Introduction
Determining accurate age in crustaceans remains difficult because the exoskeleton is periodically moulted, shedding evidence of age with each consecutive moult. Unlike fish or other ectothermic organisms that retain evidence of age within bony structures (i.e. otoliths), crustaceans lack such structures, which has posed a significant problem for ecologists and fisheries managers who require detailed information regarding life-history characteristics to evaluate population dynamics (Sheehy & Prior, Reference Sheehy and Prior2008; Bosley & Dumbauld, Reference Bosley and Dumbauld2011). Size frequency information is often used as a proxy for age, however, strong variation in size-at-age within a cohort for some crustaceans can lead to significant bias in estimation of life-history parameters such as mortality and maturity (Campana, Reference Campana2001; Hufnagl et al., Reference Hufnagl, Temming, Siegel, Tulp and Bolle2010; Hirose et al., Reference Hirose, Fransozo, Tropea, López-greco and Negreiros-Fransozo2013). Indirect methods of ageing have been introduced that attempt to circumvent inherent biases in length-based methods, particularly analysis of the biochemical ageing pigment, lipofuscin. More recently, a direct ageing approach has been developed that uses measurement of growth increments within the crustacean gastric mill (Kilada et al., Reference Kilada, Sainte-Marie, Rochette, Davis, Vanier, Campana and Gillanders2012; Leland & Bucher, Reference Leland and Bucher2017).
Lipofuscin is an autofluorescent pigment that is deposited in post-mitotic (non-proliferating) cells in the neural tissues of a variety of animals as they age (Terman & Brunk, Reference Terman and Brunk2002). Lipofuscin content increases with age and shorter-lived animals accumulate the pigment at a faster rate than long-lived animals (Terman & Brunk, Reference Terman and Brunk2002; Fonseca et al., Reference Fonseca, Brancato, Prior, Shelton and Sheehy2005). Previous studies have verified the use of extractable lipofuscin as an accurate measure of age for several decapod crustaceans (Ju et al., Reference Ju, Secor and Harvey1999; Bosley & Dumbauld, Reference Bosley and Dumbauld2011; Pinchuk et al., Reference Pinchuk, Harvey and Eckert2016). The formation of lipofuscin occurs through metabolic processes (Sheehy et al., Reference Sheehy, Greenwood and Fielder1995), which has led to some criticism of the method because it reflects the physiological history of the organism that can be greatly influenced by environmental factors such as temperature (Sheehy, Reference Sheehy2008; Bosley, Reference Bosley2016). Some studies have examined environmental effects on lipofuscin accumulation rate by conducting controlled growth experiments with known-age animals under a range of thermal conditions (Tully & Fletcher, Reference Tully and Fletcher2000; Sheehy, Reference Sheehy2002), but these relationships tend to be highly variable and species-specific. Ageing methods can also be verified by corroborating results from different independent ageing techniques, provided that alternative methods are available. This approach can provide support for an ageing method and confirm accuracy in age estimation (Campana, Reference Campana2001).
Recent work on crustacean ageing methods has focused on the use of gastric mill structures for direct ageing (Kilada et al., Reference Kilada, Agnalt, Arboe, Bjarnason, Burmeister, Farestveit, Gíslason, Guðlaugsdóttir, Guðmundsdóttir, Jónasson, Jónsdóttir, Kvalsund, Sheridan, Stansbury and Søvik2015; Sheridan et al., Reference Sheridan, O'Connor and Henderson2016; Leland & Bucher, Reference Leland and Bucher2017). The crustacean gastric mill consists of a number of chitinous ossicles that support the posterior dorsolateral cardiac foregut, where food grinding takes place (Claiborne & Ayers, Reference Claiborne, Ayers, Selverston and Moulins1987). The primary components that make up the gastric mill structure are the pterocardiac, zygocardiac and mesocardiac ossicles, which also contain the dominant grinding teeth of the gastric mill (Factor, Reference Factor and Factor1995). Previous studies have demonstrated that these ossicles contain rings or layers of calcified growth features, much like fish otoliths, which could potentially be used as a method of direct ageing (Kilada et al., Reference Kilada, Sainte-Marie, Rochette, Davis, Vanier, Campana and Gillanders2012; Leland & Bucher, Reference Leland and Bucher2017). More recently, chemical staining and rearing studies have provided additional support that the ‘ring-like’ structures observed within these ossicles may contain chronological information (Kilada et al., Reference Kilada, Agnalt, Arboe, Bjarnason, Burmeister, Farestveit, Gíslason, Guðlaugsdóttir, Guðmundsdóttir, Jónasson, Jónsdóttir, Kvalsund, Sheridan, Stansbury and Søvik2015; Leland & Bucher, Reference Leland and Bucher2017), however there continues to be a debate as to whether or not the gastric mill is actually retained through the moulting process (Vatcher et al., Reference Vatcher, Roer and Dillaman2015; Sheridan et al., Reference Sheridan, O'Connor and Henderson2016; Becker et al., 2018; Crook et al., Reference Crook, Adair, Grubert, Saunders, Morrongiello, Douglas and King2018). Gastric mill growth features have been observed in an increasing number of species (Kilada & Acuña, Reference Kilada and Acuña2015; Kilada et al., Reference Kilada, Agnalt, Arboe, Bjarnason, Burmeister, Farestveit, Gíslason, Guðlaugsdóttir, Guðmundsdóttir, Jónasson, Jónsdóttir, Kvalsund, Sheridan, Stansbury and Søvik2015; Kilada & Driscoll, Reference Kilada and Driscoll2017), but few long-term studies have been conducted to confirm the temporal periodicity of these marks. Given the potential for widespread application of gastric mill growth features for accurately assessing age in crustaceans, cross-validation of the methods with other established direct ageing techniques (i.e. lipofuscin ageing) would provide a useful step in verification of the approach.
The ghost shrimp, Neotrypaea californiensis, is an important member of soft sediment communities in US Pacific Northwest estuaries and is a deposit-feeding crustacean that builds complex burrows within intertidal sediments (MacGinitie, Reference MacGinitie1934; Griffis & Suchanek, Reference Griffis and Suchanek1991; Dumbauld et al., Reference Dumbauld, Armstrong and Feldman1996). Bioturbation by N. californiensis negatively affects the Pacific oyster (Crassostrea gigas) which is a commercially important species grown in aquaculture beds along the Pacific Northwest coast of North America (Feldman et al., Reference Feldman, Armstrong, Dumbauld, DeWitt and Doty2000; Dumbauld et al., Reference Dumbauld, Booth, Cheney, Suhrbier and Beltran2006). Oysters are typically produced in hatcheries and often planted as juveniles directly on the surface of the mudflats, where they are vulnerable to sinking into the soft sediment from the burrowing action of N. californiensis (Chew, Reference Chew2002; Dumbauld et al., Reference Dumbauld, Booth, Cheney, Suhrbier and Beltran2006). As a result, shellfish growers treat these shrimp as pests and are actively working towards an integrated pest management plan which requires more detailed information about the life history and ecology of the shrimp, including population estimates and age structure (Booth, Reference Booth2003; Dumbauld et al., Reference Dumbauld, Booth, Cheney, Suhrbier and Beltran2006).
Due to interest in the shrimp's ecology and the economic interest in controlling them for shellfish culture, N. californiensis has been the focus of several studies aimed to improve understanding of its life-history characteristics including age and growth (Feldman et al., Reference Feldman, Armstrong, Dumbauld, DeWitt and Doty2000, Bosley & Dumbauld, Reference Bosley and Dumbauld2011). Bosley & Dumbauld (Reference Bosley and Dumbauld2011) applied lipofuscin ageing to establish age structure and longevity across multiple populations. They found that length-based estimates of age can be highly imprecise because of strong differences in growth among locations. These conclusions were based on a lipofuscin accumulation rate determined from a short-term (10-month) growth study with animals whose ages were not truly known. In the current study, we sought to further verify the lipofuscin ageing method for N. californiensis by establishing lipofuscin accumulation rate from known-age animals reared in outdoor mesocosms over a multi-year period. In addition, we investigate the relationships between lipofuscin concentration, body size and internal gastric mill growth features within the mesocardiac ossicle from both reared and wild-caught animals to establish how these potential ageing metrics vary with chronological time. Although previous studies have focused on growth marks that are assumed to be annual deposits, some of these studies have made reference to the paired structural lamellate layers within the ossicle (i.e. structural lamellae) and the role they play in formation of the growth marks used for direct ageing (Leland et al., Reference Leland, Bucher and Coughran2015; Leland & Bucher, Reference Leland and Bucher2017). Our study looks at the lesser examined intracuticular lamellae and how these features relate to other previously established ageing techniques, namely body size and lipofuscin concentration. This is the first study to apply, verify and compare these separate ageing methods within the same study. We identify whether these techniques are useful for determining age in the burrowing shrimp, N. californiensis, but also establish a methodology for using multiple ageing techniques in conjunction with each other to improve understanding of age and growth in future crustacean studies.
Materials and methods
Field sampling
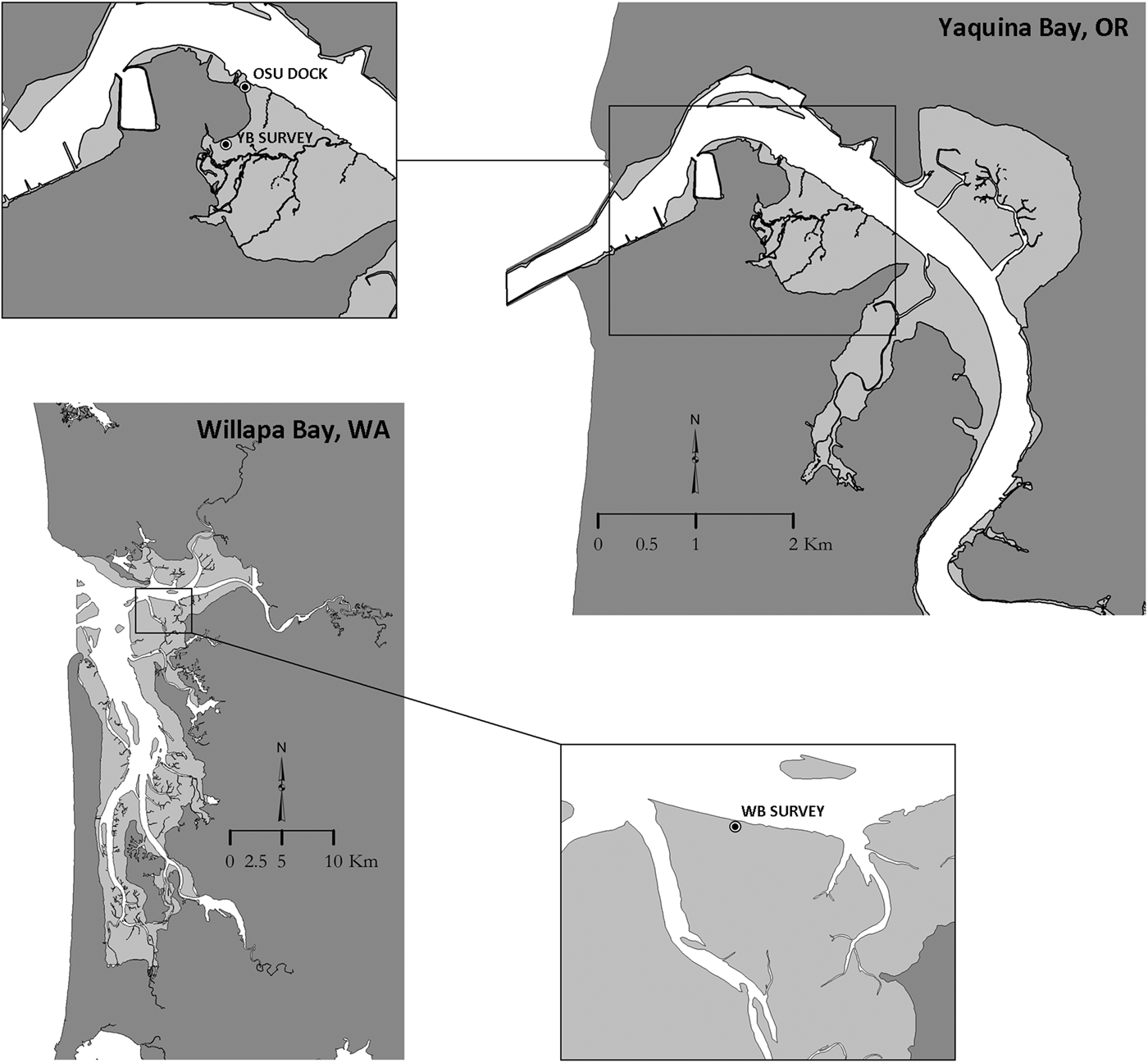
Shrimp samples were collected from Yaquina Bay, a small estuary located on the central Oregon US coast (44.6181°N −124.0302°W) and Willapa Bay (46.6622°N −124.0106°W) a large estuary located on the US southern Washington coast. Shrimp from Yaquina Bay included 109 Neotrypaea californiensis specimens collected using a clam gun (a coring tool commonly used to harvest clams from soft intertidal sediments) from the south side of the estuary in summer 2014 (OSU Dock, Figure 1). Annual recruitment had been observed at this location beginning in 2010 and shrimp here were presumably ≤4 years old (Dumbauld & Bosley, Reference Dumbauld and Bosley2018). Twenty shrimp sampled as part of a 2013 demographic survey in Yaquina Bay on Idaho Flats were also included in the study. For a comparison group, 13 shrimp from a similar survey conducted in Willapa Bay in 2014 were included (B. Dumbauld unpubl.). Shrimp collected during demographic surveys were obtained by sampling with a large stainless steel core (40 cm diameter × 60 cm depth) and sieving the contents with a 3 mm mesh. All shrimp were measured and sexed prior to being frozen at −80 °C until they could be processed for further analysis. Lipofuscin concentration was obtained for all shrimp greater than 6.0 mm carapace length (CL) and gastric mill lamellae counts (see below for methods) were measured from these same shrimp collected during these demographic surveys. In addition, lamellae counts were recorded for 30 randomly selected shrimp from the 2014 OSU dock samples representing a range of sizes and possible ages.
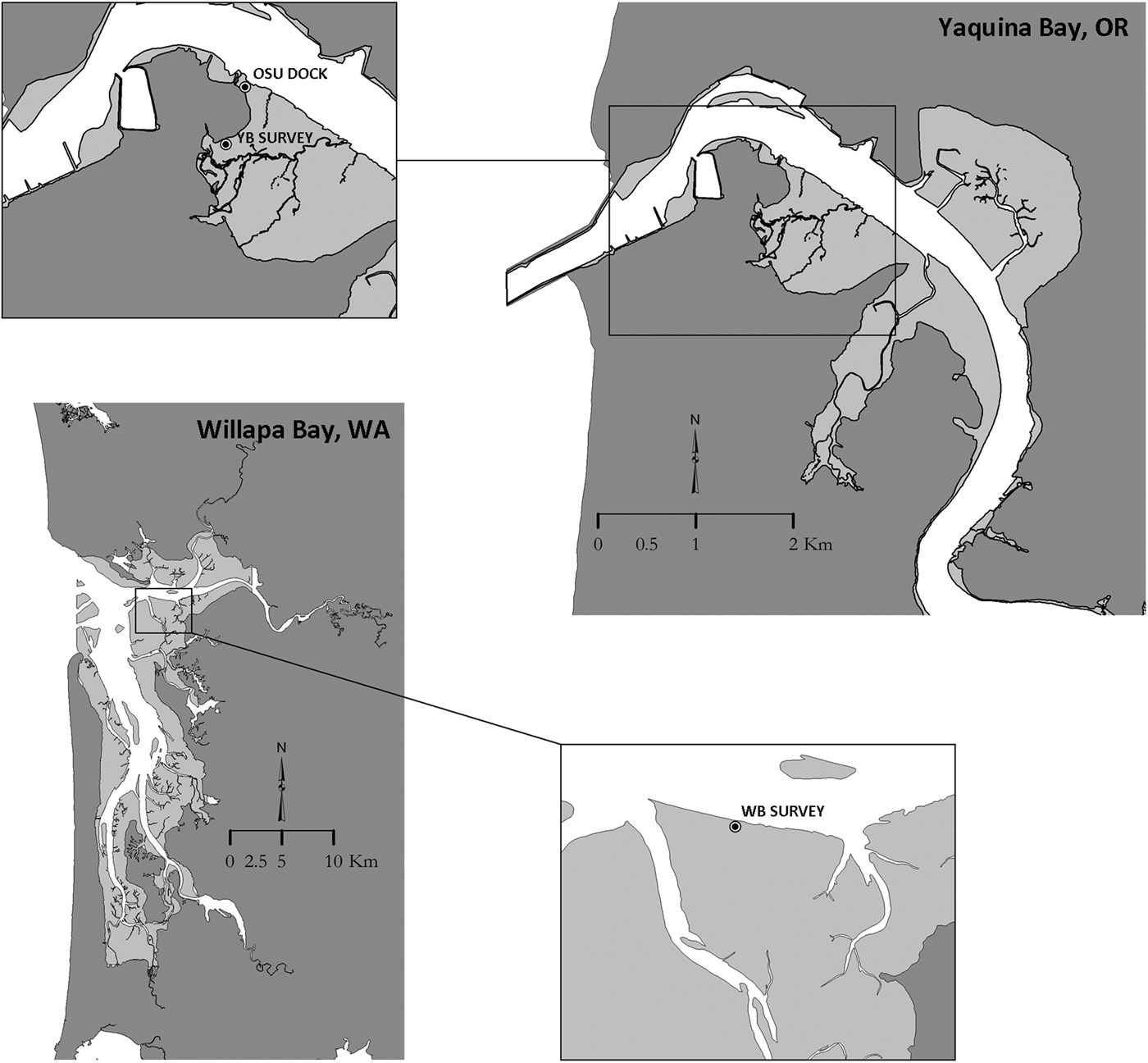
Fig. 1. Maps of Yaquina Bay, Oregon and Willapa Bay, Washington showing sample locations where Neotrypaea californiensis were collected for the lipofuscin analysis and gastric mill lamellae counts. Juvenile shrimp for the mesocosm study were collected at the OSU dock location where shrimp were presumably ≤4 years of age.
Mesocosm experiments
Mesocosm experiments took place over a 5-year time period, encompassing approximately half of the total lifespan for the species (Bosley & Dumbauld, Reference Bosley and Dumbauld2011). Juvenile shrimp (age 0+) were collected from the OSU dock site (Figure 1) in November 2010 and placed in outdoor mesocosms located at the Hatfield Marine Science Center in Newport, Oregon. These small shrimp (1.73 ± 0.21 mm CL, mean ± SD) represented the ‘known-age’ animals in this study. Six open-system mesocosm tanks each contained four 5-gallon buckets filled with sand collected from Yaquina Bay that was sieved through 3 mm mesh to remove adult shrimp and other macrofauna. The tanks received a constant flow of marine water (~3 l min−1) pumped from Yaquina Bay filtered to 50 µm before entering the tanks and a HOBO® temperature probe was placed in one tank to continually record ambient temperature (Tank 3; Figure S1). Shade cloth (40%) was also placed over each tank to reduce the severity of algal accumulation during the summer growing season. Excess macroalgae was removed from tanks every 2–3 weeks during summer months (April–Sept) to allow sedimentary microalgae to proliferate and also to reduce build-up of decaying macroalgal material. Because of the significant amount of macro and micro algae that occurred naturally within the tanks, no additional food was added to the tanks over the course of the study.
Shrimp were sampled twice annually, by removing a bucket from at least two separate tanks and sieving the contents through a 3 mm sieve. Wet weight and CL were recorded for all shrimp before they were frozen at −80 °C for further processing. Both lipofuscin concentration and lamellae counts were obtained from shrimp greater than 6.0 mm CL collected from mesocosms over the 5-year grow-out period. Shrimp less than 6.0 mm CL were not analysed for lipofuscin because their brain tissue was too small for protein detection with the established technique.
Measurement of lipofuscin concentration
Lipofuscin concentration was determined with methods modified from Bosley & Dumbauld (Reference Bosley and Dumbauld2011). Shrimp brains were dissected, placed in a pre-combusted 1.5 ml amber vial and topped with 1.0 ml dichloromethane:methanol (2:1) solution. Samples were sonicated for 30 s at 18% with a microprobe sonicator then stored in the freezer overnight to ensure complete extraction of lipofuscin. Samples were then dried completely with pure N2 and reconstituted with 0.25 ml HPLC-grade methanol. Lipofuscin was measured with an Agilent 1100 scanning fluorescence detector at excitation wavelength 281 nm and emission wavelength 615 nm using methanol as a carrier solvent. Fluorescence peaks were maximized with a sample volume of 15 µl and a flow rate of 0.8 ml min−1. Lipofuscin concentration was quantified by calibrating fluorescence values to a standard of quinine sulphate in 0.1 N H2SO4. Following lipofuscin measurement, samples were prepared for protein analysis by evaporating samples to dryness with pure N2, and reconstituting with 1 ml of 16% deoxycholic acid. Samples were sonicated in an ice-water bath sonicator for 30 min and stored in a refrigerator overnight before protein quantification. Protein concentration was measured with an Agilent 1100 fluorescence detector at excitation 280 nm and emission 345 nm using nanopure water as a carrier solvent. Peaks were maximized with a 12 µl sample volume and 0.8 ml min−1 flow rate. Fluorescence intensity of extracted protein was calibrated with a standard of bovine serum albumin (BSA) in 16% deoxycholic acid (Harvey et al., Reference Harvey, Ju, Son, Feinburg, Shaw and Peterson2010). Lipofuscin concentration was normalized to protein concentration in each sample to account for differences in body size and variability in brain tissue dissections. The lipofuscin-based age metric is hereafter termed the ‘LF Index’:
Gastric mill ossicular lamellae counts
Gastric mill ossicles were carefully dissected from shrimp and separated with forceps into three major sections, the central mesocardiac and the two zygocardiac ossicles, which contain grinding teeth (Figure 2A). Previous studies have embedded ossicles into resin and cut thin sections with an isomet saw to count internal growth bands (see Leland et al., Reference Leland, Bucher and Coughran2015). The gastric mill ossicles of N. californiensis are very small and therefore difficult to maintain a consistent orientation when embedded in thermoplastic resin. We chose to use a different approach for measuring the ossicular lamellae by make counts using the flat, blade-like portion of the mesocardiac ossicle and developed a protocol for embedding the structure on microscope slides that could be tightly controlled and standardized across samples (Figure 2B). The mesocardiac ossicle was broken cleanly at the joint to the median tooth and embedded in melted thermoplastic resin placed on a glass slide so that the entire ossicle was lying flat in a plane parallel to the slide and underneath a thin resin layer (Figure 2B). Once embedded, the top layer of resin was slowly and carefully removed using 800-μm sandpaper until the parallel internal lamellae were clearly visible under a compound microscope from the thin, fan-like outer edge of the structure along the entire medial plane. Scratches and other reflective obstacles were polished off using alumina buffing powder mixed into a solution with deionized water so the internal features could be viewed clearly. Extra care was taken during the buffing process to be sure that the exocuticle was not removed from mesocardiac ossicle and was clearly visible under the microscope to ensure accurate and complete lamellae counts throughout the entire structure.
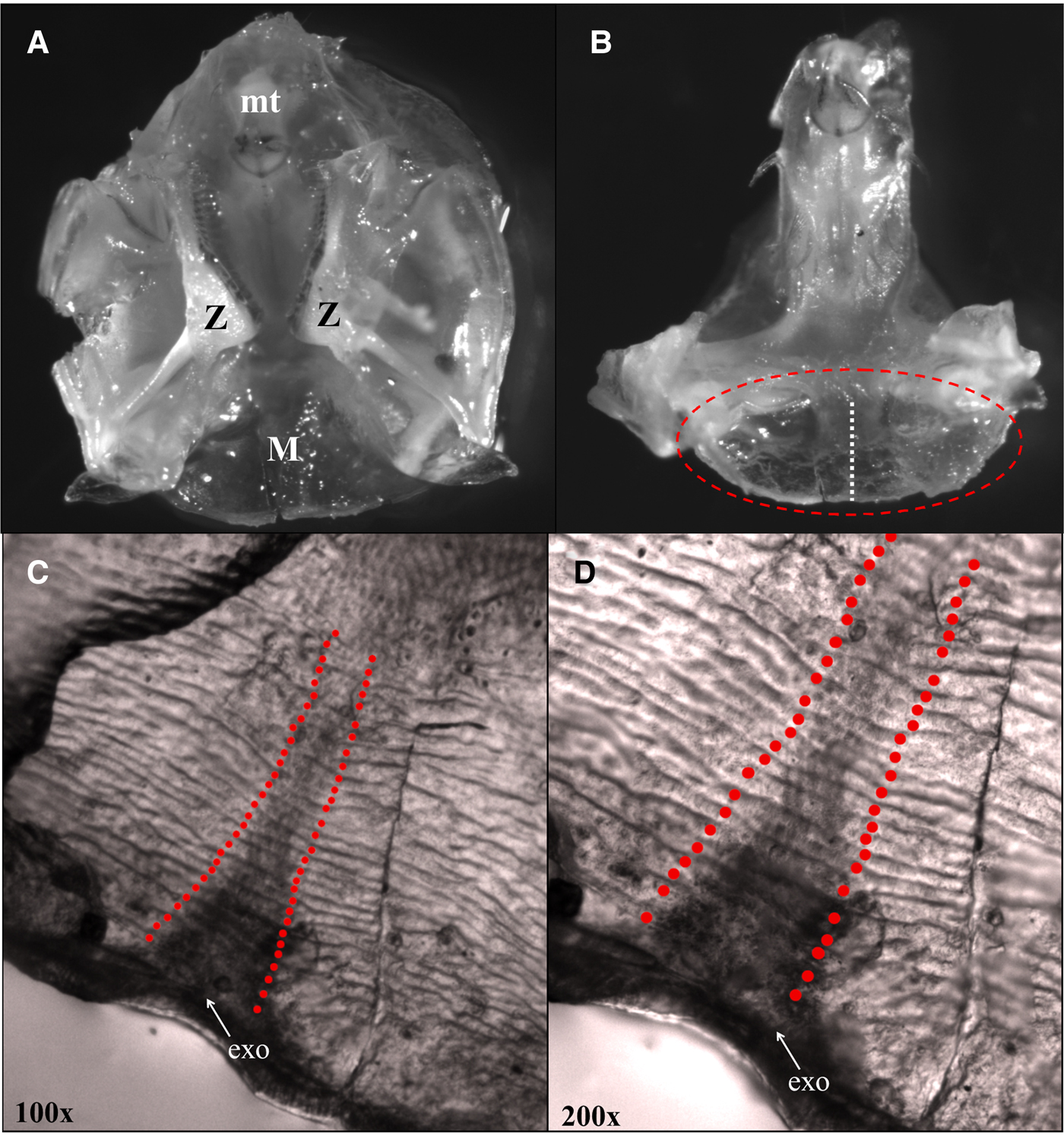
Fig. 2. (A) Vertical anterior view of the complete gastric mill with all ossicles and grinding teeth from Neotrypaea californiensis. The gastric mill is comprised of several calcified structures including the mesocardiac ossicle (M) from which lamellae counts were taken and holds the median tooth (mt) and the zygocardiac ossicles (Z) with the zygocardiac teeth. (B) Vertical anterior view of the entire median tooth and mesocardiac ossicle. The oval denotes the flat, blade-like section of the mesocardiac ossicle that was removed, embedded in thermoplastic resin and prepared for polishing. The vertical line shows the axis along which lamellae measurements were taken. (C) Polished mesocardiac ossicle at 100× magnification showing striated structure of internal lamellae and the perpendicular axis over which duplicate counts were made beginning at the interior edge of the exocuticle (exo). (D) Alternating light and dark lamellae layer shown at higher magnification (200×).
Lamellae counts were made along the medial axis of the mesocardiac structure beginning interior to the fan-like exocuticular boundary, counting each pair of alternating light and dark components, counting inward to the base of the median tooth where the structures were no longer present (Figure 2C, D). Counts were made from photographs taken with a Leica® camera attached to a Leica® compound light microscope at 200× and 400× magnification. Leica Application Suite (LAS) imaging software was used to make measurements of the growth features in the photographs. Duplicate counts were completed for each ossicle, both on the left and right sides of the median axis to ensure that counts were the same throughout the measured region of the structure and for reproducibility (Figure 2C). An unbiased second reader recounted 25 ossicles to ensure counts matched and were not subjective.
Statistical analysis
Small individuals appeared in the mesocosms several years after the start of the experiment that led us to assume reproduction had occurred within the mesocosms. Although this result was unexpected, we accounted for the new age classes statistically and used the reproductive events as an opportunity to evaluate the ability for each ageing metric to resolve population age structure. This analysis was completed by fitting a mixture of normal distributions to frequency histograms of lipofuscin index, gastric mill lamellae number and carapace length from each sample period using the ‘mixdist’ package (Du, Reference Du2002; MacDonald & Du, Reference Macdonald and Du2015) in the software program R (R Core Team, 2016). The mixdist algorithm provides estimates for mixing proportions (π), mean (μ) and standard deviations (σ) by fitting finite mixture distribution models to frequency histograms. A bin width of 0.5 ng−1 µg−1 was used for LF Index-frequency histograms and a bin width of two lamellae for gastric mill histograms. CL measurements were binned in 0.5 mm increments. Model fits were improved by constraining the algorithm to maintain a constant coefficient of variation for normal components determined in each analysis. Modes that were present in histograms were assigned ages (in years) based on sampling date and an assumed birth date of 1 September 2010 for the initial cohort. Successive reproductive events were categorized as ‘cohorts’ with the initial animals introduced to the experiment assigned as cohort A and the following year classes as cohorts B, C and D. Modal analyses were completed independently for lipofuscin concentration, lamellae count and CL datasets.
Multiple linear regression was used to test the effect of age (estimated from modal analysis) on lipofuscin accumulation rate and gastric mill lamellae count using cohort and tank as covariates. Models were constructed using tank means for both age metrics to account for non-independence of shrimp grown in the same tank. Models containing age, tank and cohort were compared against reduced models that included both age and tank or only age as variables. The best models were selected based on lowest Akaike's Information Criterion (AIC) and Bayesian Information Criterion (BIC) values. Ageing methods were compared by creating a matrix of age class designations using each method and calculating the proportion of observations that were estimated to be in the same or different age class depending on the metric used.
Linear mixed effects models (LME) were used to determine the relationship between gastric mill lamellae count and body size and also between lipofuscin concentration and body size in known-age shrimp (from mesocosms) with tank set as a random variable to account for non-independence of shrimp grown in the same tank. LME models were fitted using maximum likelihood estimation with untransformed data using the ‘lme4’ package in R (Bates et al., Reference Bates, Maechler, Bolker and Walker2015). Significance of predictor variables in LME models was determined by comparing the full model containing the variable of interest to an intercept-only model with ANOVA (Bates, Reference Bates2010). The most parsimonious lipofuscin accumulation model determined from analysis of known-age shrimp was then applied to estimate ages of shrimp collected in field surveys.
Linear regression models were applied to evaluate the relationship between lamellae count and lipofuscin-based age and lamellae count and body size for wild shrimp from estuarine collections. Varying-intercept LME models were applied that included a sample location random variable. This accounted for variation in the lamellae count-at-age and lamellae count-at-size across locations but also used the entire pooled dataset to boost sample numbers and derive a general model. Similar to the analysis of mesocom data, the significance of the predictor variables in LME models was determined by comparing a full model containing the variable of interest to an intercept-only model with ANOVA (Bates, Reference Bates2010). Regression analyses were completed on non-transformed data using R (R Core Team, 2016). Residuals of all regression analyses were visually assessed to ensure data met assumptions of normality and equal variance.
Results
Analysis of mesocosm-grown shrimp
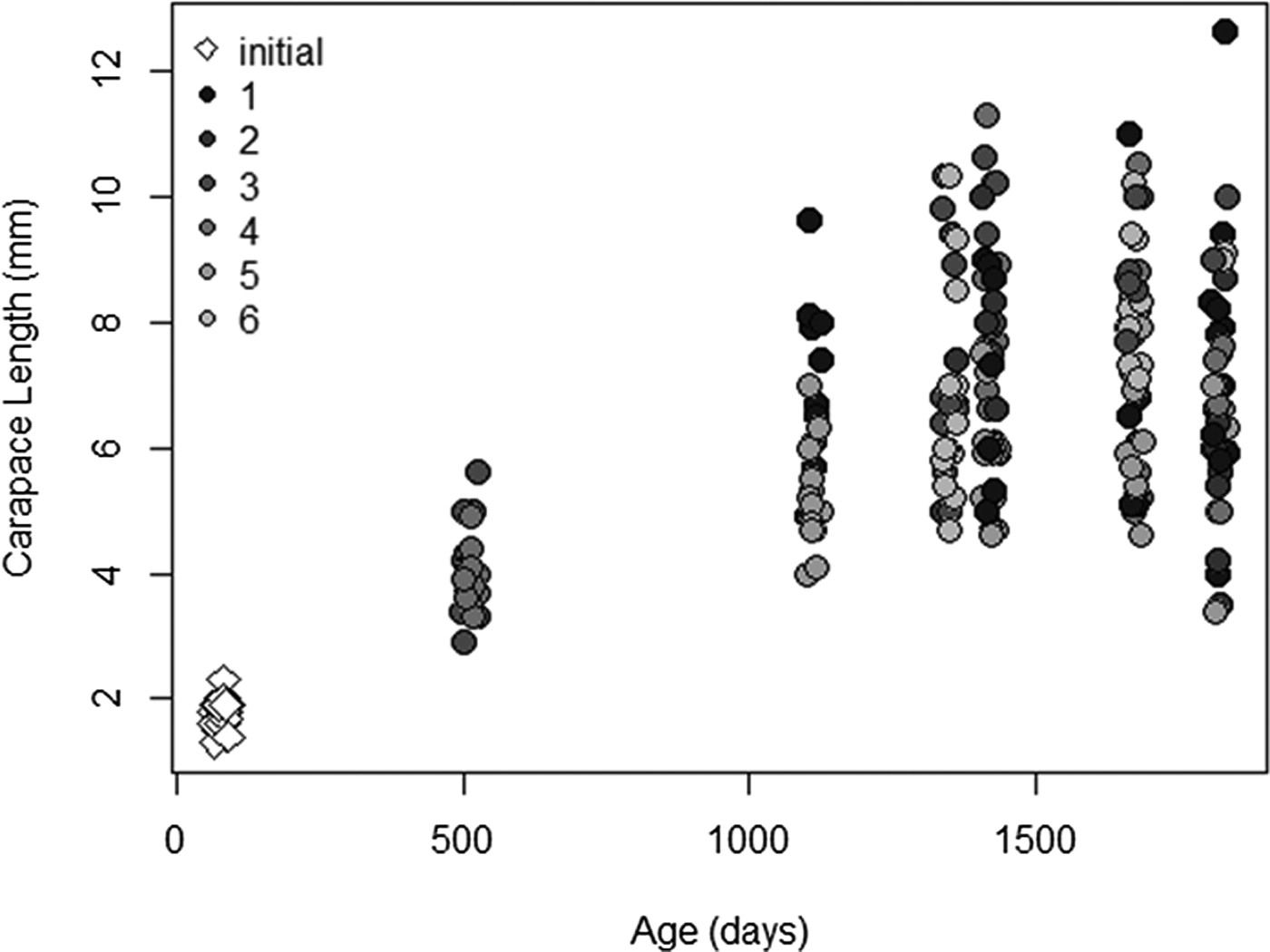
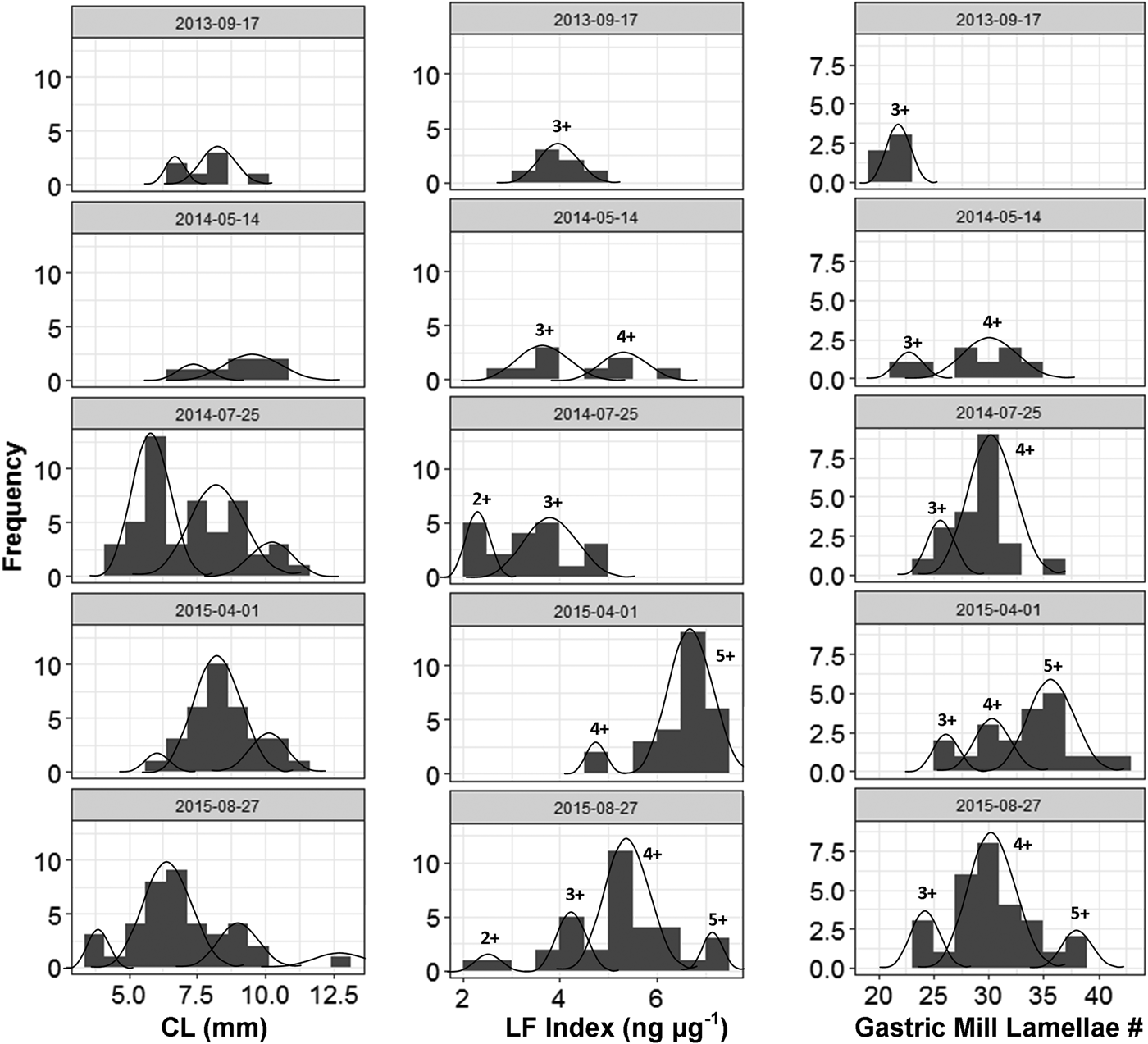
A total of 126 known-aged shrimp were collected from the mesocosms during five sample periods from 17 September 2013 to 26 August 2015. The range in shrimp body size for mesocosm grown animals increased over time and at later sampling events, but smaller and presumably younger shrimp appeared in the tanks, which led to a wide range of shrimp sizes (3.4–12.6 mm CL) by the final sampling date (Figure 3). Lamellae counts also increased with time and, like body size, a wide range of values were observed in the later sampling date (25–40 lamellae). The cohort analysis of lipofuscin index showed a clear progression of modes in frequency distributions representing multiple cohorts (Figure 4 & Table S1 in the supplemental material). Modal analysis of lamellae count data also showed a progression of assumed cohorts, but the groupings were less clear with a greater degree of overlap (Figure 4 & Table S2). Once age classes were assigned to individuals, the age structure for both metrics were strikingly similar, however analysis of lipofuscin showed a younger (2+) cohort present in years 4 and 5 where gastric mill lamellae did not (Figure 5A, B). Modal analysis of carapace length data also revealed a mixture of size classes at each sample period but the progression of the size groups was less clear than observed with LF Index or lamellae counts (Figure 4 & Table S3).

Fig. 3. Plot showing carapace length (CL, mm) vs age (approximate number of days since recruitment) for Neotrypaea californiensis grown in outdoor mesocosms. Points are shaded corresponding to tank number and are jittered to reduce overlap.

Fig. 4. Results from modal progression analysis of carapace length (mm; left), LF Index values (ng µg−1; middle column) and gastric mill ossicular lamellae count (#; right column) for shrimp sampled from outdoor mesocosms over the course of the study. Gaussian curves represent the estimated proportion of individuals comprising cohorts within the tanks (See supplemental material Tables S2 & S3). Age classes are estimated for cohorts using LF Index and gastric mill lamellae counts based on the approximate length of time the shrimp would have been living in the tanks.
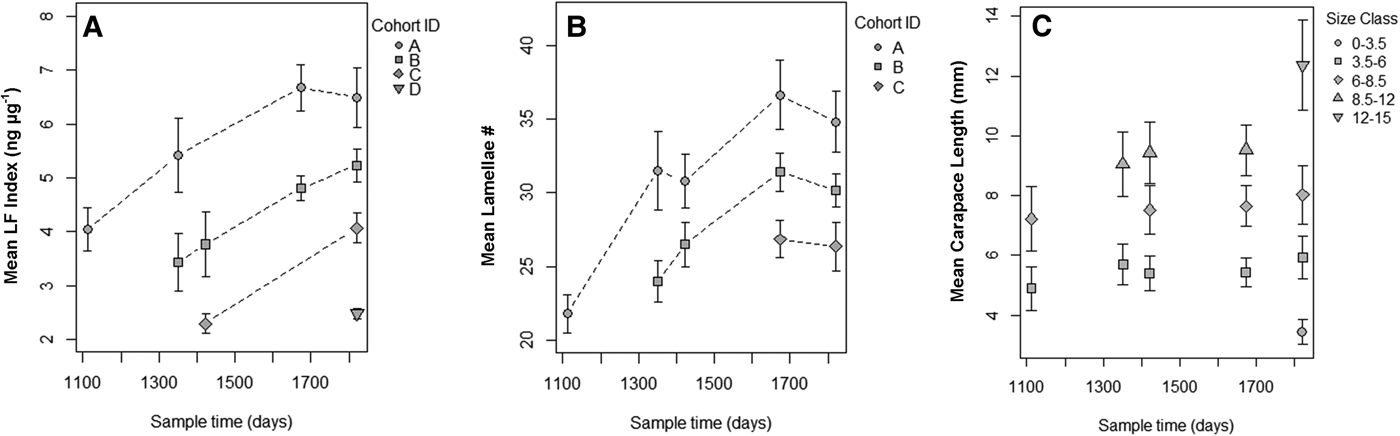
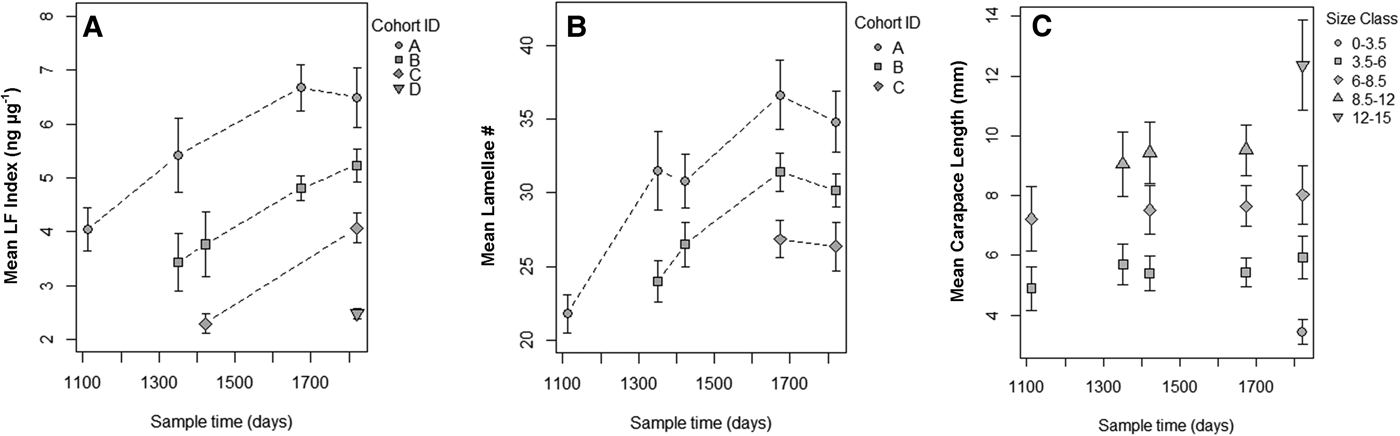
Fig. 5. (A) Mean LF index (ng µg−1) vs time elapsed (days) from beginning of outdoor mesocosm grow-out experiments showing cohorts identified by modal progression analysis. (B) Mean lamellae number vs time elapsed (days) from beginning of outdoor mesocosm grow-out experiments showing cohorts identified by modal progression. (C) Mean carapace length (mm) vs time elapsed (days) from beginning of outdoor mesocosm experiments. Results of modal analysis of carapace length are presented in Table S3.
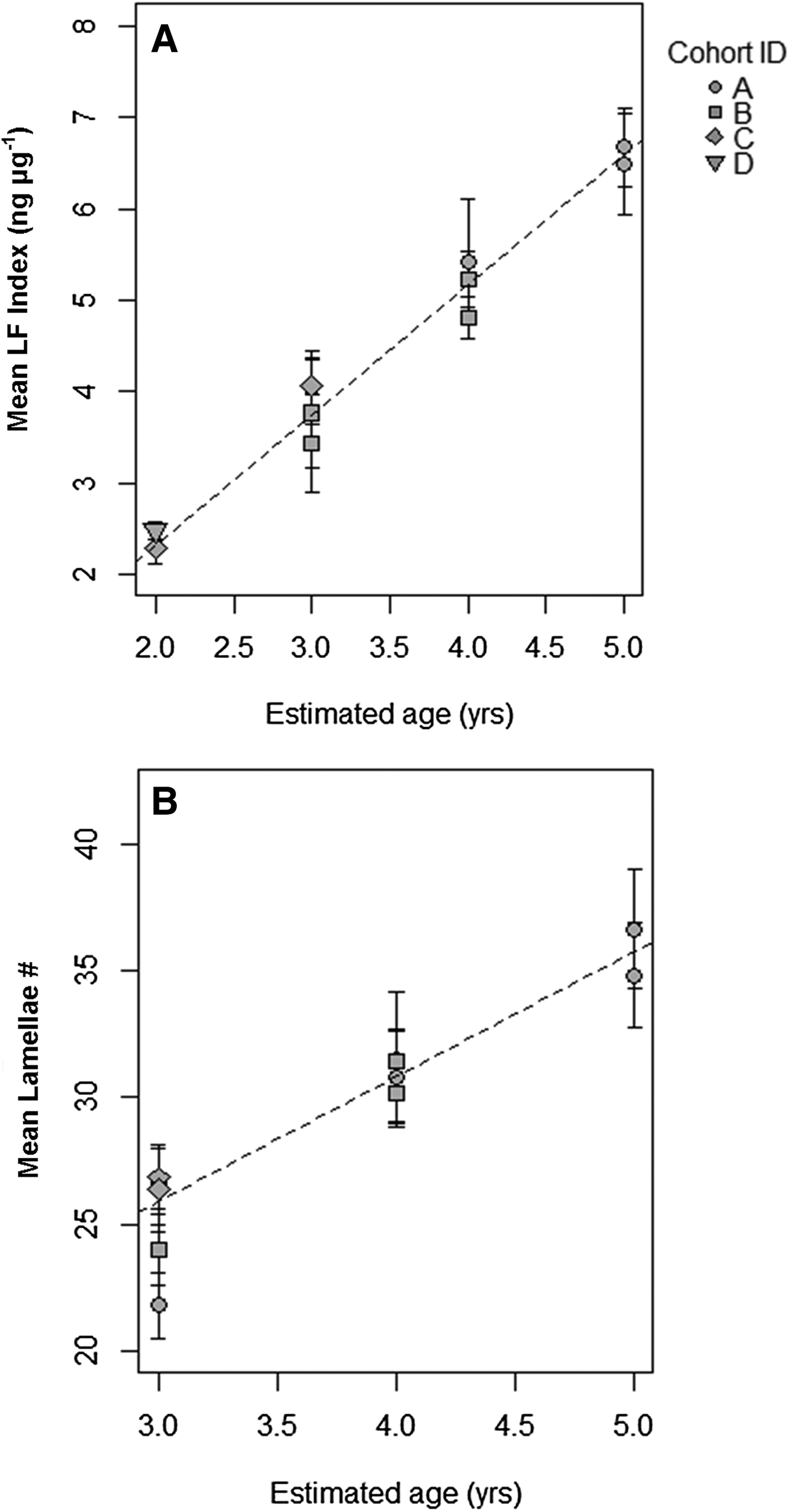
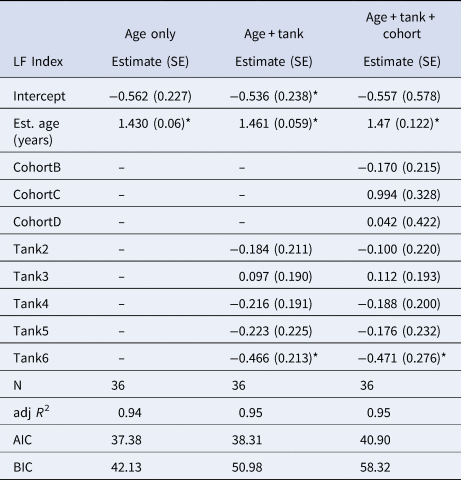
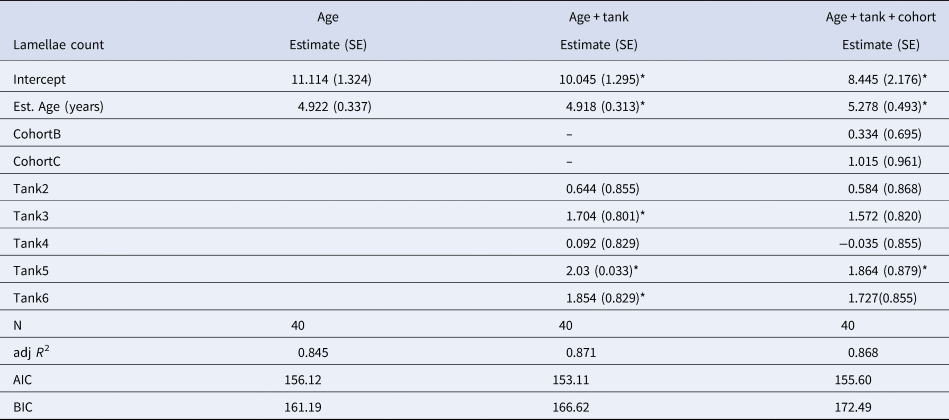
Average LF Index was positively correlated with actual age (in years) determined from the cohort progression model and lipofuscin accumulation rate was not significantly different across cohorts or tanks (Figure 6A & Table 1). Lipofuscin accumulation was linear at a rate of 1.430 ± 0.060 ng µg−1 year−1 in a basic model. Lamellae counts also increased with chronological time in known-age shrimp and was not significantly different across cohorts, but the effect of tank was significant, a result primarily driven by tank 1 having lower lamellae counts-at-age (Figure 6B & Table 2). Average lamellae count increased linearly at a rate of 4.922 ± 0.337 lamellae year−1 as estimated from the reduced model.
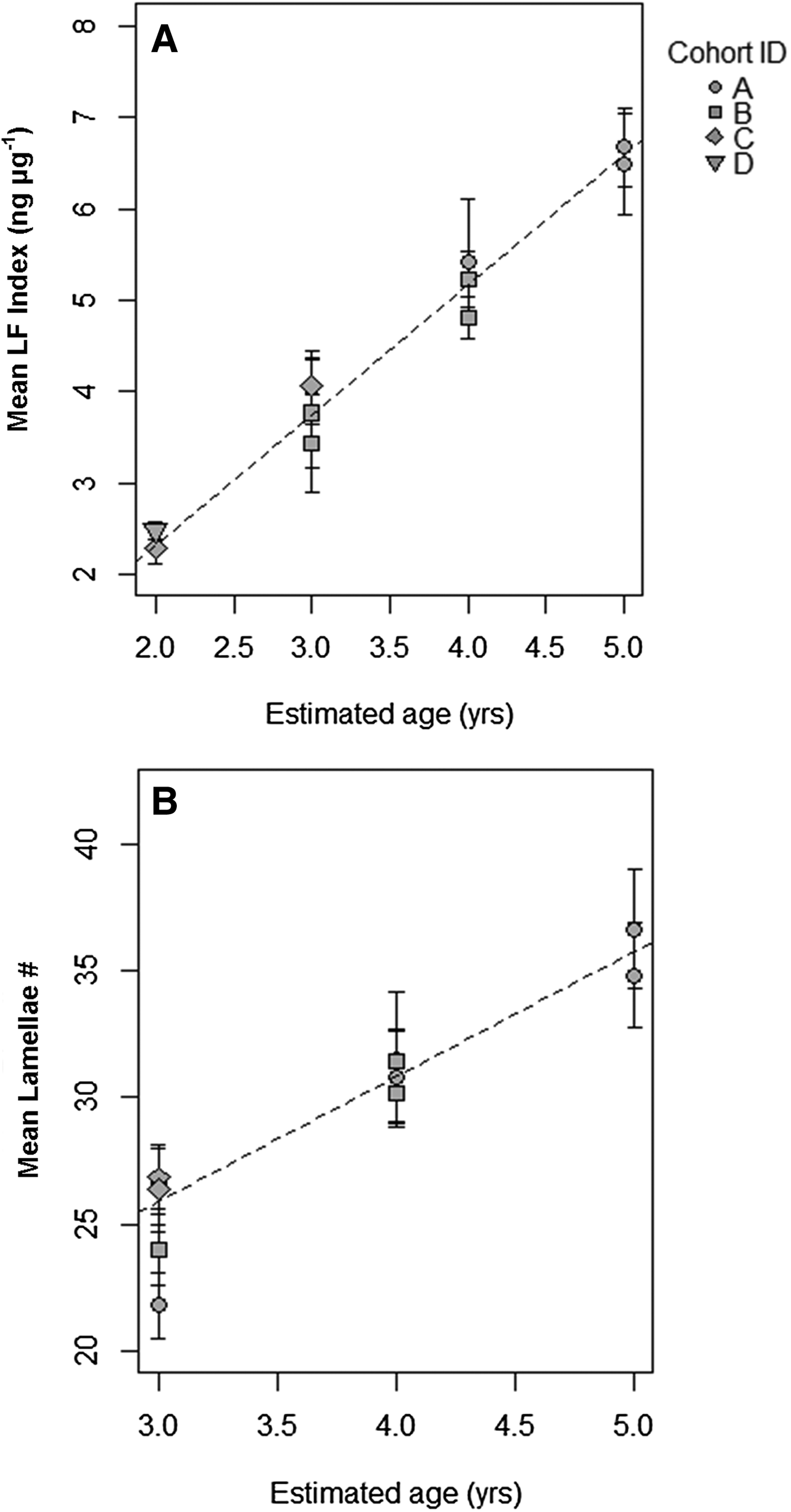
Fig. 6. (A) Linear correlation of mean LF Index (ng µg−1) with assumed age (in years) for each cohort determined from modal progression analysis. (B) Linear correlation of mean lamellae count with assumed age (years) for each cohort determined from modal progression analysis. The coefficients were not significantly different among cohorts in each analysis.
Table 1. Results of multiple regression models investigating the effect of age (years), tank and cohort on LF Index (ng µg−1) for shrimp sampled from mesocosms

SE, Standard error; N, sample size; AIC, Akaike's Information Criterion; BIC, Bayesian Information Criterion.
*Indicates statistically significant parameters (P < 0.05). Both cohort and tank are coded as factors in the regression models.
Table 2. Results of multiple regression models investigating the effect of age (years), tank and cohort on gastric mill lamellae count for shrimp sampled from mesocosms

SE, Standard error; N, sample size; AIC, Akaike's Information Criterion; BIC, Bayesian Information Criterion.
*Indicates statistically significant parameters (P < 0.05). Both cohort and tank are coded as factors in the regression models.
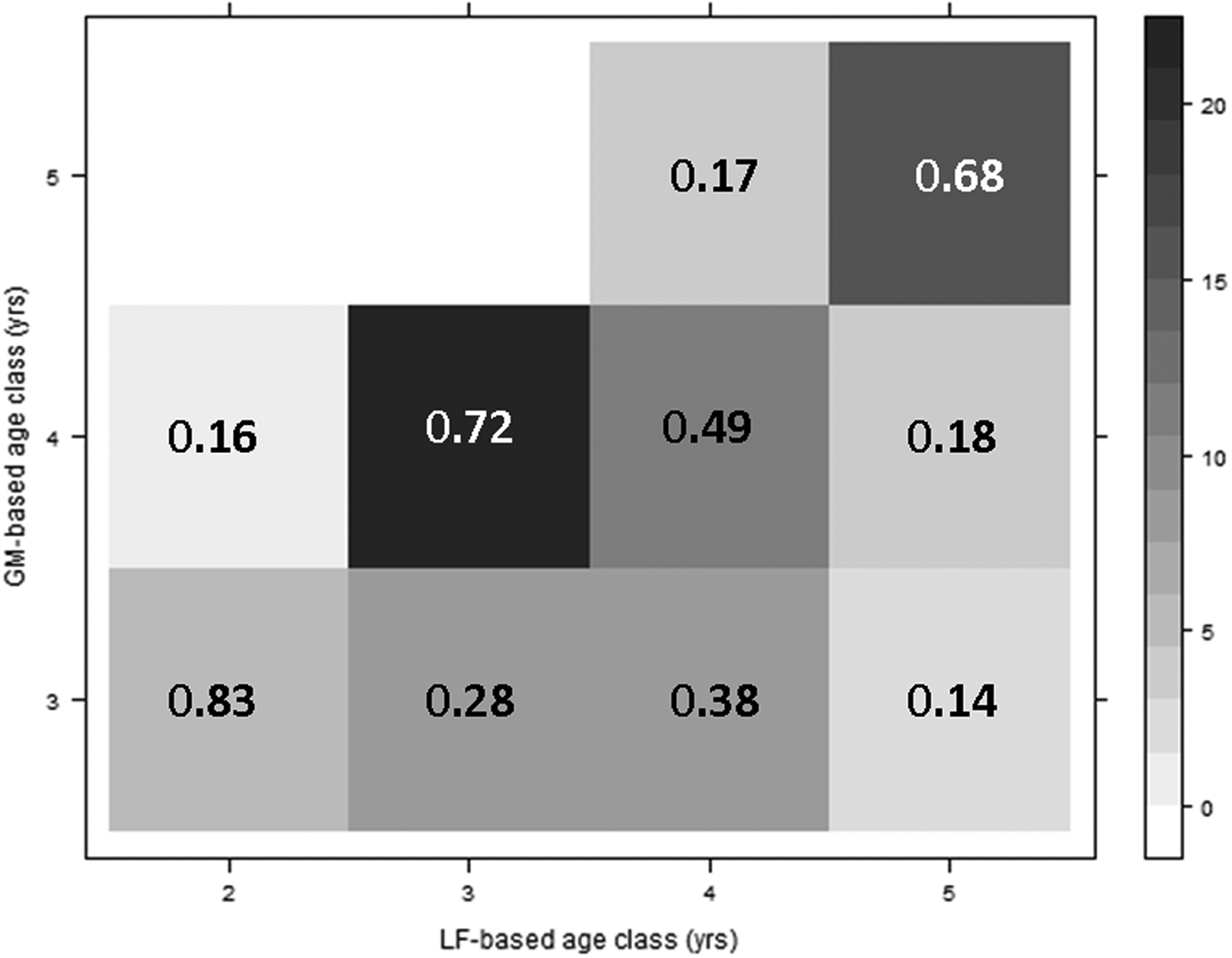
A matrix of age class assignments showed consistency in age determination between the two methods (Figure 7). A total of 68% of samples that were assigned to age 5+ based on lipofuscin concentration were also assigned to age 5+ based on lamellae counts. Similarly, 49% of samples that were assigned to age 4+ based on lipofuscin were also classified as age 4+ with gastric mills. There was a greater discrepancy between the two methods in the younger age groups. A total of 72% of the samples that were classified as 3+ based on lipofuscin concentration were also estimated to be age 4+ using lamellae counts.
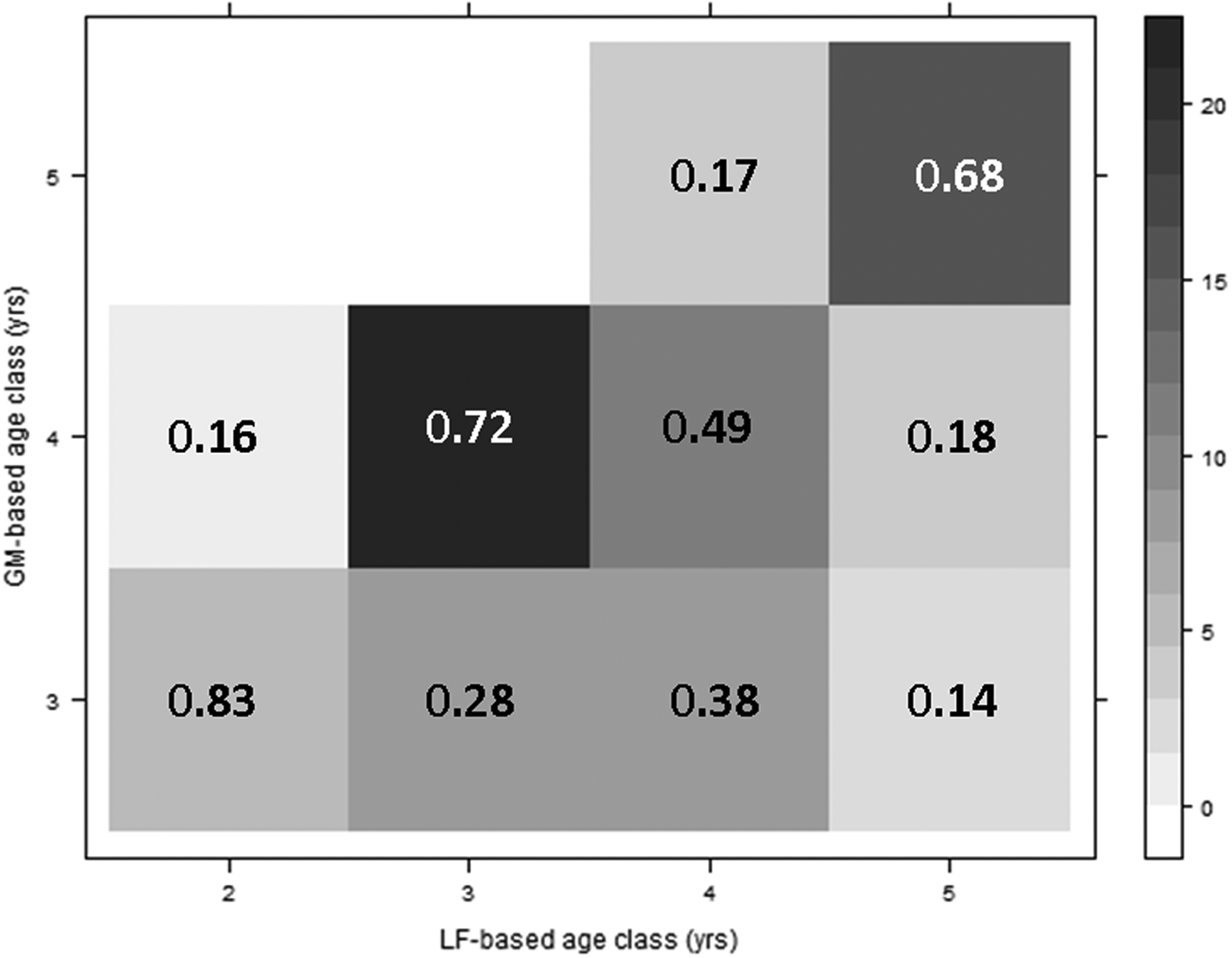
Fig. 7. A matrix of age class assignments using both the gastric mills (GM-based) and lipofuscin ageing (LF-based) methods for shrimp sampled from outdoor mesocosm. Shading indicates the number of observations in each age category combination (Total N = 81) and values represent the proportion of samples that occur in each age group based on LF index that were assigned to each age group based on lamellae count (columns sum to 1).
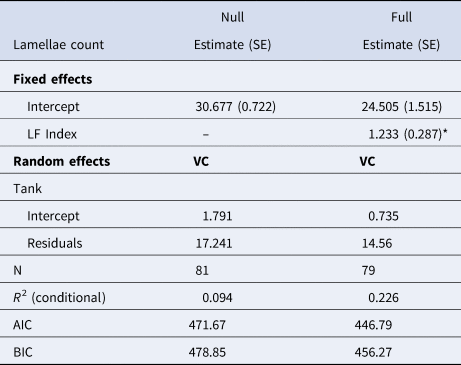
Comparison of null (intercept only) models for lamellae count and LF Index versus models containing CL showed that inclusion of CL in the model did not improve the fits to the observed data, suggesting no significant relationship between lamellae count and CL (χ2, P = 0.264) or LF Index and CL (χ2, P = 0.06). However, lamellae count was positively correlated to LF Index in shrimp collected from the outdoor mesocosms (Table 3 & Figure 8). The linear mixed effects model containing LF Index provided a better fit to the data than the intercept-only model based on AIC and BIC values and showed lamellae number increased at 1.233 ± 2.287 lamellae ng−1 µg−1 LF, but the relationship showed considerable scatter.
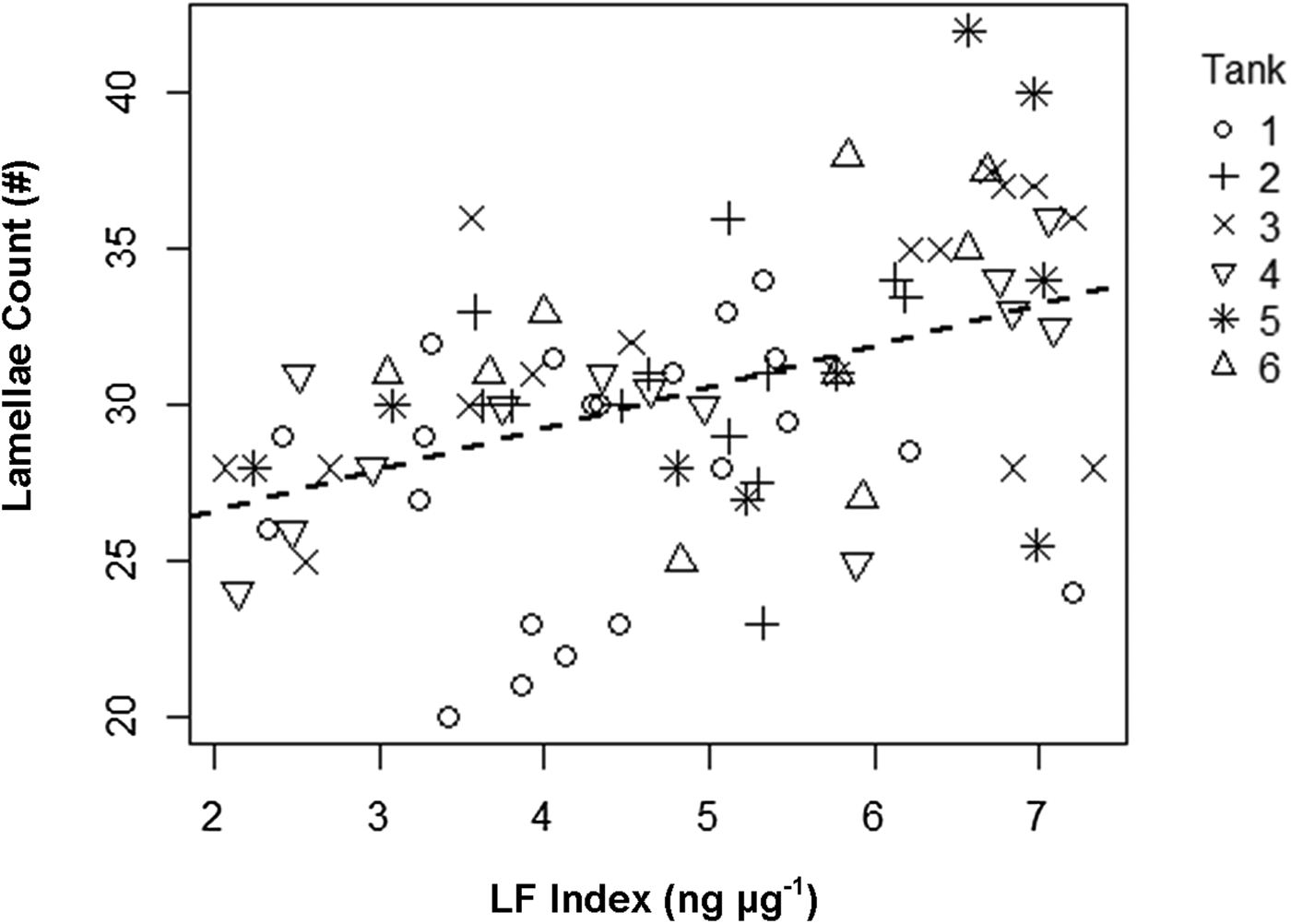
Fig. 8. Linear correlation of gastric mill lamellae counts (#) as a function of LF Index (ng µg−1). Regression line shows the positive relationship determined with a linear mixed effects model (Table 3). Sample tanks are denoted with different symbols.
Table 3. Results of linear mixed effects models used to determine the relationship between LF Index (ng µg−1) and gastric mill lamellae number while accounting for non-independence of shrimp collected from the same tank
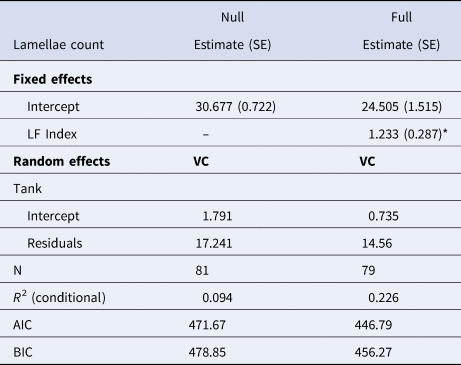
SE, Standard Error; VC, Variance components of random effect; N, sample size; AIC, Akaike's Information Criterion; BIC, Bayesian Information Criterion.
The null models is an intercept-only model and full model includes the dependent variable, lamellae count.
*Indicates statistically significant parameters (P < 0.05).
A total of 25 gastric mills were measured by more than one person to test for reproducibility in lamellae counts. A paired t-test on the duplicate measurements indicated no significant differences in the average count (t = −0.413, df = 20, P = 0.68). The mean difference in lamellae number among readers was 0.66 ± 0.81, which is high precision considering that the overall average lamellae count was 30.
Analysis of field-collected shrimp
The simple (reduced) model of lipofuscin accumulation rate was applied to estimate ages for shrimp collected from field surveys in Yaquina Bay and Willapa Bay because it provided a more precise method of estimating age than gastric mill lamellae count (lower error estimates and few significant tank effects, Tables 2 & 3). Lipofuscin-based age structure of shrimp collected from the OSU dock site showed a significant portion of the shrimp taken there were aged 2–4 (Figure S2), which agrees with our assumption that this site was colonized following a period of steady recruitment beginning in 2010 (Dumbauld & Bosley, Reference Dumbauld and Bosley2018).
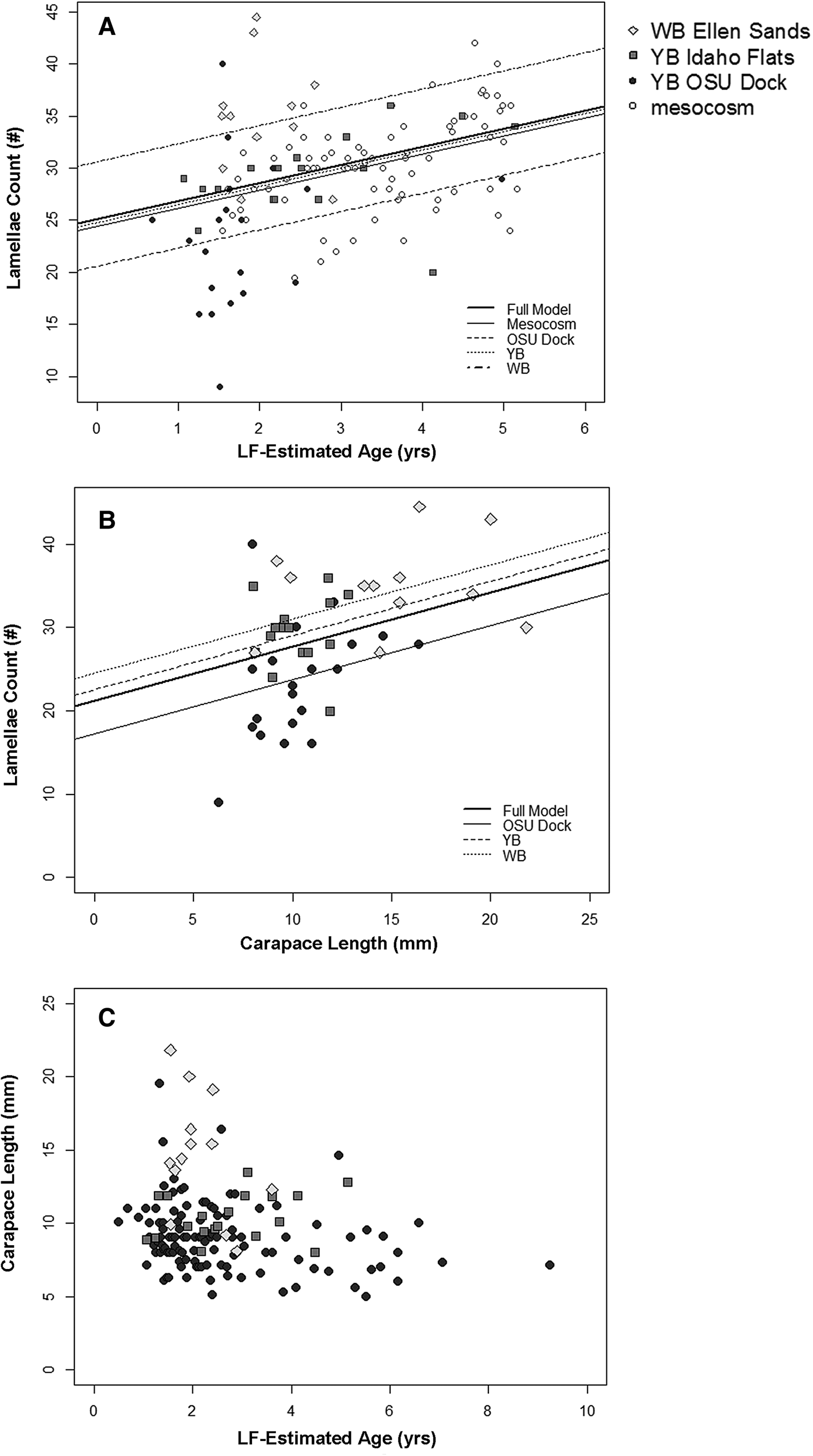
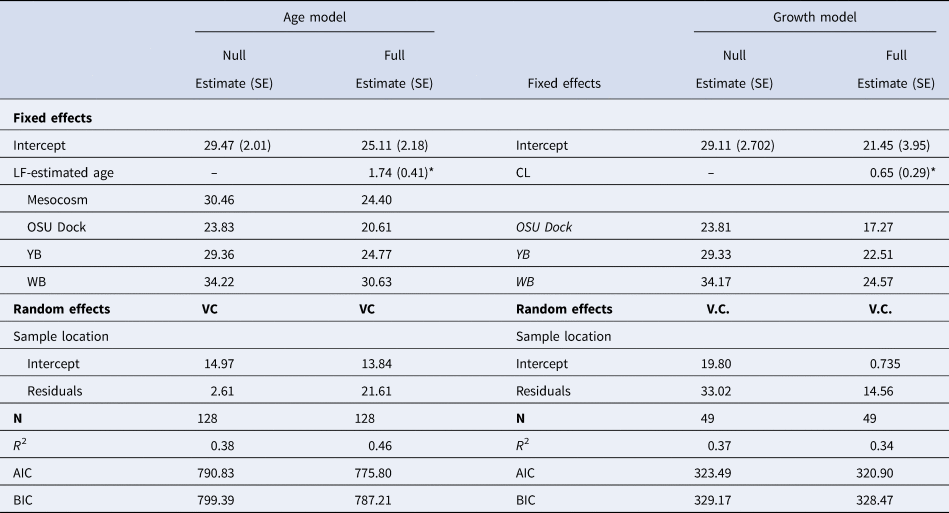
Results from the LME models indicated age was a significant predictor of lamellae count but the relationship varied by location (χ2, P < 0.001; Table 4). The LME analysis also showed carapace length to be a weak predictor of lamellae number (χ2, P = 0.035) but also showed strong differences among locations (Table 4). Shrimp collected from Willapa Bay generally had more lamellae at a given age or size than shrimp from the other locations (Figure 9), where shrimp from the OSU Dock location had fewer lamellae. Interestingly, no significant difference was found in the lamellae count vs assumed age relationship between the mesocosm and the YB Idaho flats location (Figure 9A).

Fig. 9. (A) Linear growth model for gastric mill lamellae count-at-age data. The lines represent linear relationships for individual sites determined from a mixed effects model with varying intercepts. (B) Linear relationships for lamellae count vs carapace length for wild shrimp sampled from different locations determined with a mixed-effects model. (C) Scatterplot of carapace length vs lipofuscin-estimated age for wild-caught shrimp. Sample locations are denoted by plotting symbol.
Table 4. Table of parameter values from linear mixed effects models representing the relationship between gastric mill lamellae count and both lipofuscin (LF)-estimated age and body size (CL) for field-collected Neotrypaea californiensis. The table includes intercept coefficients for the individual sample groups which includes mesocosms shrimp in the age model
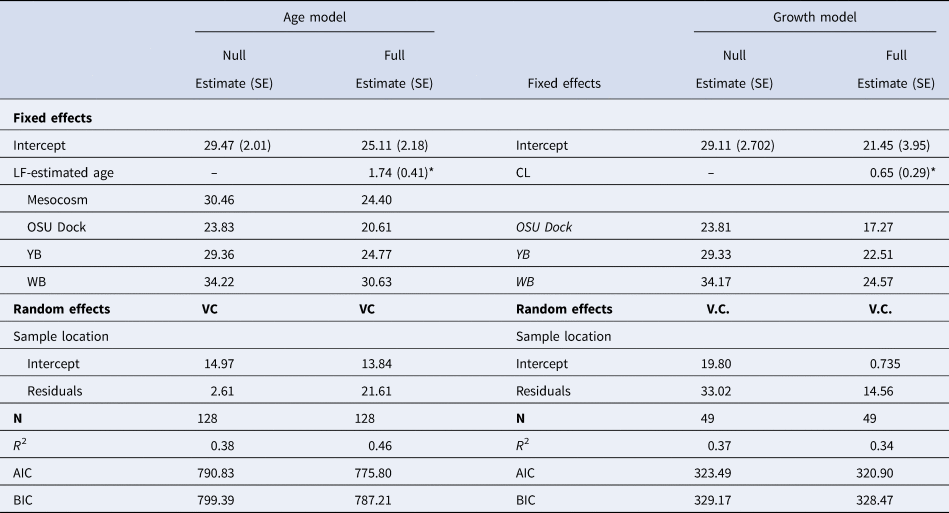
SE, Standard Error; VC, Variance components of random effect; N, sample size; AIC, Akaike's Information Criterion; BIC, Bayesian Information Criterion.
*Indicates statistically significant parameters (P < 0.05).
Carapace length was not related to lipofuscin-estimated age in field-collected animals (Figure 9C), which agreed with findings from the mesocosm experiment. On average, the shrimp from Willapa Bay were much larger (14.59 ± 4.14 mm; 2–4-year-olds) for their age than shrimp collected from Yaquina Bay (8.89 ± 2.33 mm; 1–8-year-olds).
Discussion
The overall goal of this study was to further validate the previously applied lipofuscin ageing technique to ghost shrimp, Neotrypaea californiensis, of known age and to establish whether gastric mill ossicular features (i.e. structural lamellae) can be used as an additional method of age determination in this species. Through a combination of long-term growth experiments and field surveys, along with the concurrent evaluation of lipofuscin concentration and gastric mill ossicular lamellae, our work provides clear evidence that these alternative methods of age determination provide more reliable estimates of age than body size measurements in N. californiensis. Additionally, use of the two methods together shows promise for widespread evaluation of crustacean age and growth.
Transplanting newly recruited juvenile shrimp into an outdoor mesocosm allowed us the opportunity to observe temporal changes in lipofuscin index with known-age animals. The shrimp increased in body size and lipofuscin index, but the distribution of both of these values became more widely spread with many individuals having much lower LF index values and smaller sizes at later time points. This surprised us as we initially expected to follow one age class through time, but suggested possible recruitment of new individuals in the tanks. Shrimp sampled from the tanks had eggs on at least one occasion, suggesting that the smaller and presumably younger animals were offspring of the original shrimp placed in the tanks. The modal analysis of LF index indicated up to 3 or 4 age cohorts in the tanks after 5 years. N. californiensis are estimated to reach maturity in 18–24 months in Oregon estuaries (Bird, Reference Bird1982) and perhaps slightly longer in Washington where minimum size at oviposition is ~9 mm CL (Dumbauld et al., Reference Dumbauld, Armstrong and Feldman1996). While smaller shrimp (CL <6.0 mm) from the mesocosms were not analysed for lipofuscin because their brain tissue was too small for protein detection, they presumably represented additional cohorts entering the population. If shrimp were reproductively mature in the mesocosms at ~18 months, two cohorts could be present after 3 years and up to 4 cohorts by the end of the experiment if shrimp reproduced each year thereafter, which is what we observed.
Analysis of multiple cohorts from our mesocosm study showed average lipofuscin accumulation rate to be 1.430 ng ug−1 year−1. When this rate was applied to estimate population age structure at the OSU dock location in Yaquina Bay, Oregon, results showed the site was dominated by young shrimp, with most of the shrimp sampled from that location <4 years old (Figure S2). This age structure is consistent with measurements of post-larval settlement at this location since 2010 (Dumbauld & Bosley, Reference Dumbauld and Bosley2018). Prior to 2010, shrimp were fewer at this site, which has since been subject to regular strong recruitment events. The lipofuscin-based age structure determined from the Yaquina Bay field survey reflects this temporal trend in recruitment and further verifies the method for assessing population demographics in N. californiensis.
The rate of LF accumulation measured from the long-term mesocosm experiment was substantially greater than that estimated by Bosley & Dumbauld (Reference Bosley and Dumbauld2011) for N. californiensis in Yaquina Bay from field samples (≈0.37 ng µg−1 year−1). There are several potential reasons for this difference. First, Bosley & Dumbauld (Reference Bosley and Dumbauld2011) used methods developed by Ju et al. (Reference Ju, Secor and Harvey1999) where lipofuscin extracts were split prior to fluorescence measurement. This would cause the ratio of lipofuscin to protein concentration to be generally lower as the protein fraction of these samples was not also split. Further, the previous study was short term, taking place over only 10 months from July to April and lipofuscin accumulation may have slowed over this period due to winter environmental conditions (i.e. cooler temperatures) which could result in a lower accumulation rate (O'Donovan & Tully, Reference O'Donovan and Tully1996; Tully & Fletcher, Reference Tully and Fletcher2000; Pinchuk et al., Reference Pinchuk, Harvey and Eckert2016). The long-term study of lipofuscin accumulation presented here equates to approximately half of the total predicted lifespan of N. californiensis (Bosley & Dumbauld, Reference Bosley and Dumbauld2011), taking place over many seasons. While this study provides evidence that estimated lipofuscin accumulation rate can be applied generally to wild populations, additional long-term field studies should be completed to confirm these findings, preferably taking place over a broader spatial scale.
There is conflicting evidence as to whether the gastric mill is retained or lost during ecdysis (reviewed in Kilada & Driscoll, Reference Kilada and Driscoll2017; Becker et al., Reference Becker, Dick, Cunningham, Schmitt and Sigwart2018). Two previous studies used calcein dye to track the formation of growth marks in the gastric ossicles of lobsters and crayfish, both of which suggested that the mesocardiac and pterocardiac ossicles are retained for at least one moult cycle (Kilada et al., Reference Kilada, Sainte-Marie, Rochette, Davis, Vanier, Campana and Gillanders2012; Leland et al., Reference Leland, Bucher and Coughran2015). Conversely, work by Brösing (Reference Brösing2014) and Vatcher et al. (Reference Vatcher, Roer and Dillaman2015) found differences among some crab species regarding which gastric mill ossicles and teeth are lost during moulting, with indications that some may be partially reabsorbed prior to moulting, and their remnants found in the exuviae. Sheridan et al. (Reference Sheridan, O'Connor and Henderson2016) provided evidence that gastric mill ossicles are not lost in the exuviae, but instead, are lost internally within the stomach at moulting and contribute to recalcification of the gastric mill structure postmoult. More recently, Becker et al. (Reference Becker, Dick, Cunningham, Schmitt and Sigwart2018) showed that the gastric mills of several crustacean species were lost during ecdysis and that marks contained within these ossicles may not represent the putative annuli suggested by previous research. Irrespective of whether the gastric mill in N. californiensis is lost during ecdysis, our results clearly demonstrate that the lamellate features within the gastric mill mesocardiac ossicle are deposited sequentially over time and with increasing age. These results support the hypotheses presented by Leland et al. (Reference Leland, Bucher and Coughran2015) suggesting that ossicular (and likely the lamellar) growth marks in moulted structures may be duplicated in newly formed structures, retaining previously recorded internal growth rate variations, before new growth features are added with consecutive moults.
The lamellar structure we measured in the mesocardiac ossicle of N. californiensis closely resembled the lamellar structure of the mesocardiac ossicle in blue crabs with alternating light and dark rings stacked parallel to one another in the endocuticle (Vatcher et al., Reference Vatcher, Roer and Dillaman2015). These lines are rigid chitinous structures that are believed to act as a support or scaffolding in the cuticle during moulting and are a general feature common to many arthropods, including insects (Neville, Reference Neville1965). Lamellae in the endocuticle and exocuticle in some arthropods have shown a daily pair of growth marks, a non-lamellate day layer and a lamellate night layer, with the amount of chitin deposited slowly decreasing with increasing age (Neville, Reference Neville1965). In addition, the width of each light/dark pair of lamellae in several species of insects is ~10 µm which is consistent with the growth features we found in N. californiensis (Figure 2D). The mechanism through which crustacean lamellae are formed is currently unknown and may be species-specific. Future work should be done to evaluate not only the periodicity in which the lamellae are deposited within gastric mill ossicles but also the mechanism that allows this growth record to be retained over time and through consecutive moults.
Our results provide evidence that the structural lamellae within the mesocardiac ossicle of N. californiensis contain a chronological record that is a more precise indicator of true age than body size measurements in N. californiensis. These findings complement the growing body of literature showing that measurement of growth marks within the gastric mill provides a method for ageing crustaceans. While previous validation studies using known-age animals have interpreted intercuticular growth marks as putative annuli (Kilada et al., Reference Kilada, Reiss, Kawaghchi, King, Matsuda and Ichii2017), the lamellae we measured are probably deposited at a rate that varies depending on moulting frequency and environmental conditions. Previous studies have shown that moult increment can be very small in crustaceans, with animals staying the same size after moulting (Hartnoll, Reference Hartnoll2001). In some cases, crustaceans may actually shrink (i.e. moult to a smaller size) to conserve metabolic resources when under environmental stress (Marinovic & Mangel, Reference Marinovic and Mangel1999). There is a limited amount of information regarding moulting frequency or moult increments in N. californiensis but recent work has indicated that moult increments are small in larger shrimp (K. Bosley, unpubl.) and shrimp can actually shrink in size under poor food conditions (Bosley, Reference Bosley2016). In light of this, it may be feasible for a small shrimp to accumulate 30 or more lamellae over a lifetime if the features are added with consecutive moults, despite a limited change in overall body size. This may explain why some small shrimp may have many lamellae, as was observed in the mesocosms.
We observed differences in mean lamellae count-at-age and lamellae-at-size among our sample groups. Similar growth differences were documented by Bosley & Dumbauld (Reference Bosley and Dumbauld2011), where N. californiensis from Willapa Bay, WA were much larger at a given age than shrimp from Yaquina Bay, Oregon. These differences probably reflect variation in habitat quality and environmental conditions across locations. Because food availability is a primary driver of growth in crustaceans (reviewed in Hartnoll, Reference Hartnoll2001), it has been hypothesized that spatial variation in food quantity and quality results in differences in growth rate and body condition among spatially discrete populations of N. californiensis (Bosley, Reference Bosley2016; Bosley et al., Reference Bosley, Copeman, Dumbauld and Bosley2017). Willapa Bay shrimp were collected at a location close to the estuary mouth that receives an abundance of high quality oceanic food sources (Newton & Horner, Reference Newton and Horner2003). Increased moult frequency and shorter intermoult period would explain why shrimp from this location generally had higher body size and lamellae count for a given age. Interestingly, the relationship between lipofuscin-estimated age and gastric mill lamellae was not statistically different between the mesocosms and the Yaquina Bay sampling location. The shrimp that were grown in the mesocosms were collected from Yaquina Bay and water intake for the mesocosms was nearby to the locations where wild shrimp were sampled. The similarity in the results from the mesocosms and YB shrimp may reflect a consistency in environmental conditions (e.g. food, temperature) between locations. Despite spatial differences, we observed a positive correlation between lamellae number and assumed age where carapace length was not correlated with age. These results suggest that lamellae provide a better indicator of relative age, but the individual relationship may be location specific.
Combined use of methods to assess intracellular lipofuscin concentration and gastric mill lamellae in this study provide a useful step toward the development of an alternative to the traditional length-based approaches of crustacean ageing. Body size in N. californiensis showed no relationship to LF Index or lamellae count in known-age animals from the mesocosm experiment and relatively rapid divergence in body size within the tanks confirms that body size measurements are a poor predictor of age for this deposit-feeding shrimp. This supports the hypothesis that growth of individual N. californiensis may be governed by extrinsic factors (e.g. resource limitation, density dependence, physical factors) that ultimately decouple the relationship between age and body size (Bosley & Dumbauld, Reference Bosley and Dumbauld2011; Bosley et al., Reference Bosley, Copeman, Dumbauld and Bosley2017). This presents a challenge to researchers interested in obtaining accurate age estimates needed for population demographic assessments for these crustaceans. Correlation of lamellae counts to lipofuscin age estimates for shrimp collected in demographic surveys revealed a significant positive relationship. These findings indicate that with proper validation, these two methods have the potential to improve age estimation for N. californiensis and potentially other crustacean species, while eliminating or reducing the reliance on body size, a parameter that varies significantly over space and time for many species.
The ability to apply multiple approaches to assess age (i.e. lipofuscin, ossicular lamellae), creates an opportunity to advance understanding of crustacean growth dynamics and population ecology. Management procedures often require robust estimates for population vital rates and the methodology that we present shows potential to overcome challenges in age estimation for N. californiensis, which could also be applied to other decapod crustacean species. Prior to broad-scale application of these ageing methods, further studies should be completed using known-age shrimp with mesocosms designed to prevent successful reproduction in the laboratory (e.g. daily water flushes), in order to determine whether lamellae number and lipofuscin concentration exhibit the same relationship with age without the need to statistically account for multiple cohorts. Additionally, further work should evaluate whether lipofuscin formation and gastric mill lamellae formation are influenced by the same environmental processes, which may result in biased age estimates. Future application of these methods would benefit from developing a thorough understanding of the mechanisms through which gastric mill ossicular lamellae are formed, how their formation relates to putative annuli using the previous established methodology and also the influence of variable environmental conditions on these alternative approaches to crustacean ageing. Despite these uncertainties, our unique study represents an important step in the quest to develop and hone direct ageing methods for N. californiensis and other crustacean species whose populations would benefit from improved conservation and management.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000742.
Acknowledgements
We would like to thank Lee McCoy, Daniel Sund and an army of REU student volunteers for their help with sample collection. We offer our sincere appreciation to Dr Jessica Miller and her lab group for teaching us how to polish the gastric mills and providing the equipment needed to prepare the samples for measurements. Many thanks also to Patrick McDonald at NWFSC ageing laboratory for allowing us access to the use of their digital camera. Thank you to the three anonymous reviewers who improved this manuscript greatly through their insightful and constructive comments.
Financial support
This work was funded by the USDA-ARS (CRIS project 5358-63000-002-00D) and the 2014 NSF funded Research Experience for Undergraduates program.