INTRODUCTION
Global terrestrial biodiversity reaches its peak in the tropical forests of South-East Asia and the neotropics (Fine & Ree Reference FINE and REE2006, Gentry Reference GENTRY1992, Slik et al. Reference SLIK, ARROYO-RODRÍGUEZ, AIBA, ALVAREZ-LOAYZA, ALVES, ASHTON, BALVANERA, BASTIAN, BELLINGHAM, VAN DEN BERG, BERNACCI, DA CONCEIÇÃO BISPO, BLANC, BÖHNING-GAESE, BOECKX, BONGERS, BOYLE, BRADFORD, BREARLEY, BREUER-NDOUNDOU HOCKEMBA, BUNYAVEJCHEWIN, MATOS, CASTILLO-SANTIAGO, CATHARINO and CHAI2015, ter Steege et al. Reference TER STEEGE, PITMAN, SABATIER, BARALOTO, SALOMÃO, GUEVARA, PHILLIPS, CASTILHO, MAGNUSSON, MOLINO, MONTEAGUDO, NÚÑEZ VARGAS, MONTERO, FELDPAUSCH, HONORIO CORONADO, KILLEEN, MOSTACEDO, VASQUEZ, ASSIS, TERBORGH, WITTMANN, ANDRADE, LAURANCE, LAURANCE and MARIMON2013). However, studies of historical biogeography have identified contrasting scenarios of change in geology and climate leading to high diversity in each region (Jaramillo et al. Reference JARAMILLO, HOORN, SILVA, LEITE, HERRERA, QUIROZ, DINO, ANTONIOLI, Hoorn and Wesselingh2010, Morley Reference MORLEY2000). Therefore, to understand the processes involved in the assembly of high diversity in South-East Asia requires knowledge of the tectonic and climatic histories of the region (de Bruyn et al. Reference DE BRUYN, STELBRINK, MORLEY, HALL, CARVALHO, CANNON, VAN DEN BERGH, MEIJAARD, METCALFE, BOITANI, MAIORANO, SHOUP and VON RINTELEN2014, Hall Reference HALL2002, Reference HALL2009; Morley Reference MORLEY2000), and patterns of diversification within individual clades. Evaluation of all these processes is on-going, with the latter continuing to be clarified from molecular studies.
The deep time history of tropical forests can be evaluated from macrofossil and fossil pollen records (Morley Reference MORLEY2000) and these are examined within the current perspective of the tectonic and palaeogeographic evolution of the region (De Bruyn et al. Reference DE BRUYN, STELBRINK, MORLEY, HALL, CARVALHO, CANNON, VAN DEN BERGH, MEIJAARD, METCALFE, BOITANI, MAIORANO, SHOUP and VON RINTELEN2014, Hall Reference HALL, Hall and Holloway1998, Reference HALL2002, Reference HALL2009; Morley et al. Reference MORLEY, MORLEY and SWIECICKI2016, Reference MORLEY, MORLEY and SWIECICKI2017). Pollen records provide information on the time of appearance and patterns of dispersal of families, genera and some species based on characteristic pollen, and, by whole-assemblage analyses, provide a glimpse of the history of biomes. The latter allows differentiation of the past distribution of perhumid megathermal forests versus seasonally dry vegetation. There are some aspects of the present-day distribution of tropical forests that are poorly understood, and this has hampered our understanding of how to interpret the significance of past vegetation and climate changes. The poleward limit of tropical forests is determined by the occurrence of occasional killing frosts (Ashton Reference ASHTON2014, Morley Reference MORLEY2000), and the term ‘subtropical’, referring to present day forests outside the tropics, is, in that context, unnecessary (Ashton Reference ASHTON2014, Webb Reference WEBB1959). Similarly, the term ‘paratropical’ introduced by Wolfe (Reference WOLFE1979) for the same forests, has never been taken up by ecologists (Morley Reference MORLEY2000), and hence is not used here. Seasonal vegetation classifications are also inconsistent, especially with respect to seasonally dry forests and savanna. This is particularly the case with respect to classifications from India (Champion Reference CHAMPION1936), and Indochina (Blasco et al. Reference BLASCO, BELLAN and AIZPURU1996, Reference BLASCO, WHITMORE and GERS2000; Gaussen Reference GAUSSEN1978). The broad overview of tropical Asian forests provided by Ashton (Reference ASHTON2014) places the seasonal forests across India, Indochina and Sunda into a single coherent perspective for the first time and reveals that the main divisions of the original forest cover can be characterized physiognomically (sensu Dansereau Reference DANSERAU1957, Daubenmire Reference DAUBENMIRE1968, Webb Reference WEBB1959) and floristically (Table 1).
Table 1. Main characters of principal vegetation types; based on Ashton (Reference ASHTON2014).

A primary control on physiognomy and floristics is the length of the dry season, which impacts on flowering regimes, and the regularity and intensity of fire affecting the forest canopy, which determines the representation of evergreen versus deciduous trees (Ashton Reference ASHTON2014). The forests of Indochina are often visualized as consisting of ‘monsoon forests with patches of tropical rain forest’ (Richards Reference RICHARDS1996). The ‘patchiness’ relates to the presence of uplands on the one hand, which attract orographic rain but also create rain shadows, and the impact of the East Asian monsoon on the other, that brings additional moisture to the north-eastern part of the region (Figure 1). Also important is the orographic effect of the Himalaya, and their heat-retaining capacity, which pulls rain forests further poleward in northern Myanmar and Assam than anywhere else (Ashton Reference ASHTON2014).
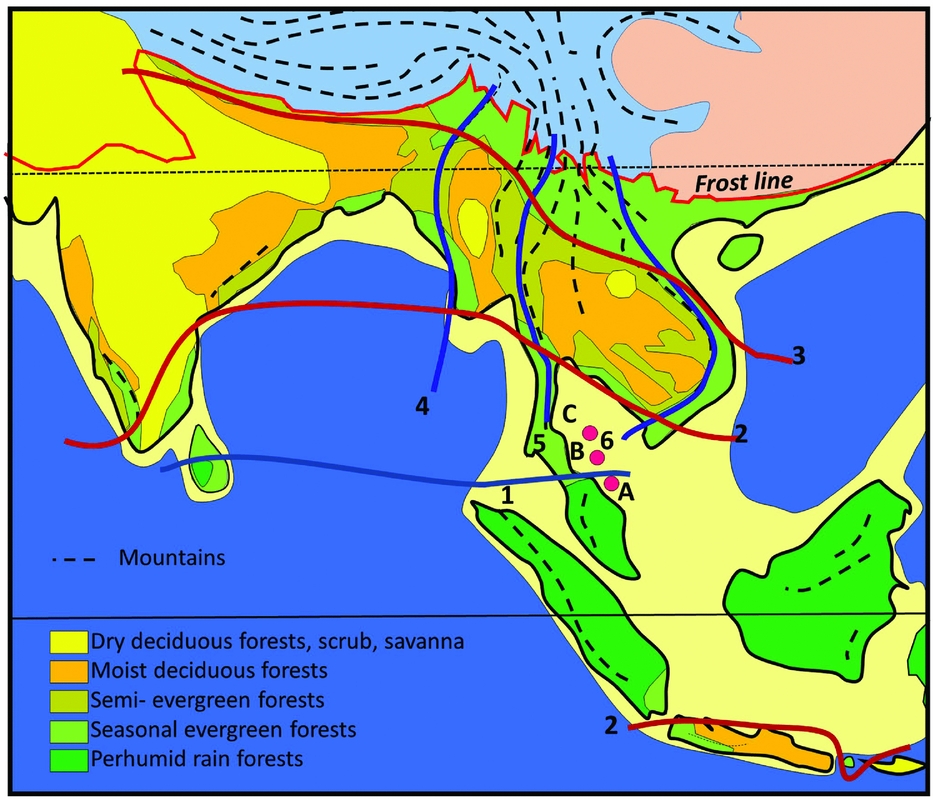
Figure 1. Vegetation map of South and South-East Asia, simplified from Ashton (Reference ASHTON2014). (1) Kangar-Pattani Line, marking the northern boundary of the perhumid tropics, with flowering periodicity driven by the southern oscillation; (2) equatorward limit of seasonally dry climates, which control the distribution of semi-evergreen and deciduous forests and align with the Kra Isthmus; (3) northern limit of seasonally dry climates in the lowlands; (4) lineament of Indo-Burmese Range, dividing the deciduous forest belt: (5) lineament of Sino-Burmese Ranges, which align with the Kra Isthmus; (6) lineament of Annamite range, that coincide with the western limit of seasonal evergreen forests in western Indochina. Temperate mountain climates, blue; temperate lowland climates, pink. Mountain ranges as dashed lines, and in the tropics, these are characterized by tropical mountain climates. A, B, C, locations for wells in Figure 6.
This paper begins with a brief overview of the Cretaceous and Paleocene flora and vegetation of South and South-East Asia, which is becoming clarified following geological studies of West Sarawak (Breitfield et al. Reference BREITFELD, GALIN, HALL, SEVASTJANOVA, FORSTER and LISTER2014) in the same area that Muller (Reference MULLER1968) undertook palynological work. With respect to the Eocene collision of India with Asia, microfossil and macrofossil data from South-East Asia and India as well as a meta-analysis of molecular data of clades that occur in both areas (Klaus et al. Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016) are reviewed to illustrate the likely scenario of dispersals of plant taxa between these land masses. The enrichment of the South-East Asian flora then continued with immigration from Australasia following the mid-Cenozoic collision of the Australian Plate with Asia, the impact of the Himalayan uplift on the development of the Indian Monsoon and its effect on dispersals into India, and the effect of tectonic uplift on the climate and the flora and vegetation of Sunda. This is followed by an overview of the effect of late Neogene global cooling and drying and how it affected the region, and the likelihood of a Quaternary seasonal climate corridor across equatorial Sunda. In conclusion, 12 dispersal and vicariance events, are proposed into or out of the region, from the time of origin of angiosperms until the Quaternary, that help to account for the floristic diversity of this region.
This review has been prepared for the Journal of Tropical Ecology primarily for, but not restricted to, an audience of tropical ecologists, and hence geological jargon is kept to a minimum.
CRETACEOUS AND PALEOCENE VEGETATION
Armen Takhtajan (Reference TAKHTAJAN1969) suggested that the region between ‘Assam and Fiji’ was the cradle of the flowering plants (reviewed in Briggs Reference BRIGGS1995). The current perspective based on fossil and molecular data suggests the opposite, that the South-East Asian area was indeed a backwater of angiosperm evolution until the collision of the Indian Plate with Asia during the early Cenozoic.
The precise area of origin of flowering plants remains unclear, because the first angiosperms have left no fossil record (Muller Reference MULLER1970, Soltis et al. Reference SOLTIS, BELL, KIM and SOLTIS2008). As a result, the time of appearance based on molecular evidence is considerably older than the first fossils, with current estimates based on molecular data ranging from 183–147 Ma within the Middle to Early Jurassic (Bell et al. Reference BELL, SOLTIS and SOLTIS2010). However, the earliest basal angiosperm pollen records are from the Late Valanginian and Hauterivian (130–138 Ma) of North Africa and the Middle East (Brenner Reference BRENNER1974, Gubeli et al. Reference GUBELI, HOCHULI and WILDI1984, Thusu et al. Reference THUSU, VAN DER EEM, EL-MEHDAWI, BU-ARGOUB, El-Arnauti, Owens and Thusu1988), and thus suggest a low-latitude, palaeotropical area of origin (Barrett & Willis Reference BARRETT and WILLIS2001). From the Barremian (125–130 Ma) onward, angiosperm occurrences are widespread, with records from Gabon, Brazil, England, Russia, China and North America (Barrett & Willis Reference BARRETT and WILLIS2001, Hickey & Doyle Reference HICKEY and DOYLE1977) and Japan (Legrand et al. Reference LEGRAND, YAMADA and NISHIDA2014). They arrived about 10 Ma later in Australia (Dettmann Reference DETTMANN and Hill.1994) and India (Srivastava Reference SRIVASTAVA and Maheswari1983, Morley Reference MORLEY2003).
On the other hand, eudicots clearly originated in western Gondwana during the Late Barremian or Aptian (112–125 Ma) with widespread pollen records from Gabon, Brazil and the Middle East (Doyle Reference DOYLE2012), and subsequently dispersed poleward during the Albian (99.5–12 Ma) and Cenomanian (94–99.5 Ma, Hickey & Doyle Reference HICKEY and DOYLE1977).
South-East Asian Early Cretaceous pollen assemblages are characterized mainly by abundant gymnosperm pollen with Cheirolepidaceae (extinct gymnosperms) and Araucaria (Araucariaceae), suggesting a seasonally dry vegetation with minimal representation of angiosperms (Morley Reference MORLEY2000, Racey et al. Reference RACEY, GOODALL, LOVE, POLACHAN, JONES, Angsuwathana, Wongwanich, Tansathien, Wongsomsak and Tulyatid1994). An Early Cenomanian assemblage from Myanmar yielded common Sequoia-type pollen but rare angiosperms (Cruickshank & Ko Reference CRUICKSHANK and KO2003), contrasting with African and American contemporaneous assemblages that contain increasing numbers of angiosperm pollen (Crane Reference CRANE, Friis, Chaloner and Crane1987). The Myanmar locality has also yielded beautifully preserved flowers of Ceratopetalum (Cunoniaceae) (Chambers et al. Reference CHAMBERS, POINAR and BUCKLEY2010, Poinar & Chambers Reference POINAR and CHAMBERS2017), a family presently restricted to Gondwana but formerly widespread (Shoenberger & Friis, Reference SCHÖNENBERGER and FRIIS2001).
Muller (Reference MULLER1968) found a low diversity of triaperturate pollen in Santonian (83.5–86 Ma) (Morley Reference MORLEY, Hall and Holloway1998) sediments from Sarawak that also exhibit much reduced morphological diversity than assemblages from age-equivalent sediments in West Africa (Belsky et al. Reference BELSKY, BOLTENHAGEN and POTONIE1965, Jardine & Magloire Reference JARDINE and MAGLOIRE1965, Morley Reference MORLEY2000).
A low-diversity lowland flora is also suggested for the Paleocene (56–66 Ma) of Sarawak (Figure 2a), which was characterized by taxa with Laurasian affinities, based on pollen assemblages which include east Asian aspect Aquilapollenites (possibly Santalaceae), probable Ulmaceae and Ilex (Aquifoliaceae), and a few widely dispersed megathemal elements, such as Anacolosa (Olacaceae), Calamoidae, Apocynaceae, Myrtaceae and the mangrove palm Nypa (Morley Reference MORLEY, Hall and Holloway1998, Reference MORLEY2000; Muller Reference MULLER1968). Upland areas, such as the Schwaner Mountains in Borneo, are likely to have had vegetation with diverse Laurasian conifers. This is suggested because the bisaccate-rich assemblages described by Muller (Reference MULLER1968) were obtained from alluvial fan deposits shown from zircon analysis to be derived from the Schwaner Mountains (Breitfeld et al. Reference BREITFELD, GALIN, HALL, SEVASTJANOVA, FORSTER and LISTER2014). This mountain range must, at that time, have been in the order of 2000–3000m in elevation to support such a flora at such low latitudes.
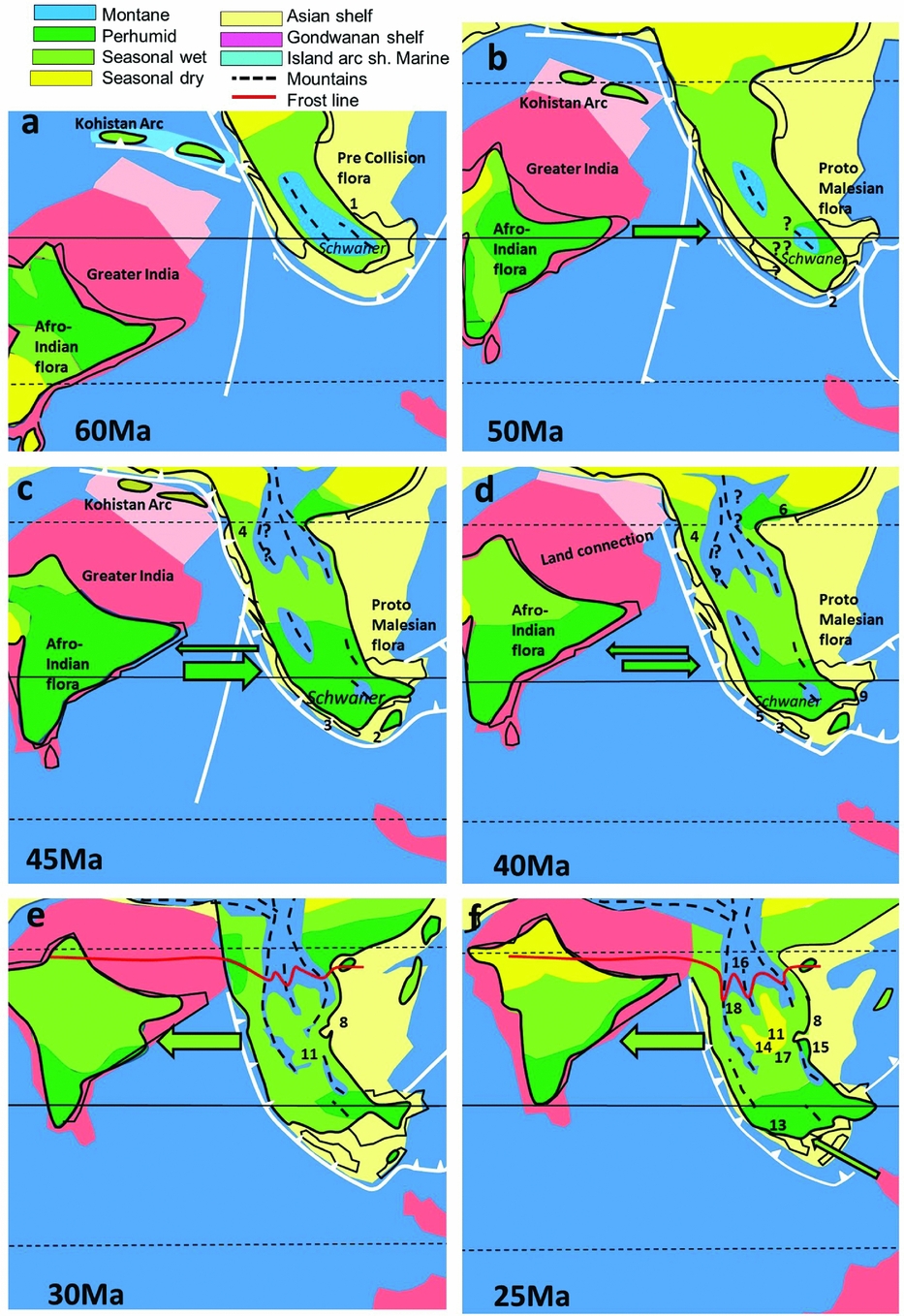
Figure 2. Palaeoclimate maps and plant dispersals, 60–25 Ma. Tectonic reconstructions from Hall (Reference HALL, Gower, Johnson, Richardson, Rosen, Rüber and Williams2012b), palaeoclimate reconstructions, this paper. Numbers refer to localities with palaeoclimate data, listed in Appendix 1. Solid arrows show direction of plant dispersals, faunal dispersals dotted. Mid Paleocene reconstruction, prior to collision of India with Asia (a); latest early/earliest middle Eocene, India and South-East Asia are at the same latitude but without a land connection (b); mid middle Eocene, India and South-East Asia at same latitude and within same perhumid climate zone, but still without a land connection (c); end of middle Eocene, land connection between India and South-East Asia (d); India drifts into northern high-pressure zone during Oligocene, seasonally dry climates predominate across India and South-East Asia (e) and (f).
The depauperate nature of Cretaceous and Paleocene South-East Asian lowland vegetation is emphasized further by the fact that none of the main angiosperm families that characterize the region today actually evolved in the area. Examples include: Annonaceae (Doyle et al. Reference DOYLE, SAUQUET, SCHARASCHKIN and LE THOMASY2004, Richardson et al. Reference RICHARDSON, CHATROU, MOLS, ERKENS and PIRIE2004), Arecaceae (Couvreur & Baker Reference COUVREUR and BAKER2013), Dipterocarpaceae (Heckenhauer et al. Reference HECKENHAUER, SAMUEL, ASHTON, TURNER, BARFUSS, TAE-SOO, TEMSCH, MCCANN, ABU SALIM, ACHALA, ATTANAYAKE and CHASE2017, Moyersoen Reference MOYERSOEN2006), Sapotaceae (Richardson et al. Reference RICHARDSON, BAKAR, TOSH, ARMSTRONG, SMEDMARK, ANDERBERG, SLIK and WILKIE2013) and Myrtaceae (Berger et al. Reference BERGER, KRIEBEL, SPALINK and SYTSMA2016, Thornhill et al. Reference THORNHILL, HO, KÜLHEIM and CRISP2015) and probably Anacardiaceae, Burseraceae, Ebenaceae and Moraceae that likely originated in West Gondawana; Meliaceae in Africa (Muellner et al. Reference MUELLNER, SAVOLAINEN, SAMUEL and CHASE2006) and Myristicaceae in Africa-Madagascar (Doyle et al. Reference DOYLE, SAUQUET, SCHARASCHKIN and LE THOMASY2004). In addition, Clusiaceae and possibly Rubiaceae may be Boreotropical families (Davis et al. Reference DAVIS, WEBB, WURDACK, JARAMILLO and DONOGHUE2005).
In suggesting the area of origin for angiosperms, Takhtajan was heavily influenced by the richness of primitive angiosperms in the seasonal evergreen and warm temperate rain forests in Indochina and the area surrounding the Coral Sea. The concentration of basal angiosperms and Magnoliidae in these areas has since been shown to relate to global tectonics and Cenozoic climate change (Morley Reference MORLEY, Metcalfe, Smith, Morwood and Davidson2001a). The survival of such diverse archaic Boreotropical taxa in the rain forests of Indochina may have been possible due to the long-term presence of north-south Sino-Burmese and Annamite Ranges, which have provided appropriate habitats and opportunities for plants to migrate and shift in altitude, in response to changing global climates, throughout the Cenozoic. In contrast, the concentration of primitive taxa in the region surrounding the Coral Sea relates to their retention on southern landmasses as they drifted into warmer latitudes as global climates cooled during the Neogene (Morley Reference MORLEY, Metcalfe, Smith, Morwood and Davidson2001a).
THE COLLISION OF INDIA WITH ASIA AND EOCENE DIVERSIFICATION OF THE SOUTH-EAST ASIAN FLORA
It was the collision of the Indian Plate with Asia in the Eocene that was most influential on the eventual development and subsequent diversification of the South-East Asian flora.
After being juxtaposed with Australia and Antarctica during the early Early Cretaceous (Berriasian/Valanginian) the Indian Plate drifted northward (starting around 135 Ma) together with Madagascar until the Coniacian (86–88.5 Ma), during which time many elements of the African flora dispersed via Madagascar to India (Morley Reference MORLEY, Hall and Holloway1998, Reference MORLEY2000). The Indian Plate then separated from Madagascar (Storey et al. Reference STOREY, MAHONEY, SANDERS, DUNCAN, KELLEY and COFFIN1995) and rapidly drifted northward as an isolated island, splitting from the Seychelles at about 66 Ma (Chatterjee et al. Reference CHATTERJEE, GOSWAMI and SCOTESE2013) before slowing down in the late Paleocene as it collided with the Kohistan-Ladakh Island Arc (Hall Reference HALL2012a, Khan et al. Reference KHAN, WALKER, HALL, BURKE, SHAH and STOCKLI2009), although some authors place this collision earlier (Chatterjee & Bajpai Reference CHATTERJEE and BAJPAI2016). The subsequent collision with Asia was most likely during the middle Eocene (Ali & Aitchinson Reference ALI and AITCHISON2008, Klaus et al. Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016) although older (Beck Reference BECK1995, Rage et al. Reference RAGE, CAPPETTA, HARTENBERGER, JAEGER, SUDRE, VIANEY-LIAUD, KUMAR, PRASAD and SAHNI1995) and younger (van Hinsbergen et al. Reference VAN HINSBERGEN, LIPPERT, DUPONT-NIVET, MCQUARRIE, DOUBROVINE, SPAKMAN and TORSVIK2012) collision ages have been proposed.
As the Indian Plate drifted northward, it passed through the southern-hemisphere high-pressure zone, and then into equatorial latitudes during the Paleocene and early Eocene (48–56 Ma). After transgressing different climatic zones India was not left with a generalized flora, as suggested by Raven & Axelrod (Reference RAVEN and AXELROD1974) but showed a rapid diversity increase with time (Morley Reference MORLEY2000, Figure 8.2). Prior to collision with Asia it carried a flora with three distinct components: (1) an ancient autochthonous gondwanic component; (2) an allochthonous component from Africa consisting of megathermal angiosperms; and (3) a diverse endemic component, which evolved as India drifted across many climatic zones. Immediately prior to collision with Asia, the Indian Plate was characterized by a perhumid climate and bore a rich, and rapidly diversifying megathermal angiosperm flora rooted in Africa.
The Kohistan-Ladakh Island Arc
Vertebrate fossils suggest that the Indian Plate was not wholly isolated during its northward passage, but had connections with Africa and Europe (Briggs Reference BRIGGS2003, Chatterjee & Scotese Reference CHATTERJEE and SCOTESE1999). This apparent conflict is beginning to be resolved with the clarification of the role of the Kohistan-Ladakh Island Arc (Treolar et al. Reference TREOLAR, REX, GUISE, COWARD, WINDLEY, PETERSON, JAN and LUFF1989) that may have provided a corridor to Africa (Kapur et al. Reference KAPUR, DAS, BAJPAI and PRASAD2017) and Europe (Smith et al. Reference SMITH, KUMAR, RANA, FOLIE, SOLÉ, NOIRET, STEEMAN, SAHNI and ROSE2017) immediately prior to collision with Asia. Recent studies of vertebrates from the early Eocene Cambay Shale in Gujarat show a surprising fauna with European affinities (Figure 3), including frogs (Folie et al. Reference FOLIE, RANA, ROSE, SAHNI, KUMAR, SINGH and SMITH2013), snakes (Rage et al. Reference RAGE, FOLIE, RANA, SINGH, ROSE and SMITH2008), a parrot-like bird (Mayr et al. Reference MAYR, RANA, ROSE, SAHNI, KUMAR, SINGH and SMITH2010), a primate (Rose et al. Reference ROSE, RANA, SAHNI, KUMAR, MISSIAEN, SINGH and SMITH2009), bats (Smith et al. Reference SMITH, RANA, MISSIAEN, ROSE, SAHNI, SINGH and SINGH2007) and perissodactyl-like mammals (Rose et al. Reference ROSE, HOLBROOK, RANA, KUMAR, JONES, AHRENS, MISSIAEN, SAHNI and SMITH2014). It is unclear whether these groups originated in India or Europe, except for the perissodactyls, which are now thought to have originated in India since fossils of their sister group form part of the Cambay Shale fauna (Rose et al. Reference ROSE, HOLBROOK, RANA, KUMAR, JONES, AHRENS, MISSIAEN, SAHNI and SMITH2014). Amber from this locality yields insects with European affinities (Rust et al. Reference RUST, SINGH, RANA, MCCANN, SINGH, ANDERSON, SARKAR, NASCIMBENE, STEBNER and THOMAS2010).

Figure 3. Dispersal routes from Africa to India, and to Europe, along the Kohistan/Ladakh Island Arc. 1, from Madagascar to India prior to Coniacian; 2 from Africa to India via Kohistan/Ladakh Island Arc during the Paleocene; C, between India and Europe during the Early Eocene via Kohistan/Ladakh Island Arc (modified from Smith et al. Reference SMITH, KUMAR, RANA, FOLIE, SOLÉ, NOIRET, STEEMAN, SAHNI and ROSE2017).
It is therefore appropriate to review the origin of the early Eocene London Clay flora, long believed to be derived from Malesia (Chandler Reference CHANDLER1964, Collinson Reference COLLINSON1983, Reid & Chandler Reference REID and CHANDLER1933) as there are many taxa which are common between the London Clay flora and those from the Deccan Traps. However, a full assessment of this issue is outside the scope of this paper. Taxa common to both areas include members of Anacardiaceae, Annonaceae, Euphorbiaceae, Salicaceae, Vitaceae and possibly Burseraceae, Elaeocarpaceae, Meliaceae and Rutaceae (see below). An early Eocene dispersal route to Europe would explain the occurrence of twigs of Anisoptera (Dipterocarpaceae) in the London Clay (Poole Reference POOLE1993), which has a molecular age of about 51 Ma (Morley & Ashton in Ashton Reference ASHTON2014), although their identity is disputed by Ashton, pers. comm. It would also account for the numerous London Clay records of Vitaceae, with latest Cretaceous records in India by Manchester et al. (Reference MANCHESTER, KAPGATE and WEN2013), who proposed an ‘out of India’ dispersal by birds prior to collision with Asia.
It is considered that the collision of India with the Kohistan-Ladakh Island Arc did not have a significant effect on migrations of flora to Asia, as suggested also from vertebrates (Rose et al. Reference ROSE, HOLBROOK, RANA, KUMAR, JONES, AHRENS, MISSIAEN, SAHNI and SMITH2014).
Indian climate and flora prior to collision with Asia
Fossil woods from the Intertrappean beds (67–64 Ma) provide a remarkable perspective of the Indian flora as the Indian Plate approached Asia and these have recently been reviewed by Wheeler et al. (Reference WHEELER, SRIVASTAVA, MANCHESTER and BAAS2017), who consider the flora to be ‘surprisingly modern’ based on the rarity of scalariform perforations. The flora contains a diversity of angiosperm orders, families and genera from many clades with magnoliids (one Annonaceae) and the rosid and asterid core eudicot clades all present. The Malpighiales, Malvales and Sapindales are especially well represented. Intertrappean fossil woods provide the earliest fossil records for the families Anacardiaceae, Salicaceae, Lamiaceae, Lecythidaceae, Malvaceae, Moraceae, Myrtaceae, Simaroubaceae and Vitaceae.
The low incidence of scalariform perforations suggests xeric conditions, which would be in keeping with the late Maastrichtian palaeolatitude of about 19oS. However, the low incidence of distinct growth-ring boundaries suggests an aseasonal (typically perhumid) climate; or perhaps rainfall was uniformly low throughout the year. Clearly the palaeoclimate for the Deccan traps requires further investigation.
During the early Eocene, with the Indian Plate drifting northward to straddle the equator, the climate became perhumid to seasonally wet from north to south, as indicated by the widespread occurrence of coals, bauxites and laterites (Boucot et al. Reference BOUCOT, XU, SCOTESE and MORLEY2013), but drier climates may have been present in the north-west where they reported evaporites.
The early Eocene flora is well documented from the palynological analysis of coal-bearing successions across India (Monga et al. Reference MONGA, KUMAR, PRASAD and JOSHI2015, Prasad et al. Reference PRASAD, FAROOQUI, TRIPATHI, GARG and THAKUR2009, Samant & Phadare Reference SAMANT and PHADARE1997). The pollen flora suggests a setting with a perhumid climate, and tall, dense, closed-canopy rain forests of modern aspect. The diverse flora included Arecaceae and Malvaceae as dominants; and other angiosperm families such as Alangiaceae, Celastraceae, Clusiaceae, Euphorbiaceae, Meliaceae, Myrtaceae, Rubiaceae and Sapotaceae were also widely represented. Podocarpus is the only representative among gymnosperms. Late Paleocene and early Eocene sediments contain the oldest pollen and geochemical fossils attributable to the family Dipterocarpaceae (Dutta et al. Reference DUTTA, TRIPATHI, MALLICK, MATHEWS, GREENWOOD, RAO and SUMMONS2011, Paul et al. Reference PAUL, SHARMA, SINGH, SARASWATI and DUTTA2015). Remarkably, the insect fauna from early Eocene amber contains many elements characteristic of present-day South-East Asian dipterocarp forests (Rust et al. Reference RUST, SINGH, RANA, MCCANN, SINGH, ANDERSON, SARKAR, NASCIMBENE, STEBNER and THOMAS2010). The arrival of Dipterocarpaceae in India might have been either via Madagascar, but with a fossil record for the Late Cretaceous still wanting, or from East Africa via the Kohistan-Ladakh Island Arc (Kapur et al. Reference KAPUR, DAS, BAJPAI and PRASAD2017) during the Paleocene. Maastrichtian (74–66 Ma) palynomorph assemblages from Sudan include pollen which closely resembles that of Dipterocarpus, such as Periretisyncolpites phosphaticus sensu Cole et al. (Reference COLE, ABDELRAHIM, HUNTER, SCHRANK and MOHD SUHAILI BIN2017, Plate 1, Figures 6 & 7) and Leptolena (Sarcolaenaceae) that compares to unidentified ‘tetrad pollen’ (Plate 1, Figure 3). Confirmation requires further evaluation using LM and SEM and if confirmed as dipterocarp and Leptolena pollen, would support the Paleocene dispersal of dipterocarps from Africa via the Kohistan/Ladakh Island Arc. Dispersals from the horn of Africa, which was characterized by a perhumid climate during the Maastrichtian (Upchurch et al. Reference UPCHURCH, OTTO-BLIESNER and SCOTESE1998), may help to explain the apparently sudden diversification of the Indian flora by the Paleocene.
Dispersals between India and South-East Asia
Dispersals between India and South-East Asia are indicated from the palynological record (Morley Reference MORLEY, Hall and Holloway1998, Reference MORLEY2000, Reference MORLEY2003) and augmented by macrofossils. Trends in dispersal between these areas over time are suggested also from a meta-analysis of molecular data from clades which are currently present in both India and Asia (Klaus et al. Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016). Klaus et al. (Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016) determined the mean number of dispersal events (MDE) between the two areas based on the evaluation of 37 phylogenetic datasets (flora and fauna). Deviations in MDE with time are thought to reflect changes in rates of dispersal between the two areas that may interpreted in relation to tectonics and climate.
Middle Eocene
Middle Eocene (37–48 Ma) pollen records from Sulawesi and Java suggest that initial dispersals of plant taxa from the Indian Plate occurred in two stages (Morley Reference MORLEY2014). The first ‘Indian’ elements occur close to the early/middle Eocene boundary, with the appearance of palm pollen referable to Iguanurinae, Lagerstroemia (Lythraceae) and sonneratioids, with taxa dispersing at about 49 Ma, closely followed by Alangium section Conostigma (Alangiaceae) and Durio type (Malvaceae) and then Barringtonia (Lecythidaceae) (Figure 4). The climate was probably distinctly seasonal, suggested by the common occurrence of Restionaceae pollen (Figure 2b).
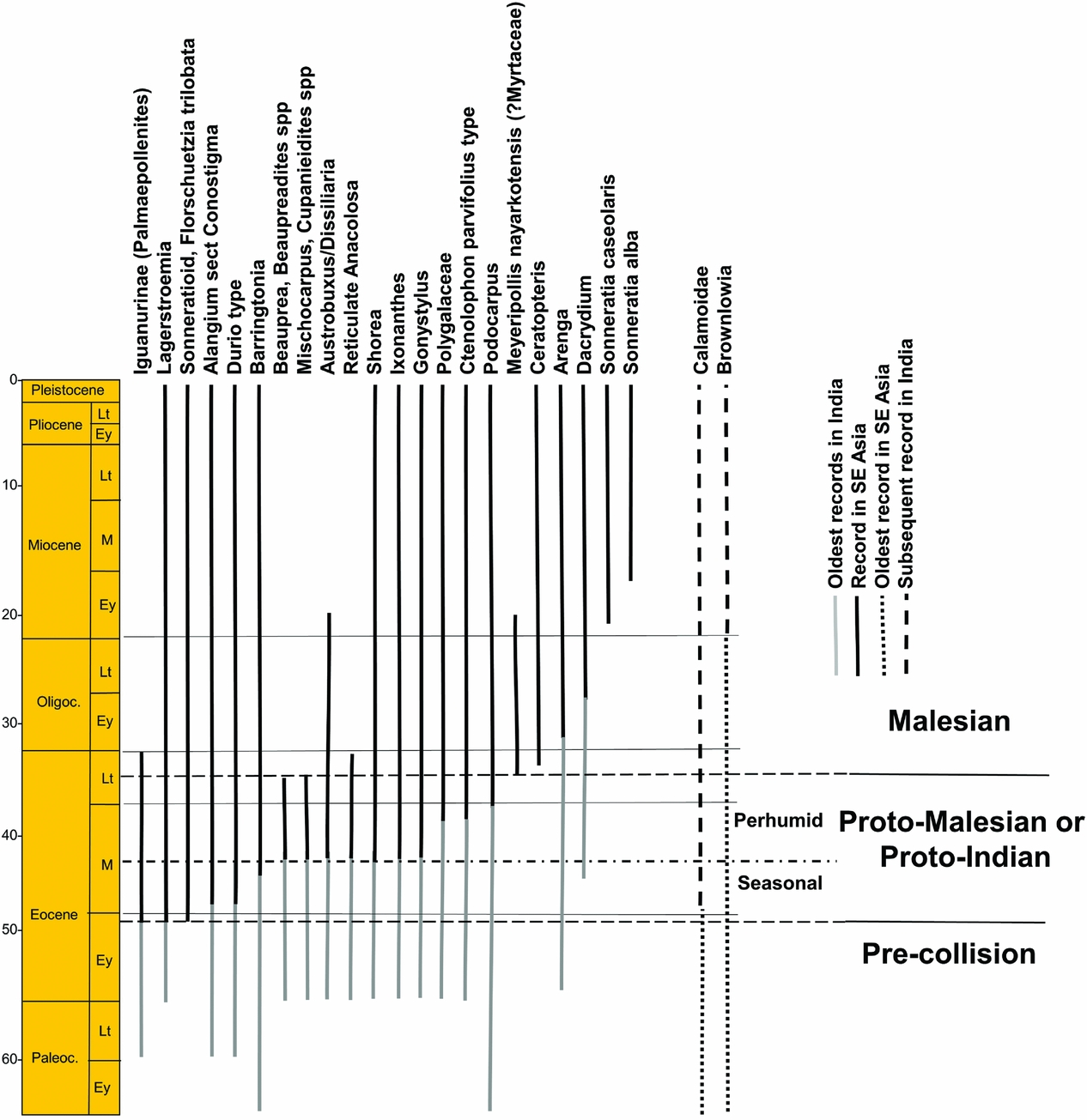
Figure 4. Stratigraphic ranges of pollen (and fossil wood for Shorea and Indian Barringtonia) that occur both in South-East Asia and India (ranges for South-East Asia modified from Morley (Reference MORLEY2014) and van Gorsel et al. (Reference VAN GORSEL, LUNT and MORLEY2014).
There was a change to a more perhumid climate after about 45 Ma at lower-latitude localities, (Figure 2c), coinciding with the appearance of Gonystylus (Gonystylaceae) and Ctenolophon (Ctenolophonaceae) pollen, an increase in abundance of palm pollen and a corresponding reduction of Restionaceae, and a significant increase in palynomorph assemblage diversity (Lelono Reference LELONO2000). Other dispersals from India are of Beauprea (Proteaceae), Mischocarpus (Sapindaceae) and Ixonanthes (Ixonanthaceae). Podocarpus (Podocarpaceae) shows its first appearance in Java at 40 Ma. MDE based on meta-analysis show a corresponding sudden increase from India at 48 Ma with minimal dispersals in the other direction (Klaus et al. Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016).
The first amphibians dispersed after 41 Ma and the first primary freshwater fishes after 38 Ma suggesting a continuous terrestrial connection from this time (Klaus et al. Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016). The likelihood is that until this time most plant dispersal was transoceanic and plants were dispersed by birds or wind prior to the establishment of a land connection. This assumes that similar climates occurred in both areas, and emphasizes the importance of niche conservatism.
Middle Eocene dipterocarps in South-East Asia are represented by Shorea wood and the geochemical biomarker bicadinane from Myanmar (Curiale et al. Reference CURIALE, KYI, COLLINS, DIN, NYEIN, NYUNT and STUART1994, Licht et al. Reference LICHT, BOURA, DE FRANCESCHI, DUCROCQ, AUNG NAING and JAEGER2014). The first dispersals were all winged taxa, with the wingless Stemonoporus, Vateria and Vateriopsis not occurring beyond Sri Lanka and the Seychelles. The presence of the perhumid winged dipterocarp genera Cotylelobium and Dryobalanops with disjunct distributions in Sri Lanka/S India and Borneo (Ashton Reference ASHTON2014) and very old molecular ages (43 and 40 Ma, Morley & Ashton, phylogeny following Gamage et al. Reference GAMAGE, DE SILVA, INOMATA, YAMAZAKI and SZMIDT2006, in Ashton Reference ASHTON2014) might suggest that these taxa could also have dispersed to Borneo during a period of middle Eocene perhumid climate, but without leaving a fossil record. The ancient disjunct taxa Axinandra (Crypteroniaceae) and Trichadenia (Achariaceae) may have followed the same route (Ashton & Gunatilleke Reference ASHTON and GUNATILLEKE1987).
After the appearance of elements of Indian affinity in South-East Asia, the characteristic Paleocene pollen taxa of Muller (Reference MULLER1968) are missing, suggesting that the Indian flora was the more successful, resulting in the extinction of many Paleocene and early Eocene plant taxa. The only Eocene dispersal into India based on pollen is of Calamoidae, which appears in the Paleocene of Sarawak, but is not recorded until the middle Eocene in India (Gupta et al. Reference GUPTA, MITRA, BERA and BANERJEE2003, Misra & Kapoor Reference MISRA, KAPOOR, Biswas, Dave, Garg, Pandey, Maithani and Thomas1994, Ramanujam et al. Reference RAMANUJAM, REDDY and RAMAKRISHNA1997). This is also supported by molecular data, with the genera with Indian representatives (Daemonorops, Plectocomia and Calamus) being deeply nested within genera endemic to South-East Asia (Baker et al. Reference BAKER, SAVOLAINEN, ASMUSSEN-LANGE, CHASE, DRANSFIELD, FOREST, HARLEY, UHL and WILKINSON2009, Barrett et al. Reference BARRETT, BACON, ANTOLELLI, CANO and HOFMANN2016).
Late Eocene and Oligocene
During the late Eocene (33.8–37 Ma) and Oligocene (23.0–33.8 Ma), the climate for much of South-East Asia was seasonal (Figures 2c, d, e). This is suggested by the reduction of perhumid elements in pollen assemblages, the common occurrence of grass pollen, the persistent indications of seasonally inundated swamps surrounding the numerous large lakes that characterize this period (Morley & Morley Reference MORLEY and MORLEY2013), and rarity of coals. Dipterocarps continued to extend their range with late Eocene records from southern China (Feng et al. Reference FENG, TANG, KODRUL and JIN2013). With the ongoing collision of India with Asia, the Tibetan Plateau attained significant elevation (Ding et al. Reference DING, SPICER, YANG, XU, CAI, LI, LAI, WANG, SPICER, YUE, SHUKLA, SRIVASTAVA, ALI KHAN, BERA and MEHROTRA2017, Dupont-Nivet et al. Reference DUPONT-NIVET, HOORN and KONERT2008) but is thought to have been too far north to impact on dispersal of megathermal plant taxa.
Molecular data (Klaus et al. Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016) suggests that dispersals from India to South-East Asia were negligible, but that there were numerous faunal dispersals into India, including frogs, freshwater fish and lizards. With India drifting into the northern subtropical high-pressure zone, and its climate most likely becoming seasonally drier over large areas, it is suggested that the South-East Asian seasonal climate biota was favoured, and successfully colonized newly established seasonally dry habitats across India.
There were a few dispersals from India during the Oligocene based on the pollen record, with Arenga (R.J. Morley, unpubl. data) occurring from the earliest Oligocene, and Dacrydium (Podocarpaceae) appearing in the Sunda region after the mid Oligocene (Morley Reference MORLEY2010).
The oldest records for the extinct pollen type Meyeripollis nayarkotensis (possibly Myrtaceae) at about 37 Ma and spores of Ceratopteris (Parkeriaceae) at about 35 Ma (Handique Reference HANDIQUE1993, Witts et al. Reference WITTS, HALL, NICHOLS and MORLEY2012), occurred simultaneously in the late Eocene of both India and South-East Asia (Morley Reference MORLEY2014), testifying to the presence of a continuous land connection by this time.
Seasonal climates became more pronounced across Sundaland during the late Oligocene, with grass pollen becoming common in many areas (Morley et al. Reference MORLEY, MORLEY and RESTREPO-PACE2003, Morley Reference MORLEY, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012) suggesting the widespread occurrence of open forest or savanna (Figure 2f).
The Australian Plate was drifting toward the South-East Asian region during the Oligocene and this resulted in the onset of dispersals from Australia to Sunda. There are just a few of these (Morley Reference MORLEY2000, Reference MORLEY, Kershaw, Bruno, Tapper, Penny and Brown2001b), but one of the more convincing is of the genus Phormium (Phormiaceae), with characteristic pollen seen also in the Australian genus Dianella, recorded from West Java (Lelono Reference LELONO2017, Morley Reference MORLEY2000). The main effect of the collision of the Australian Plate with South-East Asia was a dramatic increase in precipitation across the region (Morley Reference MORLEY, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012).
Early Miocene
The main change at the beginning of the Miocene (23.0–5.3 Ma) is the broad change across the Sunda region from seasonally wet to perhumid climates. This corresponded to the closure of the Indonesian throughflow and the onset of the East Asian monsoon (Wang et al. Reference WANG, CLEMENS and LIU2003) as the Australian Plate began its collision with South-East Asia (Morley Reference MORLEY, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012). India continued to drift into the northern subtropical high-pressure zone, and was most likely characterized by open forest and savanna (Awasthi Reference AWASTHI1992, Morley Reference MORLEY2000), except in the south (Figure 5a). There is little evidence for dispersals from fossil pollen other than for Brownlowia, which appears in India within the early Miocene (Venkatachala & Rawat Reference VENKATACHALA, RAWAT, Ghosh, Chanda, Ghosh, Baksi and Banerjee1971) and well-dispersed mangroves (e.g. Sonneratia caseolaris, at 21 Ma, and S. alba at 18 Ma), but molecular data suggest some faunal dispersals in both directions based on changes in MDE (Klaus et al. Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016), perhaps reflecting the expansion of perhumid climates in South-East Asia.
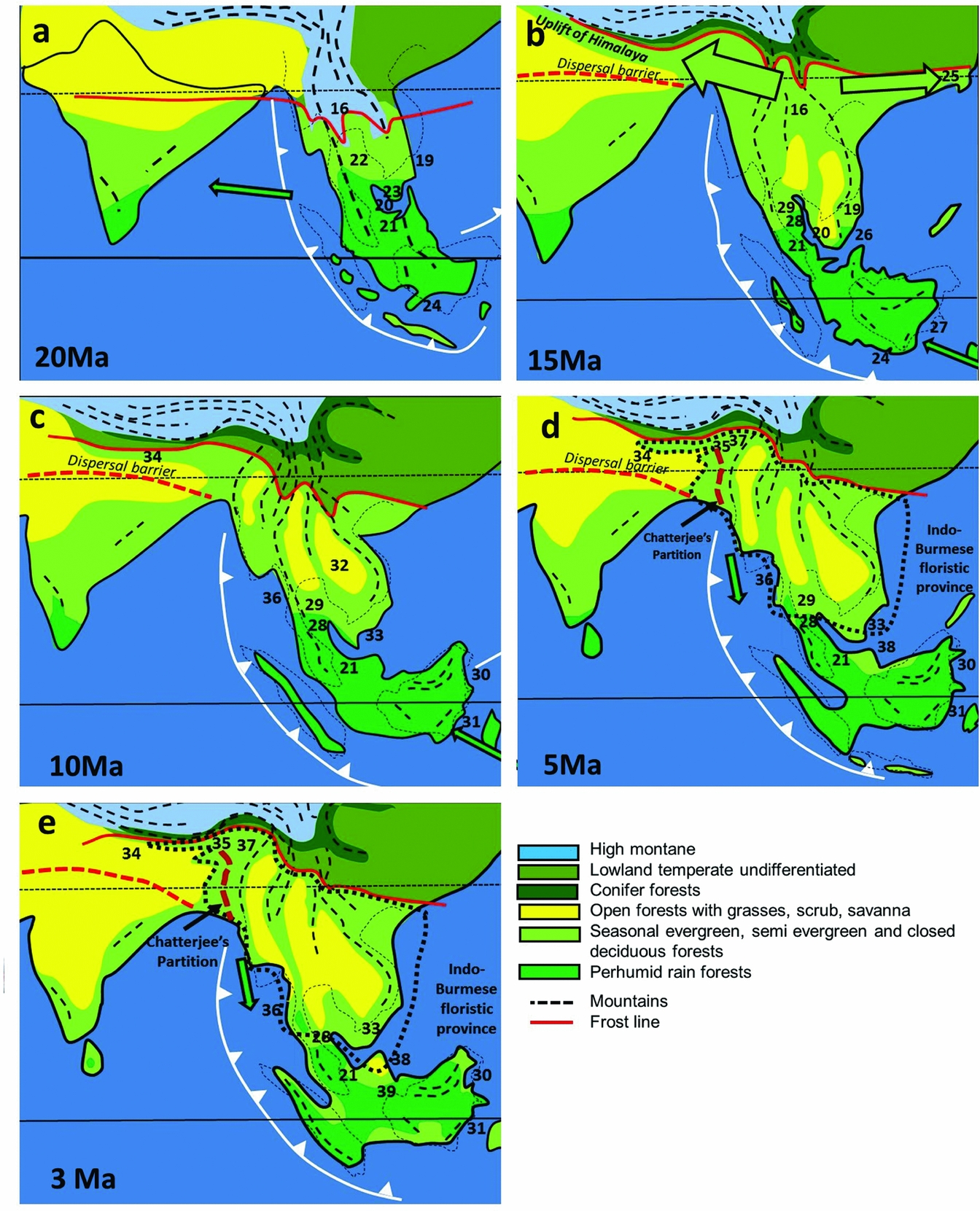
Figure 5. Palaeogeography, climate and dispersals, middle Miocene to Pliocene. tectonic model follows Hall (Reference HALL, Gower, Johnson, Richardson, Rosen, Rüber and Williams2012b); palaeogeography for South-East Asia follows Morley et al. (Reference MORLEY, MORLEY and SWIECICKI2016). The climate model utilizes palynological (Hoorn et al. Reference HOORN, WESSELINGH, TER STEEGE, BERMUDEZ, MORA, SEVINK, SANMARTÍN, SANCHEZ-MESEGUER, ANDERSON, FIGUEIREDO, JARAMILLO, RIFF, NEGRI, HOOGHIEMSTRA, LUNDBERG, STADLER, SÄRKINEN and ANTONELLI2010) and isotope (Quade et al. Reference QUADE, CERLING and BOWMAN1989) data for northern India coupled with palynological data from Thailand and unpublished data to demonstrate the likely pattern of expansion of areas of seasonally dry vegetation over time. The distribution of humid vegetation follows Morley (Reference MORLEY, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012) with updates. The position of the Fotan flora (Jacques et al. Reference JACQUES, SHI, SUA and ZHOU2015) critically positions the northern limit of megathermal forests during the middle Miocene climatic optimum. Numbers refer to localities with palaeoclimate data, listed in Appendix 2. Arrows indicate direction of plant dispersals.
Middle Miocene
At the time of the middle Miocene (16.0–11.5 Ma) thermal maximum megathermal forests probably extended further north along the East Asian seaboard than at any other time during the late Cenozoic. The primary evidence for this is the presence of a diverse flora of evergreen rain-forest elements reported from the Fotan Formation in southern China at 24oN (Figure 4b). This flora included Dipterocarpaceae (Shi et al. Reference SHI, JACQUES and LI2014), Leguminosae, Clusiaceae and other megathermal elements (Jacques et al. Reference JACQUES, SHI, SUA and ZHOU2015).
During the early and middle Miocene, the Himalaya underwent their main period of uplift, reaching 5.5 km by 15 Ma (Ding et al. Reference DING, SPICER, YANG, XU, CAI, LI, LAI, WANG, SPICER, YUE, SHUKLA, SRIVASTAVA, ALI KHAN, BERA and MEHROTRA2017). This uplift resulted in greatly increased orographic rainfall, and increased temperature in northern India due to the mountain mass elevation effect coupled with the initiation of the wet Indian monsoon, and the screening effect of the Himalaya from cold northern winter north-easterlies. In combination with globally higher temperatures at the thermal maximum this facilitated dispersal of evergreen to semi-evergreen megathermal and lower montane rain-forest elements from Myanmar westward along the Himalayan foothills. Widespread leaf and wood fossils and palynomorphs from this vegetation are preserved in the Siwalik foredeep deposits across northern India (Awasthi Reference AWASTHI1992, Ding et al. Reference DING, SPICER, YANG, XU, CAI, LI, LAI, WANG, SPICER, YUE, SHUKLA, SRIVASTAVA, ALI KHAN, BERA and MEHROTRA2017, Khan et al. Reference KHAN, SPICER, SPICER and BERA2016, More et al. Reference MORE, PARUYA, TARAL, CHAKRABORTY and BERA2016).
Many of the Siwalik taxa, such as Diospyros (Ebenaceae), Dipterocarpaceae, Koompassia, Millettia (Fabaceae) and Polyalthia (Annonaceae) are of Malesian affinity and this led to the long-held belief that the Indian flora originated from South-East Asia (Awasthi Reference AWASTHI1992). The current perspective of Himalayan uplift, coinciding with the Miocene thermal maximum creating a corridor along the northern margin of the megathermal forest belt, allowing for the dispersal of seasonal evergreen forest elements from South-East Asia to both northern India and southern China (Jacques et al. Reference JACQUES, SHI, SUA and ZHOU2015, Morley Reference MORLEY2000) is thought to be the more realistic interpretation. This is supported by the meta-analysis of Klaus et al. (Reference KLAUS, MORLEY, PLATH, YA-PING and JIA-TANG2016) which indicates that the middle Miocene saw more dispersals from Asia to India than at any other time, including freshwater fish, mammals and reptiles.
Most of the remaining Indian Peninsula was forested at that time (Guleria Reference GULERIA1992), but a belt of drier, deciduous forest and grassland was probably in place in the region of the Indo-Gangetic plain creating a dispersal barrier for evergreen elements. In support of that interpretation, and if it was not the case, Malesian relics, both lowland, and lower montane, would be expected in the rain-forest outliers of Sri Lanka and the Western Ghats (Morley Reference MORLEY2000). Only a few bird-dispersed taxa, such as Magnolia (Magnoliaceae) and Mastixia (Cornaceae), have made this crossing. The Indo-Gangetic Plain can therefore be suggested as forming the biogeographic divide (the Ganges Line) between the ‘ancient’ gondwanic-derived Indian Peninsula flora, and the Malesian-derived (originally gondwanic) flora of the Himalayan foothills. There are no ‘Malesian’ dipterocarps, Fagaceae or Pinus, and minimal Himalayan montane trees to the south of this line.
LATE NEOGENE COOLING AND DRYING AND EXPANSION OF C4 GRASSLANDS
The late Miocene (11.5–5.3 Ma) and Pliocene (1.95–5.3 Ma) were characterized by successively cooler and drier global climates, and this is expressed across South and South-East Asia by a stepwise expansion of open forest and grasslands (Figure 5c–e). This is most clearly reflected by stable isotope data, leaves and mammalian fossils from the Siwaliks (Patnaik Reference PATNAIK2015, Quade et al. Reference QUADE, CERLING and BOWMAN1989, Srivastava et al. Reference SRIVASTAVA, PAUDAYAL, UTESCHER and MEHROTRA2018) which demonstrate that for northern India C3 (mainly forest) vegetation dominated until about 8 Ma, then from 8 to 5 Ma there was a gradual increase in representation of C4 vegetation, after which time C4 grasslands dominated. This is reflected in a faunal change from predominantly frugivores and browsers prior to 6 Ma, to grazers after that time. The change to drier climates relates to the further uplift of the Himalaya, resulting in a strengthening of the South Asian monsoon, reflected by changes in foraminiferal assemblages from the Arabian Sea from this time (Kroon et al. Reference KROON, STEENS and TROELSTRA1991). Prior to this period, widespread forests with common and diverse dipterocarps occurred across most of the Indian Peninsula, but the effect of the strengthening monsoon, coupled with global drying relating to the expansion of northern hemisphere ice sheets, resulted in the extinction of dipterocarps over most of their former range (Shukla et al. Reference SHUKLA, MEHROTRA and GULERIA2013), leaving only a small but diverse group in the western Ghats and Sri Lanka (Gunatilleke et al. Reference GUNATILLEKE, GUNATILLEKE and ASHTON2017).
Natural savanna of the African type, which is sustained by browsing mammals, is rare in India today, but was probably widespread prior to the influence of hominids, who may have eliminated several of the large mammals (Patnaik Reference PATNAIK2015) that characterized the Pliocene. Savanna was widespread within flood plains (Quade et al. Reference QUADE, CERLING and BOWMAN1989) and probably also in terra firma areas, as suggested from the abundance of Poaceae pollen in distal Bengal Fan sediments (Yasuda et al. Reference YASUDA, AMANO, YAMANOI, Stewart and Winkler1990), which represent pollen sourced from the entire Ganges catchment. Savanna is also found in Indochinese seasonal fire-prone lower montane forests which are dominated by Pinus kesiya or P. roxburghii, and occasionally P. merkusii. Based on unpublished pollen records from offshore Vietnam (Figure 6) such vegetation may have been present since the Oligocene and probably also occurred at lower altitudes, and extending to lower latitudes during Pleistocene glacial maxima (Morley Reference MORLEY, Hall and Holloway1998). In East Java, Casuarina junghuhniana (Casuarinaceae) played a similar role in lower montane vegetation to Pinus in Indochina during the Pleistocene (Morley Reference MORLEY, Hoorn, Perrigo and Antonelli2018).

Figure 6. Pollen summaries from Gulf of Thailand/N Malay Basin wells. Well A south of Kangar Pattani line, Well B and C north of Kangar Pattani Line, for locations see Fig 1. Most common pollen taxa: Peat swamp group – Alangium, Austrobuxus, Blumeodendron, Calophyllum, Campnosperma, Cephalomappa, Durio, Garcinia, Gonystylus, Sapotaceae; Kerapah – Casuarina type, Dacrydium; Rain forest – calamoids, other palms, Euphorbiaceae, Leguminosae, Pandanus, Sapindaceae; Seasonal – Acanthaceae, Asteraceae, Borassus, Bombax, Lagerstroemia, Poaceae, Pterospermum, Schoutenia; Temperate angiosperms – Alnus, Engelhardia, Liquidambar, Fagaceae, Juglandaceae, Tilia; Temperate conifers – Abies, Cedrus, Picea, Tsuga.
LONGEVITY OF CLIMATE DRIVEN BIOMES
Muller (Reference MULLER, Ashton and Ashton1972) remarked that for the Neogene of north-west Borneo, there were very few extinctions as revealed from fossil pollen; the pollen record simply reflected long-term apparent stability of lowland evergreen rain forests, with a gradual increase in diversity accrued either from immigration or from localized speciation. The pollen record from areas of seasonal forests, for example, from the latitude of Vietnam, exhibits a similar degree of uniformity, perhaps extending further back in time to the early Oligocene. Here pollen assemblages are characterized by typical seasonal forest elements, especially the deciduous tree genus Lagerstroemia and Dipterocarpaceae.
An examination of pollen records from petroleum exploration wells located near the two main biogeographic divides, the Kangar-Pattani Line and the Kra Isthmus, clearly demonstrate this. The Kangar-Pattani Line marks the northern limit of astronomically driven perhumid climates, with a dry season of 2 mo or less, whereas the Kra Isthmus marks the southern limit of a longer dry season with up to six consecutive dry months (Table 1) where fire affects the canopy composition. Pollen assemblages south of the Kangar-Pattani Line throughout the Neogene are characterized by perhumid forest elements, especially elements of kerangas (forests occurring on oligotrophic sandy soils), kerapah (wet variant of kerangas) and basinal peat swamps, whereas those north of the line are characterized by deciduous and semi-evergreen elements, suggesting that the main biogeographic boundaries have shifted very little over time (Figure 6).
The importance of these two major divisions was brought to attention by De Bruyn et al. (Reference DE BRUYN, STELBRINK, MORLEY, HALL, CARVALHO, CANNON, VAN DEN BERGH, MEIJAARD, METCALFE, BOITANI, MAIORANO, SHOUP and VON RINTELEN2014) who demonstrated with a meta-analysis that although most of the region's biodiversity is a result of the accumulation of immigrants and in situ diversification, Borneo and Indochina have been the two major evolutionary centres for within-area diversification and subsequent emigration, affecting South-East Asian biodiversity since at least the early Miocene.
QUATERNARY CLIMATE CHANGE AND SEA LEVEL OSCILLATIONS
Sundaland underwent a fundamental change in character at the beginning of the Quaternary (0–1.95 Ma) during which time the amplitude of sea-level changes increased following episodic expansion of ice in the northern hemisphere (Zachos et al. Reference ZACHOS, PAGINI, SLOAN, THOMAS and BILLUPS2001). This resulted in the previously mainly submerged Sunda Shelf being exposed during successive periods of low sea levels, summarized in Cannon et al. (Reference CANNON, MORLEY and BUSH2009), Morley (Reference MORLEY, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012) and Morley et al. (Reference MORLEY, MORLEY and SWIECICKI2016). The repeated transgressions created optimal conditions for the expansion of mangrove swamps, which became much more widespread than during earlier periods (Morley & Morley Reference MORLEY2010).
Over the last million years the Sunda region effectively doubled in size during periods of lowest sea level and during the current highstand exhibits its smallest geographical area for that period, with the flora presently being in a state of refuge (Cannon et al. Reference CANNON, MORLEY and BUSH2009). At times of low sea level, the region was drier, as shown from pollen records from South Kalimantan (Kershaw et al. Reference KERSHAW, PENNY, VAN DER KAARS, ANSHARI, THANOTHERAMPILLAI, Metcalfe, Smith, Morwood and Davidson2001, Morley Reference MORLEY1981), South Sulawesi (Morley et al. Reference MORLEY, MORLEY, WONDERS, SUKARNO and VAN DER KAARS2004), North Sulawesi (Dam et al. Reference DAM, FLUIN, SUPARAN and VAN DER KAARS2001), South Sumatra (van der Kaars et al. Reference VAN DER KAARS, BASSINOT, DE DECKKER and GUICHARD2010), Java (van der Kaars & Dam Reference VAN DER KAARS and DAM1995) and Thailand (Yang & Grote Reference YANG and GROTE2017), and elevations of altitudinal boundaries lower (Flenley Reference FLENLEY1984, Morley Reference MORLEY, Hoorn, Perrigo and Antonelli2018). However, there are no comparable palynological studies from the central Sunda region and the possible nature of vegetation growing on the exposed Sunda Shelf has received wide speculation, especially with respect to whether or not there was a ‘savanna corridor’ across the submerged shelf for the dispersal of elements of the Siwalik savanna fauna to Java (Bird et al. Reference BIRD, TAYLOR and HUNT2005, Cannon et al. Reference CANNON, MORLEY and BUSH2009, Heaney Reference HEANEY1991, Kershaw et al. Reference KERSHAW, VAN DER KAARS, MOSS, WANG, Kershaw, David, Tapper, Penny and Brown2002, Medway Reference MEDWAY, Ashton and Ashton1972, Meijaard Reference MEIJAARD2003, Morley & Flenley Reference MORLEY, FLENLEY and Whitmore1987, Voris Reference VORIS2000, Wurster et al. Reference WURSTER, BIRD, BULL, CREED, BRYANT, DUNGAIT and PAZ2010). The depauperate nature of the Javanese Quaternary fauna, lacking ‘arid adapted’ or ‘open savanna’ elements of time equivalent Siwalik faunas in India (Medway Reference MEDWAY, Ashton and Ashton1972), suggests a dispersal filter for savanna elements, rather than a corridor.
This issue was recently addressed by Ashton (Reference ASHTON2014), who emphasized that the presence of a savanna corridor was unlikely due to the presence of Indo-Burmese semi-evergreen elements in Java but absence (except for taxa such as teak (Tectona grandis, Lamiaceae), that is likely to have been introduced by humans) of deciduous-forest elements, posing limits for dispersal of seasonal-climate elements across the equator. The absence of Pinus from Java is also noteworthy in this respect, as its most southerly location is in the uplands of central Sumatra at 2oS (Meijer & Withington Reference MEIJER and WITHINGTON1981), and its distribution in Sumatra was likely to have been greater during the last glacial maximum (van der Kaars et al. Reference VAN DER KAARS, BASSINOT, DE DECKKER and GUICHARD2010). Its lowland continental range extended as far south as the Malay Peninsula at some stage during one or more Quaternary dry-climate phases (Morley Reference MORLEY, Hall and Holloway1998), so why did it fail to disperse to Java? Bodribb & Field (Reference BODRIBB and FIELD2007) indicate that evergreen tropical forests pose an impenetrable ecological barrier to the southward movement of Pinus. Its absence further south is best explained by the permanent presence of closed forest in the region of the equator. Continuous forest cover across Sundaland is also suggested by climate modelling (Cannon et al. Reference CANNON, MORLEY and BUSH2009) and tree population modelling for dipterocarps (Raes et al. Reference RAES, CANNON, HIJMANS, PIESSENSE, SAW, VAN WELZEN and SLIK2014). However, the absence of lowland peat accumulation anywhere in the region at the time of the LGM (Anshari et al. Reference ANSHARI, KERSHAW and VAN DER KAARS2004, Dommain et al. Reference DOMMAIN, COUWENBERG, GLASER, JOOSTEN, NYOMAN and SURYADIPUTRA2014) does suggest that drier, presumably more-seasonal climates indeed characterized the whole of Sunda during periods of lowest sea level, and this could be explained by the reduction in occurrence of ‘superwet’ climates (sensu Richards Reference RICHARDS1996). Hunt et al. (Reference HUNT, GILBERTSON and RUSHWORTH2012) suggest ‘highly disturbed rather open forest’ during the last glacial at Niah Caves, but this is likely due to prolonged human disturbance at this site. Advocates of a savanna corridor give little consideration to other seasonally dry vegetation types, such as semi-evergreen and deciduous forests.
In summary, a drier, or seasonal climate corridor at times of lowest Quaternary sea level was highly probable, but the region is unlikely to have supported savanna vegetation; perhaps riverine grasslands were present and may have been the main open habitat. A corridor with semi-evergreen forests should be considered, possibly following the line of ferricretes extending from western Malay Peninsula to South Sumatra (Figure 7). It is most likely that a seasonal climate corridor would have been more strongly developed during the late Pliocene or early Pleistocene when climates across the region were probably drier than those of the late Quaternary, but such a corridor may not have been present during the last glacial maximum, as suggested by Morley (Reference MORLEY, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012) and Raes et al. (Reference RAES, CANNON, HIJMANS, PIESSENSE, SAW, VAN WELZEN and SLIK2014). This would be consistent with palynological analyses of the Perning Java Man site which indicates the presence of early Pleistocene open savanna in East Java (R.J. and H.P. Morley unpubl. data in Huffman Reference HUFFMAN2003). Early Pleistocene increased seasonality of climate in areas such as Sarawak is inferred from molecular analyses of bird populations (Sheldon et al. Reference SHELDON, LIM and MOYLE2015). It would also be consistent with the long-term isolation of taxa currently occurring on Sumatra and Borneo, such as elephants (Fernando et al. Reference FERNANDO, VIDYA, PAYNE, STUEWE, DAVISON, ALFRED, ANDAU, BOSI, KILBOURN and MELNICK2003). The search should therefore continue for Pleistocene cores for palynological analysis, both through the LGM and older, Pleistocene glacial maxima.
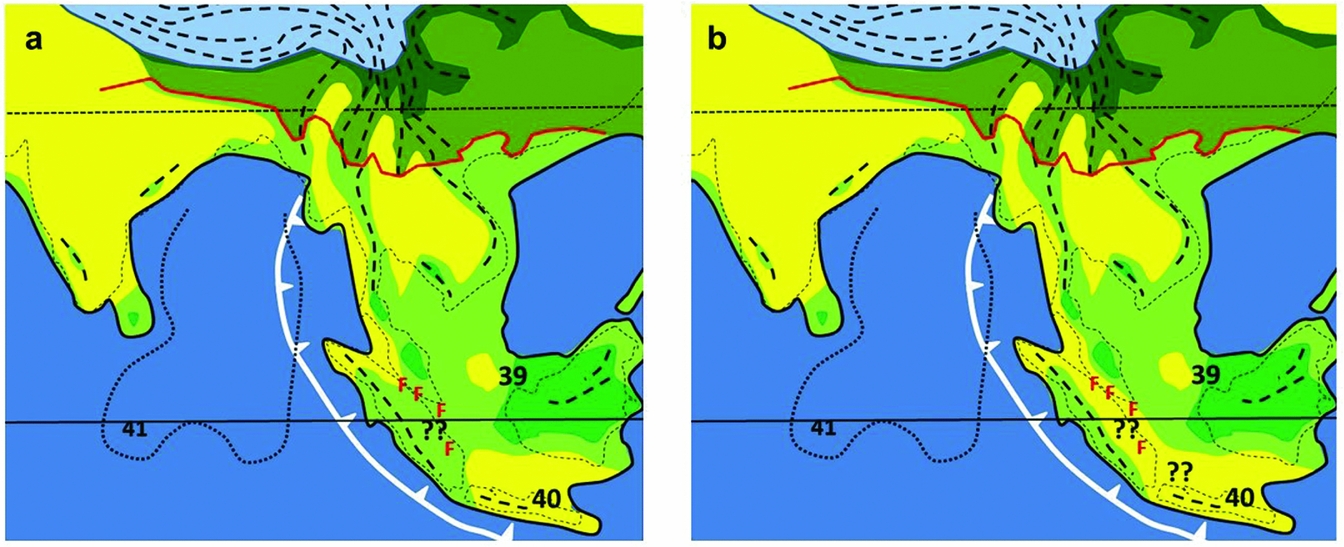
Figure 7. Vegetation at 1 Ma, (a) without and (b) with a ‘savanna’ corridor. Legend for vegetation types as for Fig. 5. Numbers refer to localities with palaeoclimate data, listed in Appendix 1. ‘F’ = areas with ferricretes. Fine dotted line, Bengal Fan.
The South-East Asian region has thus experienced repeated drowning and exposure coupled with changes from wetter to drier climates, and this is likely to have created a ‘species pump’. Molecular studies of taxa such as Rafflesia (Bendiksby et al. Reference BENDIKSBY, SCHUMACHER, GUSSAROVA, NAIS, MAT-SALLEH, SOFIYANTI, MADULID, SMITH and BARKMAN2010), Begonia (Thomas et al. Reference THOMAS, HUGHES, PHUTTHAI, ARDI, RAJBHANDARY, RUBITE, TWYFORD and RICHARDSON2012) and Sapotaceae (Richardson et al. Reference RICHARDSON, BAKAR, TOSH, ARMSTRONG, SMEDMARK, ANDERBERG, SLIK and WILKIE2013) indeed suggest that this process may have triggered speciation. The glacial refuge theory (Haffer Reference HAFFER, Whitmore and Prance1987, Prance Reference PRANCE1979) discredited in the Neotropics (Colinvaux et al. Reference COLINVAUX, IRION, RASANEN, BUSH and NUNES DE MELLO2001), is clearly tenable across Sunda!
UPLIFT OF THE INDO-BURMESE RANGES DURING THE PLIOCENE AND ESTABLISHMENT OF THE INDO BURMESE FLORISTIC PROVINCE
The Indo-Burmese wedge was subject to initial uplift during the Eocene and early Miocene (Acharyya Reference ACHARYYA2007, Allen et al. Reference ALLEN, CARTER, NAIMAN, BANDOPADHYAY, CHAPMAN, BICKLE, GARZANTI, VEZZOLI, ANDÒ, FOSTER and GERRING2007), but was then subject to erosion and extensively covered by sediments during the middle and late Miocene. The current topography of the Arakan Mountains is largely due to Plio-Quaternary uplift, and is still ongoing (Maurin & Rangin Reference MAURIN and RANGIN2009, C. Rangin pers. comm.). The Arakan Mountains, clothed mainly with seasonal evergreen forests, form a continuous belt from the eastern Himalaya to the coast west of the Irawaddy Delta, and their late Neogene uplift essentially resulted in the bisection of the seasonal evergreen and deciduous forests which previously spread widely from the Indo-Gangetic Plain to Indochina as noted by Jacques et al. (Reference JACQUES, SHI, SUA and ZHOU2015). This biogeographic boundary was first recognized by Chatterjee (Reference CHATTERJEE1939) and was subsequently called ‘Chatterjee's Partition’ by Ashton (Reference ASHTON2014). It marks the western boundary of the Indo-Burmese floristic province, which extends across the whole of tropical Indochina north of the Kangar-Pattani Line and along the Himalayan foothills (Figure 5d, e). The boundary coincides with the limits of many species, especially elements of the deciduous forests, with Shorea robusta occurring as a dominant to the west, and Tectona grandis and many deciduous dipterocarps to the east. Also, the commonly occurring genera Lagerstroemia and Dalbergia share different species either side of the boundary (summarized in Ashton Reference ASHTON2014). The late Neogene uplift of the Indo-Burmese range thus compares with the coeval uplift of the Andes that resulted in the bisection of Neotropical perhumid forests (Hoorn et al. Reference HOORN, WESSELINGH, TER STEEGE, BERMUDEZ, MORA, SEVINK, SANMARTÍN, SANCHEZ-MESEGUER, ANDERSON, FIGUEIREDO, JARAMILLO, RIFF, NEGRI, HOOGHIEMSTRA, LUNDBERG, STADLER, SÄRKINEN and ANTONELLI2010) and formed one of the primary drivers for Neotropical speciation (Miller et al. Reference MILLER, BERMINGHAM, KLICKA, ESCALANTE, RAPOSO DO AMARAL, WEIR and WINKER2008). The uplift of the Indo-Burmese Range is likely to have had a similar effect on the evolution of elements of tropical Asian deciduous forests, but its impact remains virtually unstudied (Ashton Reference ASHTON2014).
At this time semi-evergreen and seasonal evergreen elements are likely to have dispersed to the Andamans, suggesting a dispersal route which ultimately was followed by montane elements along the Sumatra ‘Track’, or corridor, of van Steenis (Reference VAN STEENIS1936) (see below).
UPLIFT ACROSS INDOCHINA AND SUNDA
South-East Asian mountain ranges divide into two distinct groups (Figure 8). The first group includes the Sino-Burmese and Annamite ranges in Indochina, the Main Range of the Malay Peninsula, and former uplands that followed the Con Son Rise, Natuna Arch, West Sarawak and the Schwaner. The second group includes the younger uplands of insular Sundaland and Wallacea. The first group carried widespread upland forests with Laurasian conifers, especially of Abies, Tsuga and Picea, during the Palaeogene, left today as relict patches in Thailand (Werner Reference WERNER1997) and Vietnam (Phan Ke Loc et al. Reference PHAN KE, PHAM VAN, PHAN KE, REGALDO, AVERANYO and MASLIN2017), whereas the second group is particularly characterised (in the uplands) by Gondwanan Podocarpaceae (Turner & Cernusak Reference TURNER and CERNUSAK2011).
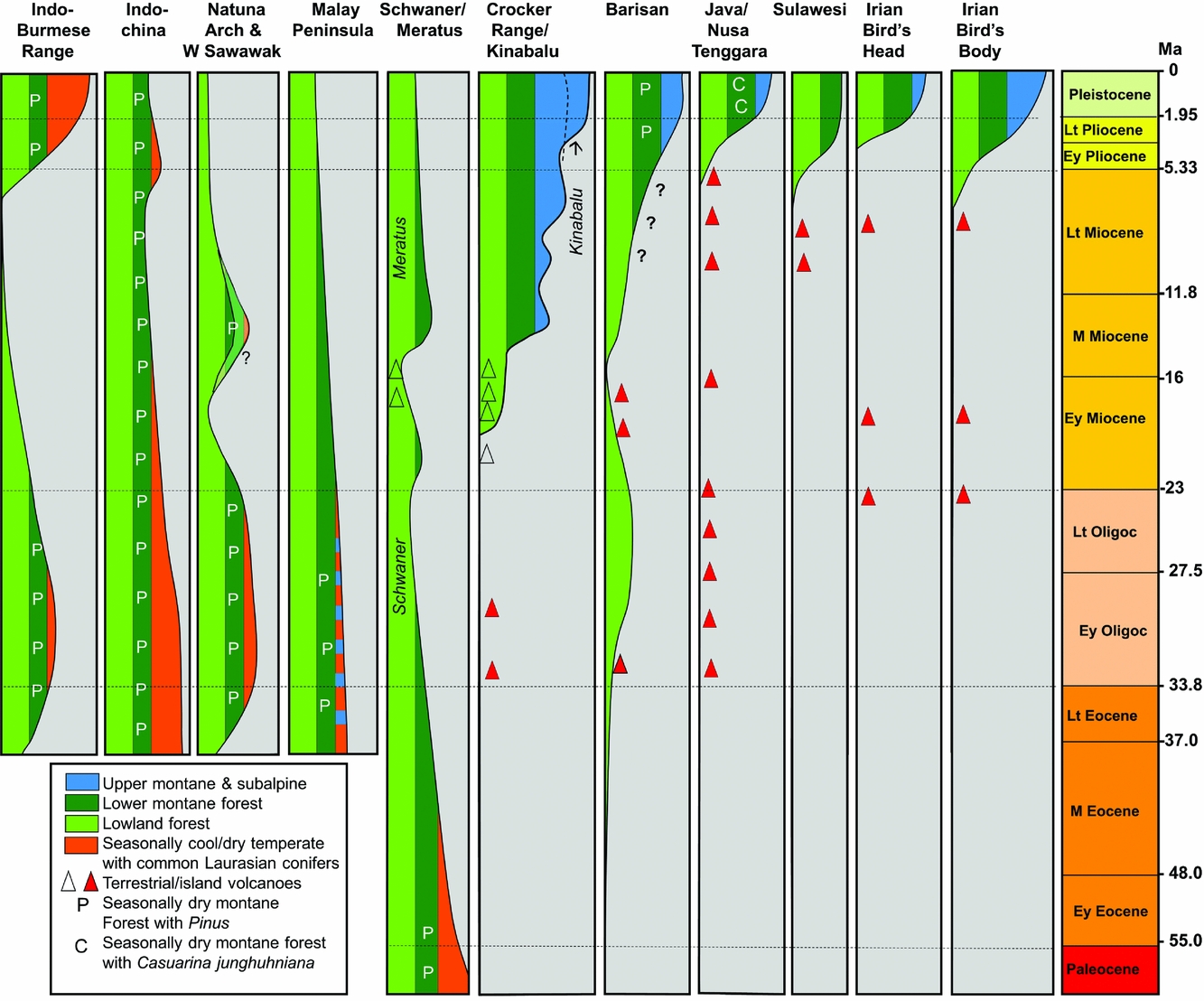
Figure 8. Timing of uplift across Sunda, Sahul and Indochina (in part from Morley Reference MORLEY, Hoorn, Perrigo and Antonelli2018).
The Sino-Burmese and Annamite mountain ranges probably date from prior to the collision of the Indian Plate during the Paleocene or Eocene (Fyhn et al. Reference FYHN, GREEN, BERGMAN, ITTERBEECK, TRI, DIEN, ABATZIS, THOMSEN, CHEA, PEDERSEN, MAI, TUAN and NIELSEN2016). These mountains are likely to have reached considerable elevation during the mid-Cenozoic. An example of the supporting evidence for this is found in an intermontane lake bed at Nong Ya Plong near the Kra Isthmus (13oN) that yielded abundant Alnus pollen (Watanasak Reference WATANASAK1988). This suggests that Alnus carr grew around the lake, a setting analogous to the Sabana de Bogota in Colombia during the Quaternary (Hooghiemstra Reference HOOGHIEMSTRA and Cramer1984), making an elevation for this lake bed of at least 1000 m likely. There are other Oligocene or early Miocene localities in Thailand dominated by temperate pollen, and these have sometimes been explained as reflecting the southward extrusion of Indochina following the Indian collision (Songtham et al. Reference SONGTHAM, RATANASTHIEN, MILDENHALL, SINGHARAJWARAPAN and KANDHAROSA1993), but as demonstrated above, deposition in intermontane basins is likely to be a more important factor. Fyhn et al. (Reference FYHN, GREEN, BERGMAN, ITTERBEECK, TRI, DIEN, ABATZIS, THOMSEN, CHEA, PEDERSEN, MAI, TUAN and NIELSEN2016) and Carter et al. (Reference CARTER, ROQUES and BRISTOW2000) indicate that Indochina underwent widespread regional denudation during the earlier Cenozoic, but with subsequent late Miocene uplift. This would tie with sedimentation patterns seen in surrounding basins, and with reduced sedimentation during the middle Miocene (Morley et al. Reference MORLEY, MORLEY and SWIECICKI2016).
The former uplands of the Natuna Arch and West Sarawak are thought to have formed a dispersal route for montane taxa from Indochina to Borneo during the mid Cenozoic for taxa such as stone oaks (Lithocarpus spp). The presence of diverse endemic Lithocarpus spp. in the eroded remnants of the former West Sarawak mountains in the Semitau region (Cannon & Manos Reference CANNON and MANOS2002) is testimony to this ancient dispersal route (Morley Reference MORLEY, Hoorn, Perrigo and Antonelli2018).
The Central Ranges, Crocker and Meratus Mountains of Borneo, on the other hand, are likely to have formed since the middle Miocene, with widespread early Miocene volcanic activity immediately prior to this uplift. The Meratus Mountains probably attained significant elevation at this time and were subsequently denuded (Morley et al. Reference MORLEY, MORLEY and SWIECICKI2016, Witts et al. Reference WITTS, DAVIES, MORLEY and ANDERSON2015). The Central Ranges of Borneo began uplift after about 15 Ma, indicated by increased sedimentation in the Mahakam Delta and the onset of appearance of Ericaceae pollen in circum-Borneo marine sediments. The uplift of the Crocker was probably later, after 13 Ma (Morley et al. Reference MORLEY, MORLEY and SWIECICKI2016). A peculiar feature of the pollen record from circum-Borneo petroleum exploration wells is the abundant occurrence of Eugeissona utilis type pollen from about 13 Ma onward (Morley & Morley Reference MORLEY2010). This was previously noted by Muller (Reference MULLER, Ashton and Ashton1972) in Brunei as ‘palm pollen peaks’, but (in both cases) with lack of common pollen of montane taxa. It is thought that this reflects abundant Eugeissona thickets along deeply dissected steep-sided rapidly eroding valleys across the region, and that for most areas, elevations were not particularly high due to the high erosion rates. Increased elevation subsequently took place in north-east Borneo with the emplacement of the Kinabalu Granite after 8 Ma (Cottam et al. Reference COTTAM, HALL, SPERBER and ARMSTRONG2010) with further Kinabalu uplift to its current elevation in the Pliocene (Merckx et al. Reference MERCKX, HENDRIKS, BEENTJES, MENNES, BECKING, PEIJNENBURG, AFENDY, ARUMUGAM, DE BOER, BIUN, BUANG, CHEN, CHUNG, DOW, FEIJEN, FEIJEN, FEIJEN-VAN SOEST, GEML, GEURTS, GRAVENDEEL, HOVENKAMP, IMBUN, IPOR, JANSSENS, JOCQUE, KAPPES, KHOO, KOOMEN, LENS, MAJAPUN, MORGADOL, NEUPANE, NIESER, PEREIRA, RAHMAN, SABRAN, SAWANG, SCHWALLIER, PHYAU-SOON, SMIT, SOL, SPAIT, STECH, STOKVIS, SUGAU, SULEIMAN, SUMAIL, THOMAS, VAN TOL, TUH, YAHYA, NAIS, REPIN, LAKIM and SCHILTHUIZEN2015). The Gondwanan gymnosperms Dacrycarpus and Phyllocladus dispersed to the area from New Guinea via Sulawesi after this latter phase of uplift (Morley Reference MORLEY, Hoorn, Perrigo and Antonelli2018), following the New Guinea ‘Track’ of van Steenis (Reference VAN STEENIS1936).
The uplift history of the Barisan Range of Sumatra remains poorly understood, but probably began to form during the late Miocene (Morley et al. Reference MORLEY, MORLEY and SWIECICKI2016), reaching its present elevation during the Quaternary. Molecular analyses of Rhododendron sect Vireya (Webb & Ree Reference WEBB, REE, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012) indicate a molecular age of about 5 Ma for endemic Sumatran taxa (Morley Reference MORLEY, Hoorn, Perrigo and Antonelli2018) and so significant elevation may have been achieved prior to this time. Molecular data can thus help in resolving geological issues. The volcanos of Java mainly date from the late Pliocene and Quaternary (Lunt Reference LUNT2013). This belt of volcanoes formed the route for temperate herbs and shrubs to disperse from Asia via the Indo-Burmese Range and Andaman Islands by hopping between ‘sky islands’.
UPLIFT ACROSS NEW GUINEA AND WALLACEA
The uplift of New Guinea and islands of Wallacea, such as Sulawesi, occurred surprisingly late considering the diversity of their biota. New Guinea formed at the beginning of the late Miocene as a series of small islands, amalgamating at the beginning of the Pliocene, and reaching their current elevation during the Quaternary (Toussaint et al. Reference TOUSSAINT, HALL, MONAGHAN, SAGATA, IBALIM, SHAVERDO, VOLGER, PORIS and BALKE2014). Sulawesi showed a similar pattern, occurring as localized islands at the beginning of the late Miocene, and amalgamating into a single island with significant elevations by 3 Ma during the late Pliocene (Nugraha & Hall Reference NUGRAHA and HALL2017). The Malesian Floristic Interchange considers dispersals between Sunda, the islands of Wallacea and Sahul (Richardson et al. Reference RICHARDSON, COSTION, MUELLNER, Gower, Johnson, Richardson, Rosen, Ruber and Williams2012, Van Weltzen et al. Reference VAN WELTZEN, PARNELL and SLIK2011), the key issue being that the New Guinea flora is predominantly derived from Asia, as noted by Good (Reference GOOD1962). The process of evaluating the assembly of the New Guinea flora is ongoing, and outside the scope of this review. Montane elements which reached New Guinea from Australia and subsequently dispersed westward to Sundaland form elements of the New Guinea ‘Track’ of Van Steenis (Reference VAN STEENIS1936).
DISCUSSION AND SUMMARY
Developing an explanation of the diversity of the South-East Asian flora has demonstrated that speculations made without accounting for both biogeographic distributions and geological history may be in error. The area ‘between Assam and Fiji' was not the ‘cradle’ of the flowering plants, but an evolutionary backwater at the time of initial radiation of the angiosperms. India was not left with a generalized flora after drifting through successive climatic zones during the Late Cretaceous and Paleocene, but that process resulted in the appearance of a dynamic and ultimately successful megathermal flora. The London Clay fruit and seed fossils should not be viewed as having an affinity with Malesian flora, but with pre-collision India. The Indian flora did not originate from South-East Asia during the Miocene, but delivered diverse megathermal elements to the Sunda region during the Eocene, after India's collision with Asia, and just to confuse the picture further, Plio-Pleistocene drying caused most of India's diverse forest flora to go extinct, taking away the historically important taxa from the extant flora, except in isolated pockets, such as perhumid south-west Sri Lanka. Savanna has never played a significant role in the development of the flora of the Sunda region and the Pleistocene Refuge Theory, discredited elsewhere, explains many instances of diversification across the region and deserves a revival.
Despite reaching similar levels of biodiversity to the Neotropics, the assembly of the South-East Asian flora has followed a very different path. Tectonics has played a much greater role as the entire region lies in the most tectonically active area of the planet. Plate collisions have facilitated intermingling of floras, the formation of the multitude of islands across the region has created multiple opportunities for allopatric speciation, and different phases of uplift, to different altitudes, has created on the one hand routes for inter-regional taxon dispersal, and on the other the formation of isolated ‘sky islands’ providing opportunities for the establishment of endemic floras. Active tectonics has resulted in a diversity of soil types, each suited to different plant species, and the predominance of monsoonal climates across the region has had a distinct effect in shaping the character of the flora. Also, the ‘pull’ of the Himalaya draws megathermal forests further into the mid latitudes in this area than anywhere else, accentuating the role of climate seasonality in this region. Finally, with so much of the region being close to sea level, the ‘maritime continent’ provides the perfect setting for speciation as a result of successive Pleistocene sea level oscillations and climate fluctuations.
The process of reaching our current understanding of how the flora has come about has required a concerted effort to integrate geological and biological results. There is still a long way to go before the process of assembly of the diverse biota, which has fascinated so many natural scientists since the days of Wallace (Reference WALLACE1869), can be considered as understood. Major re-evaluations, both with respect to the geological history, and of the phylogeography of any of the constituent plant families and genera, will undoubtedly result in further changes.
Major biogeographic dispersal and vicariance events that contributed to the development of South-East Asian biodiversity
The 12 dispersal and vicariance events that have shaped the assembly of the current flora of the South-East Asian region are summarized as follows (Figure 9):
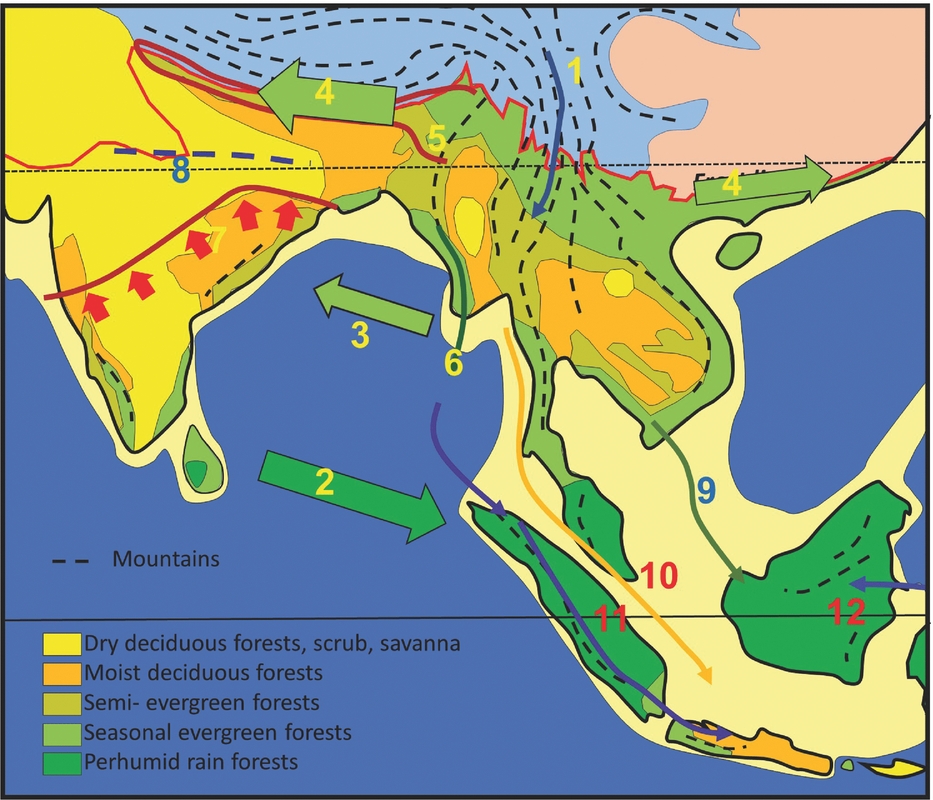
Figure 9. Dispersal routes and vicariant events. Mountain climates, blue; temperate climates, pink. Numbers indicate dispersal paths as discussed in text.
The north–south trending Sino-Burmese and Annamite mountains of Indochina are of ancient origin and most likely provided a route for boreotropical elements to reach and find refuge along a ‘survival trackway’ as climates fluctuated during the Cenozoic (1). This enabled the survival of more early angiosperms than in any other area in the northern hemisphere.
The northward drift of the Indian Plate into the perhumid equatorial zone and its eventual collision with Asia during the middle Eocene created a situation where Indian megathermal elements of gondwanic origin could disperse into South-East Asia by transoceanic dispersal immediately prior to the creation of a land bridge (2). During the late Eocene and Oligocene, the Indian Plate drifted into the northern hemisphere subtropical high-pressure zone, and the South-East Asian climate became predominantly seasonally dry. A land bridge was created after about 41 Ma, following which faunal dispersals were mainly from Asia to India based on molecular data (3).
With the uplift of the Himalaya coinciding with the middle Miocene thermal maximum, a corridor was formed for the dispersal of diverse Malesian (Gondwana-derived) lowland evergreen and semi-evergreen forest elements along foothills of the rising Himalayas (4). These elements were also able to disperse into southern China.
Early Pliocene uplift of the Indo-Burma Range divided the deciduous forests into two, forming Chatterjee's Partition (5) and establishing the Indochina biogeographic province. Evergreen forests of the Indo-Burma range provided a source for the dispersal of elements into the newly uplifted Andamans (6).
The leading edge of the Indian gondwanic flora thus extends from Goa in the west to Orissa in the east (7). The Indo-Gangetic Plain bore deciduous forests and formed a biogeographic divide between the Gondwanan and Malesian-derived (Gondwana-derived) floras (8).
The presence of a belt of uplands along the eastern margin of Sundaland, including the Con Son Rise and the Natuna Arch during the Oligocene, provided a path for the early dispersal of temperate montane elements into Borneo (9), which was removed by erosion and subsidence during the Miocene.
During the Quaternary, a belt of semi-evergreen forests probably extended across the equator, providing a route for seasonal climate elements to disperse along the Sunda Track (10), and the Indo Burmese Range and Andamans was also taking up microthermal elements following Van Steenis’ Sumatra ‘Track’ to Sumatra and Java (11).
The Malesian interchange with the uplift of New Guinea during the late Miocene and Pliocene resulted in the dispersal of diverse Sunda elements into the newly formed land areas of lowland New Guinea, but low numbers of Australia-derived taxa into Sunda (12). Australia-derived microtherm elements dispersed via upland areas along Van Steenis’ Montane ‘Track’.
Acknowledgements
This review has benefitted immensely from discussions with Peter Ashton, especially in relation to the division of deciduous forests following uplift of the Indo-Burma Range and Chatterjee's Partition, the so-called ‘savanna corridor’ and the evolution, dispersal and phenology of dipterocarps, from Rob Kooyman, providing an Australian perspective and who has greatly improved the text, from Sebastian Klaus, who has especially helped regarding the interpretation of molecular datasets, Claude Rangin for discussion of the timing of uplift of the Indo-Burmese Ranges, and Harsanti Morley, for help with regional palaeogeography and mapping. The paper was prepared for the SAGE 3 biogeography conference, held during August 2017 in Bogor, Indonesia. Thanks are also due to Thomas Von Rintelen for inviting me to the conference.
Appendix 1 Main locations used for constructing climate maps
(1) Bau-Penrissen, Sarawak, Kayan Formation Palynological analysis by Muller (Reference MULLER1968), interpretation by Morley (Reference MORLEY, Hall and Holloway1998, Reference MORLEY2000), lack of pollen of tropical families, common Laurasian conifers.
(2) Oldest Eocene well penetrations through base of Tanjung Formation with ‘Indian derived’ pollen, and common Restionaceae pollen suggesting seasonally dry climate, offshore Southwest Sulawesi (Morley Reference MORLEY2014).
(3) Nanggulan Formation, Java, regular mesic elements and presence of coals suggest perhumid climate, palynological analysis by Lelono (Reference LELONO2000) and Morley (Reference MORLEY2000).
(4) Pondaung Formation, Myanmar, fossil woods and lithologies suggest seasonally dry climate, Licht et al. (Reference LICHT, BOURA, DE FRANCESCHI, DUCROCQ, AUNG NAING and JAEGER2014).
(5) Walat Formation, West Java, palynological analysis by Morley (Reference MORLEY2000) and R. J. & H. P. Morley, unpublished poster). Mesic elements and coals suggest perhumid climate.
(6) Huangniuling Formation, Maoming Basin, South China, leaf floras with Dipterpcarpaceae suggest seasonally dry climate (Herman et al. Reference HERMAN, SPICER, ALEKSANDROVA, YANG, KODRUL, MASLOVA, SPICER, CHEN and JIN2017).
(7) Sangkarewang Formation, Ombilin Basin with coals, suggesting perhumid climate, O'Shea et al. (Reference O'SHEA, BETTIS, ZAIM, RIZAL, ASWAN, GUNNELL, ZONNEVELD and CIOCHON2015) and palynological analysis by R. J. Morley, unpublished.
(8) Tra Cu and Tra Tan Formations, Cuu Long Basin, Vietnam, palynomorph assemblages with common Lagerstroemia and Pinus suggests seasonal climate. Unpublished palynological data, R. J. Morley.
(9) Coals, Mangkalihat Peninsula, Kalimantan suggest perhumid climate. Palynological analysis by Morley (Reference MORLEY2000).
(10) Kujung Formation, East Java Sea, mesic elements, including Dacrydium, suggesting kerapah swamps, palynological analysis by Lelono & Morley (Reference LELONO, MORLEY, Hall and Wilson2010).
(11) Oligocene lakes surrounded by seasonally inundated swamps, Gabus Formation (palynological analysis by Morley & Morley Reference MORLEY and MORLEY2013).
(12) Southernmost well drilled in Gurita Basin, Natuna, only Oligocene location north of equator with clearly perhumid climate palynomorph assemblage during Oligocene, palynological analysis by R. J. Morley unpublished.
(13) Talang Akar Formation, East Java Sea, perhumid Oligocene succession with kerapah coals, palynological analysis by Morley (Reference MORLEY2000).
(14) Seismic group K, Malay Basin, common grass and pine pollen, (palynological analysis by Jaizan Md Jais 1997).
(15) Coaly Cau Formation, Nam Con Son Basin, Vietnam, palynological analysis by Morley et al. (Reference MORLEY, SWIECICKI and PHAM2011).
(16) Various intermontane basins in Northern Thailand, analysed for palynology by Songtham et al. (Reference SONGTHAM, RATANASTHIEN, MILDENHALL, SINGHARAJWARAPAN and KANDHAROSA1993).
(17) North Belut-5 well, West Natuna Basin, palynological analysis by Morley et al. (Reference MORLEY, SALVADOR, CHALLIS, MORRIS and ADYAKSAWAN2007).
(18) Nong Ya Plong, Kra Isthmus, Thailand, Oligocene intermontane lake (palynological analysis by Watanasak (Reference WATANASAK1988).
(19) Bach Ho Formation, Cuu Long Basin, Vietnam, palynomorph assemblages with common Lagerstroemia and Pinus suggests seasonal climate. Unpublished palynological data, R. J. Morley.
(20) West Natuna petroleum exploration wells, coals and common peat swamp pollen in Early Miocene suggests perhumid climate, grass and Pinus maxima in M Miocene suggests seasonal climate, palynological analysis by Morley et al. (Reference MORLEY, MORLEY and RESTREPO-PACE2003).
(21) Southern Malay Basin palynological analysis, peat swamp pollen and coals suggest perhumid climate, Yakzan et al. (Reference YAKZAN, AWALLUDIN, BAHARI and MORLEY1996).
(22) Palynological analysis of petroleum exploration wells in Gulf of Thailand, common Lagerstroemia and Pinus pollen suggests seasonal climate, Restrepo-Pace et al. (Reference RESTREPO-PACE, DALRYMPLE and MORLEY2015).
(23) Dua Formation, Nam Con Son Basin, Vietnam, coals and peat swamp pollen suggests perhumid climate, palynological analysis by Morley et al. (Reference MORLEY, SWIECICKI and PHAM2011).
(24) Barito Basin, common peat swamp and Dacrydium pollen suggests perhumid climate, palynological analysis by Amir et al. (Reference AMIR, WITTS and MORLEY2017).
(25) Fotan Formation, Fujian, southern China, leaf flora with Shorea, by Jacques et al. (Reference JACQUES, SHI, SUA and ZHOU2015).
(26) Mang Cau Formation, Vietnam, palynological analysis shows common peat swamp pollen, Morley et al. (Reference MORLEY, SWIECICKI and PHAM2011).
(27) Kutai Basin exploration wells with common peat swamp pollen suggests perhumid climate, R.J. Morley unpublished.
(28) Northern Malay Basin, exploration wells, abundant peat swamp pollen, Figure 6.
(29) Tho Chu Basin, offshore Vietnam, exploration wells, regular Lagerstroemia pollen, Figure 6, R.J. Morley unpubl. data.
(30) Tarakan Basin, well A, palynological analysis, Morley & Morley (Reference MORLEY2010).
(31) Kutai Basin, Attaka well B, palynological analysis, Morley & Morley (Reference MORLEY2010).
(32) Khorat, Thailand, palynological analysis shows common grass pollen suggest open vegetation, Sepulchre et al. (Reference SEPULCHRE, JOLLY, DUCROCQ, CHAIMANEE and JAEGER2010).
(33) Nam Con Son Formation, Vietnam, palynological analysis shows increased grass pollen compared to M Miocene, suggesting seasonal climate, Morley et al. (Reference MORLEY, SWIECICKI and PHAM2011).
(34) Siwaliks, Nepal, palynological analysis in Late Miocene, mixed tropical and warm temperate assemblages with a few grasses suggests seasonal evergreen forests with montane forests in uplands; in Pliocene, abundant grass pollen suggests savanna, Hoorn et al. (Reference HOORN, OHJA and QUADE2000).
(35) Siwaliks, Bhutan, palynological analysis suggests seasonal evergreen forest, Coutand et al. (Reference COUTAND, BARRIER, GOVIN, GRUJIC, HOORN, DUPONT-NIVET and NAJMAN2016).
(36) Andaman Sea, exploration wells, abundant grass pollen suggests open forest or savanna, palynological analysis Morley (Reference MORLEY2000).
(37) Arunchal Pradesh, leaf fossils, Khan et al. (Reference KHAN, SPICER, SPICER and BERA2016).
(38) West Sarawak, exploration well with common grass pollen, palynological analysis R. J. Morley unpubl, data.
(39) Exploration well, common peat swamp pollen suggests perhumid climate, R. J. Morley unpubl. data.
(40) Perning, East Java, palynological analysis R. J and H. P. Morley unpublished in Huffman & Zaim (Reference HUFFMAN and ZAIM2003).
(41) Bengal Fan (Yasuda et al. Reference YASUDA, AMANO, YAMANOI, Stewart and Winkler1990).
Appendix 2. Fossil pollen and macrofossils mentioned in text and oldest records.














