest fertility treatments. Schist specialist C. lividula had significantly lower LMA than all other species except soil generalist F. laurifolium (Tukey Simultaneous Tests, P < 0.001). Soil generalist M. insipida had higher LMA than all other species (Tukey Simultaneous Tests, P < 0.001). Leaf area ratio (LAR) increased by 65% across all species in response to fertilization, with schist specialist C. lividula having the most plastic response (76%) and soil generalist M. insipida having the least plastic response (33%) (Appendix 2). Increased radiation decreased LAR by 19% across all species. The hypothesis that schist specialists would demonstrate less plasticity in above-ground structural traits can be rejected, as only soil generalist C. mackinnoniana demonstrated marked plasticity (between the two lowest P treatments), whereas schist specialist C. lividula showed the greatest plasticity in LAR across all treatments.
Root and leaf physiology
Schist specialists C. virchowii and C. lividula had the highest rates of phosphorus uptake per unit root mass (UpE1) (198 and 220 mg kg−1 wk−1), whereas soil generalists C. mackinnoniana and M. insipida had the lowest UpE1 (120 and 70 mg kg−1 wk−1). Fertilization increased UpE1 by 70% (F3, 160 = 64.7, P < 0.001), with schist specialists C. virchowii and C. lividula showing the most increase (71% and 145%) and soil generalists C. mackinnoniana and M. insipida showing the least response (30% and 31%). UpE1 increased by 7% in response to radiation (F1, 160 = 6.11, P = 0.014), but soil generalist F. laurifolium exhibited a 14% decrease. Soil generalist M. insipida had the highest rate of P uptake per unit root area (UpE2), whereas schist specialist C. virchowii had the lowest (Figure 1e). Fertilization significantly increased UpE2 (F3, 12 = 47.4, P < 0.001), with soil generalist F. laurifolium showing the greatest increase and schist specialist C. virchowii showing the least increase (Figure 1e). Radiation had no significant effect on UpE2, nor were there any significant species by radiation interactions (Table 1). Only schist specialist C. virchowii provides support for the hypothesis that schist specialists would have lower and less plastic UpE2 than the soil generalist.
Amax differed significantly among species (F4, 72 = 8.95, P < 0.001), with schist specialist C. virchowii having the lowest (1.66 μmol m−2 s−1) and soil generalist F. laurifolium having the highest (3.16 μmol m−2 s−1). Fertilization increased Amax across species by 19% (F1, 72 = 7.65, P = 0.007). Increased radiation had no significant effect on Amax, nor were there any significant species by radiation interactions. Rd differed significantly among species (F4, 76 = 12.7, P < 0.001), with schist specialist C. virchowii having the lowest (0.41 μmol m−2 s−1) and soil generalist M. insipida having the highest (1.01 μmol m−2 s−1). Rd increased by 25% across species in response to fertilization (F1, 76 = 8.06, P = 0.006) and 27% in response to increasing radiation (F1, 76 = 9.12, P = 0.003) across all species (Appendix 2). AQY differed significantly among species (F4, 76 = 3.38, P = 0.013), with schist specialist C. virchowii having the highest (0.026 μmol m−2 s−1) and schist specialist C. lividula having the lowest (0.018 μmol m−2 s−1). AQY did not respond significantly to increased radiation, nor were there any significant species by radiation or species by fertilization interactions. LCP (Rd/AQY) responded similarly as its components, with schist specialist C. virchowii having the lowest LCP (Tukey Simultaneous Tests, P < 0.05). Electron transport rate efficiency (ETR/A) (units of electrons required to assimilate one unit of CO2) was lower for schist specialist C. virchowii than for soil generalists F. laurifolium and M. insipida (Tukey Simultaneous Tests, P = 0.017 and P = 0.016, respectively). ETR efficiency did not respond significantly to fertilization or radiation treatments. Only C. virchowii supported the hypothesis that schist specialists would have lower LCP than soil generalists. Contrary to this hypothesis, schist specialist C. lividula had higher Rd than soil generalists C. mackinnoniana and F. laurifolium.
P concentration, P-use efficiency and growth
Root, stem and leaf P concentrations (Table 2) increased as fertilization increased by 162%, 133% and 125%, respectively (F3, 14, P < 0.05) (Appendix 2). Increased uptake of P into roots, stems and leaves resulted in a marked decrease in PUE across all species as fertilization was increased (F3, 158 = 88.3, P < 0.001). Schist specialist C. virchowii had the lowest tissue P concentrations and, subsequently, higher PUE in the lowest fertility treatment than any other species (Tukey Simultaneous Tests, P < 0.001), but in fertility levels above 1.88 g fertilizer per pot tissue P and PUE values among species were similar (Table 1, Figure 1f). Biomass increment per crown P content (Crown-P-prod) decreased by 64% across all species with fertilization (F3, 160 = 39.6, P < 0.001). Schist specialist C. virchowii had higher Crown-P-prod (179 g g−1 wk−1) than any other species (Tukey Simultaneous Tests, P < 0.001), whereas soil generalist F. laurifolium had the lowest (77 g g−1 wk−1) (Tukey Simultaneous Tests, P < 0.001). Biomass increment per P content in biomass (Bio-P-prod) followed a similar pattern as Crown-P-prod among species and treatments. Only C. virchowii provides support for the hypothesis that schist specialists would have higher PUE in the low fertility treatments than the soil generalists. Contrary to this hypothesis, schist specialist C. lividula had high P concentrations in all treatments and similar PUE values to soil generalist C. mackinnoniana in the lowest fertility treatment.
Net assimilation rate (NAR) differed significantly among species (F4, 176 = 42.5, P < 0.001), with schist specialist C. virchowii having the lowest and soil generalist C. mackinnoniana having the highest NAR (Figure 1g). Fertilization increased NAR (F3, 176 = 62.6, P < 0.001), with C. mackinnoniana having the greatest response and C. virchowii showing the least response (Figure 1g). NAR increased by 21% in response to radiation across all species. Growth response to fertilizer was species-specific and similar to NAR, with RGR of schist specialist C. virchowii having the lowest response to fertilization and soil generalists F. laurifolium having the highest RGR response (Figure 1h). Across all fertility treatments, schist specialist C. virchowii had the lowest RGR and soil generalist C. mackinnoniana had the highest RGR (Tukey Simultaneous Tests, P < 0.001) (Figure 1h). Radiation had no significant effects on RGR, nor were there any significant species by radiation interactions. Only the response of schist specialist C. virchowii supports the hypothesis that schist specialists would have lower NAR and RGR than soil generalists. Contrary to our hypotheses, schist specialist C. lividula was equally plastic in NAR and RGR as soil generalists C. mackinnoniana and F. laurifolium.
Trait ordination
The first two principal components explained 70% of the total variance among plant traits. Considering these two principal components, there appeared to be two relatively orthogonal dimensions of trait variation across all species (Figure 2a). The first of these dimensions reflected biomass allocation patterns between roots and leaves and was associated strongly with RGR (Figure 2a, broken arrows). The second dimension of variation reflected leaf physiology, but was also associated with SRL (Figure 2a, heavy solid arrows). Fertilization strongly contributed to the variance within the first dimension (biomass allocation and RGR), whereas fertilization and radiation contributed about equally to the variance within the second dimension (leaf physiology and SRL) (Table 1, Figure 2b). PUE, an outcome of both physiology and biomass allocation, was correlated with both dimensions of trait variation.
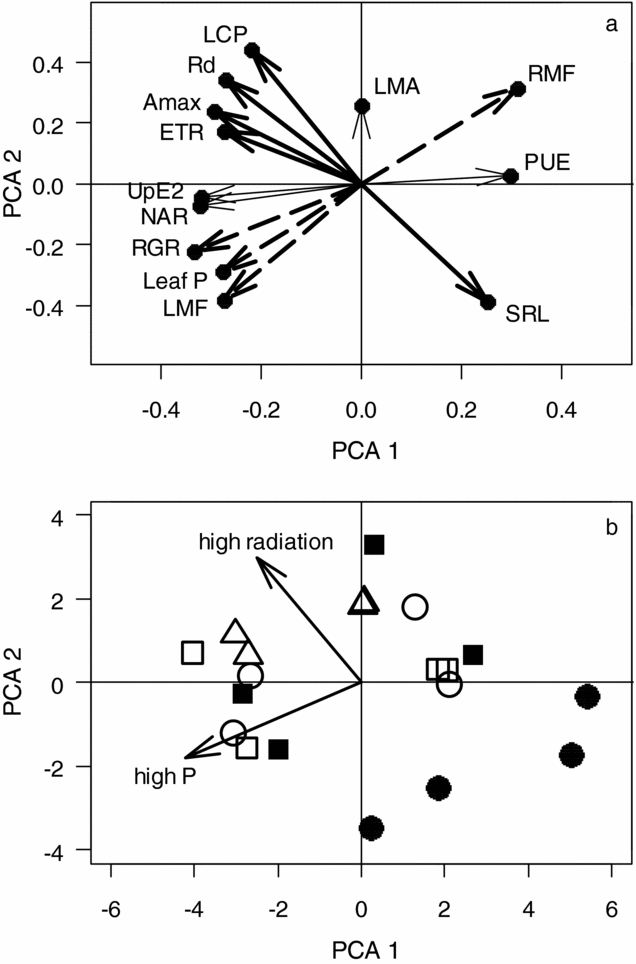
Figure 2. Principal component coefficients represented as trait eigenvectors (a), and principal component scores for all species and treatment combinations (b). The two main dimensions of variation are shown as groups of heavy solid and broken arrows (eigenvectors) (a). Symbols denote different species: infertile soil specialists Ceratopetalum virchowii (●) and Cryptocarya lividula (■), and soil generalists Cryptocarya mackinnoniana (○), Franciscodendron laurifolium (□) and Myristica insipida (∆) (b). Radiation and fertilization treatments resulted in general shifts in trait-space, as denoted by the ‘high radiation’ and ‘high P’ arrows (b). LMF = leaf mass fraction. LMA = leaf mass area. RMF = root mass fraction. SRL = specific root length. UpE2 = P uptake efficiency. NAR = Net assimilation rate. PUE = P-use efficiency. RGR = relative growth rate. ETR = electron transport rate. Amax = maximum carbon assimilation rate. Rd = dark respiration rate. LCP = light compensation point. Gas exchange descriptions are given in Appendix 1.
DISCUSSION
Structure and biomass allocation
Success of schist specialist C. virchowii seedlings on P-poor soils is likely associated with its high SRL (largely non-plastic within species) and high RMF under low-fertility conditions. SRL values for C. virchowii are among the highest we could find in the literature. High RMF coupled with high SRL resulted in a dense and fibrous root system.
Although this root system is well suited for nutrient-poor soils (Hodge Reference HODGE2004), it is not necessarily ideal for exploiting high-fertility patches (e.g. slow-release fertilizer pellets) considering that P uptake (UpE1 and UpE2) demonstrated little plasticity and was low, relative to the other species, in the higher fertility treatments.
Conversely, C. lividula did not have markedly high SRL, but had higher and more plastic RMF and UpE1 than the soil generalists, suggesting it is well-suited for exploiting nutrient-rich patches (e.g. slow-release fertilizer pellets). Plastic root morphology and allocation are traditionally associated with a competitive strategy (i.e. K-selected species; Chapin et al. Reference CHAPIN, MATSON and MOONEY2002, Grime Reference GRIME2002), but were evident for this species that is known to have a high PUE when mature, and grows exclusively on P-poor soils (Gleason et al. Reference GLEASON, READ, ARES and METCALFE2009, Reference GLEASON, READ, ARES and METCALFE2010). This suggests that coexistence of C. lividula and C. virchowii may be facilitated by different root foraging strategies on poor soils – C. lividula explores further and exploits nutrient-rich patches, whereas C. virchowii allocates similar root biomass towards extracting less labile P from more proximal sources.
Root and leaf physiology
Contrary to the hypothesis that the schist specialists would possess similar root and leaf physiology, the two schist specialists differed markedly from one another. The success of schist specialist C. virchowii seedlings on P-poor soils is likely associated with its low tissue P concentrations, and subsequently, higher PUE and markedly lower Rd than all other species. Additionally, low UpE2 of C. virchowii, relative to all other species, suggests that P uptake in C. virchowii seedlings is limited by the rates of P mineralization and diffusion through the soil matrix, rather than the rate of P uptake at the root surface (Nye Reference NYE1977). In contrast to schist specialist C. virchowii, the success of schist specialist C. lividula on P-poor soils is likely associated with markedly plastic root morphology and leaf physiology, facilitating acquisition of patchy or ephemeral sources of nutrients and radiation (i.e. sunflecks and/or canopy gaps). This markedly plastic response to fertilization (both above and below ground) in an obligate infertile soil specialist runs contrary to current theory (Grime Reference GRIME2002), and suggests the efficacy of both plastic and intrinsic traits in conferring advantage in at least one infertile habitat.
P-use efficiency and growth
High PUE is common to both schist specialists in the lowest fertility treatment, and is likely necessary for success in this environment. High RGR, on the other hand, is closely related to leaf P, biomass allocation and crown productivity (NAR), rather than leaf-level photosynthesis or root-level morphology (SRL), and is likely required for success in fertile environments (Figure 2). Moreover, traits conferring high RGR (high leaf P, high LMF, low RMF) were not traded for traits conferring shade tolerance (low LCP, low Rd). Thus, to a large extent, the seedlings in this study adjusted their leaf physiology independently from their biomass allocation patterns, with some notable caveats. SRL and LMA, traits important for nutrient and radiation acquisition, varied markedly among species, but relatively little within species. Thus, the ability to optimize either nutrient or radiation acquisition is determined by intrinsic traits of the species, in addition to plasticity in other traits (e.g. RMF, LAR). Nevertheless, success of seedlings on P-poor schist does not require high SRL, but can be accomplished by increased biomass allocation to roots (i.e. plastic RMF) coupled with leaf traits conferring shade tolerance (low LCP, Rd). Thus, success on either P-poor or P-rich soils is likely a combined outcome of different plastic and intrinsic plant traits, making generalizations about plant functional groups difficult.
Conclusion
Functional traits can vary markedly with the same functional group, as we show here, and as has been shown elsewhere (Lusk et al. Reference LUSK, CONTRERAS and FIGUEROA1997). These results agree well with the theory of limiting similarity within habitats (MacArthur & Levins Reference MACARTHUR and LEVINS1967), a concept already appreciated, but likely to be most important where/when plant growth is not markedly limited by water or nutrients (Cavender-Bares et al. Reference CAVENDER-BARES, ACKERLY, BAUM and BAZZAZ2004, Paoli et al. Reference PAOLI, CURRAN and ZAK2005, Reich et al. Reference REICH, UHL, WALTERS and ELLSWORTH1991). It is also probable that important traits that were not measured in this study (e.g. mycorrhizal dynamics, herbivory and survival, fecundity and dispersal) play important roles in excluding schist specialists from fertile soils. Regardless of the importance of these unknown factors, we found that infertile-soil specialists have different structural and physiological traits, but achieve seemingly equal success on infertile soils. Thus, the species in this study have different paths to success, each species possessing different suites of intrinsic and plastic traits that contribute substantially to their fitness and coexistence on infertile soils.
ACKNOWLEDGEMENTS
We thank Dan Metcalfe and Andrew Ford for their help with tree identification and for many productive discussions. We would also like to thank Kumi Gleason and Stuart Kerr for assisting in the glasshouse and laboratory. Gary Paoli and two anonymous reviewers provided important input on earlier versions of this manuscript. The research was supported by the Holsworth Wildlife Research Endowment, ANZ Charitable Trust, Australia, and the Monash Small Grant Scheme, Monash University, Australia. This study was completed under Queensland EPA permit no. WITK03219805.
Appendix 1. Explanation of gas exchange and fluorescence measurements.

Appendix 2. Growth, allocation, nutrient-use and gas-exchange measures for seedlings grown under four fertilization levels and two radiation levels. Data are shown as the mean ± 1 SE (n = 5). Traits that have been corrected for biomass (using a biomass covariate) have been marked with ‘‡’. CerVir = Ceratopetalum virchowii (schist specialist). CryLiv = Cryptocarya lividula (schist specialist). CryMac = Cryptocarya mackinnoniana (soil generalist). FraLau = Franciscodendron laurifolium (soil generalist). MyrIns = Myristica insipida (soil generalist). LMF = leaf mass fraction. LAR = leaf area ratio. LMA = leaf mass area. RMF = root mass fraction. SRL = specific root length. SRA = specific root area. RAR = root area ratio. UpE1 = P uptake efficiency; P uptake per unit root mass. UpE2 = P uptake efficiency; P uptake per unit root area. NAR = net assimilation rate. Crown-prod = crown P productivity. Bio-P-prod = biomass P productivity. PUE = P-use efficiency. RUE = radiation use efficiency. Bio inc = biomass increment. RGR = relative growth rate. Gas exchange descriptions are given in Appendix 1.








