INTRODUCTION
The Mediterranean endemic magnoliophyte Posidonia oceanica (L.) Delile plays the major ecological role in coastal ecosystems, contributing significantly to the productivity of shallow coastal areas (Bellan-Santini et al., Reference Bellan-Santini, Lacaze and Poizat1994; Pergent et al., Reference Pergent, Romero, Pergent-Martini, Mateo and Boudouresque1994; Costanza et al., Reference Costanza, Arge, de Groot, Farber, Grasso, Hannon, Limburg, Naeem, O'Neill, Paruelo, Raskin, Sutton and Van den Belt1997). Sea grass P. oceanica makes up dense meadows from the surface to about 40 m depth and is one of the most productive Mediterranean ecosystems (Ott, Reference Ott1980; Proccacini et al., 2003). Sea grass meadows produce large quantities of organic matter (leaves and epiphytes), which constitute the basis of the food web both within and outside the ecosystem. This production is also exported to other ecosystems, as the main source of food (Pergent et al., Reference Pergent, Romero, Pergent-Martini, Mateo and Boudouresque1994; Walker et al., Reference Walker, Pergent, Fazi and Short2001). Meadows of the endemic P. oceanica are also characterized as ‘key’ ecosystems for the Mediterranean basin (Boudouresque et al., Reference Boudouresque, Meinesz, Ballesteros, Ben Maiz, Boisset, Cinelli, Cirik, Cormaci, Jeudy De Grissac, Laboret, Lanfranco, Lundberg, Mayhoub, Panayotidis, Semroud, Sinnassamy and Span1990; Hemminga & Duarte, Reference Hemminga and Duarte2000; Larkum et al., Reference Larkum, Orth and Duarte2006).
Posidonia oceanica is legally protected in most Mediterranean countries, but despite these protection measures, significant regression of these meadows has been shown in several sectors of the Mediterranean coastal zone (Platini, Reference Platini2000; Boudouresque, Reference Boudouresque and Rodríguez-Prieto2003; Pergent-Martini et al., Reference Pergent-Martini, Boudouresque, Pasqualini and Pergent2006). In the last 40 years P. oceanica seems to have suffered a significant decline in most areas, which can be attributed to a wide array of causes, mostly related to anthropogenic activities such as industrial and urban sewage discharges (Pergent-Martini & Pergent, Reference Pergent-Martini, Pergent and Özhan1995, Reference Pergent-Martini, Pergent and Kuo1996; Pergent-Martini et al., Reference Pergent-Martini, Boudouresque, Pasqualini and Pergent2006), fish farming (Delgado et al., Reference Delgado, Grau, Pou, Riera, Massuti, Zabala and Ballesteros1997; Ruiz et al., Reference Ruiz, Pérez and Romero2001; Pergent-Martini et al., Reference Pergent-Martini, Boudouresque, Pasqualini and Pergent2006), trawl fishing (Ruiz et al., Reference Ruiz, Gutièrrez Ortega, Garcìa Charton and Pèrez Ruzafa1999), illegal fishing techniques and coastal works (Ruiz et al., Reference Ruiz, Marín, Calvo and Ramírez Díaz1993; Guidetti & Fabiano, Reference Guidetti and Fabiano2000). Recent monitoring of the sea grass Posidonia oceanica in the Croatian part of the Adriatic Sea showed significant decline in meadows density in the area influenced by human activity. The recent increase in fish farming in the Adriatic Sea has imposed additional anthropogenic pressure on P. oceanica meadows.
For P. oceanica meadows, Pergent et al. (Reference Pergent, Pergent-Martini and Boudouresque1995) proposed an evaluation scale according to meadow density in relation to depth and to other environmental factors such as turbidity and anthropogenic disturbance. The authors proposed ‘beds in equilibrium’, in which the meadow density is within normal limits (normal density) or exceptionally high (higher sub-normal density). This can be distinguished from ‘disturbed beds’ and ‘very disturbed beds’ in which the density is reduced or limited by several factors and is therefore low (lower sub-normal density) or abnormal (abnormal density). Epiphytes on P. oceanica leaves are also more sensitive to environmental changes than the plant host (Panayotidis, Reference Panayotidis1980). Delgado et al. (Reference Delgado, Ruiz, Pérez, Romero and Ballesteros1999) recorded an increase in the biomass of P. oceanica epiphytes sampled close to the sites of nutrient and organic matter discharge.
The impact of fish farming facilities on P. oceanica meadows was assessed from studies of intensive facilities carried out over the last few years (Pergent-Martini et al., Reference Pergent-Martini, Boudouresque, Pasqualini and Pergent2006). The disturbances caused by these fish farms were measured by means of both abiotic (light and sedimentation) and biotic variables (meadow density, leaf biometry and epiphytes). Fish farming induces high organic and nutrient loading into the surrounding water that cause significant degradation of water quality and are likely to be responsible for changes such as the degradation of sea grass meadows (Holmer et al., Reference Holmer, Perez and Duarte2003; Pergent-Martini et al., Reference Pergent-Martini, Boudouresque, Pasqualini and Pergent2006). The results showed significant degradation of these sea grasses or even their disappearance and the sediment showed a strong increase in organic matter that could lead to anoxia phenomena. Other factors can also negatively affect the plant, for example an epiphytic bloom that shades the sea grass leaves or grazing pressure increase caused by fish and sea urchins.
The main problem about P. oceanica in the Adriatic Sea is that the meadows are insufficiently studied and there is no precise data about their distribution and condition. Limited information is available on leaf features and epiphytic macroflora in P. oceanica meadows from the Croatian coast and Croatian islands. The lack of old historical references and the high costs of new surveys prevent any regular monitoring programme in the Adriatic Sea.
Measuring meadow density (number of shoots per m2) as a direct method is probably the most straightforward. Shoot density depends on structure and functionality of a meadow as well as on its ability to adapt to substrate variability (Scardi et al., Reference Scardi, Chessa, Fresi, Pais and Serra2006). It also plays an important role when estimates of quantitative properties of P. oceanica beds are to be calculated.
The aim of the present study was to obtain evidence on the relationships between the regression of P. oceanica meadows and environmental disturbances caused by anthropogenic pressure, which proliferated in the last decades due to the tourism and aquaculture development along most of the Mediterranean coast. The objective of the present study was to collect data on widely used P. oceanica meadow structural attributes (number of leaves per shoot, leaf surface per shoot, leaf area index, number of epiphytic taxa and their coverage), which is the first first attempt to investigate the status of the P. oceanica meadows in Croatia. Furthermore, since nearshore fish-farming activities are carried out in the vicinity of the P. oceanica meadows in the Dugi Otok channel, the other objectives were to determine if the nutrient enrichment of the environment in the Dugi Otok channel represents a source of direct disturbance to the growth and structure of the meadows, and to compare these results to oligotrophic waters, such as those of the outer coast of the Dugi Otok Island.
MATERIALS AND METHODS
Study area
Posidonia oceanica shoots were sampled in June and July 2004 at eight meadows near Dugi Otok Island in the central part of the Adriatic Sea. The research was carried out at the outer part of Dugi Otok Island at sites Veli Rat (VR), Sakarun (SK), Meanj (ME) and Brbinjšćica (BR); in the inner part of Dugi Otok Island at site Savar (SA); near the I Island at sites I (IZ) and Mrtovnjak (MR) and in the area of Fulija Islet (FU) (Figure 1). The northernmost meadow was located at the site Veli rat (VR) (44°09′11″N 14°49′13″E) and it extended from 1 to 32 m of the depth, whereas the southernmost one was located at the site I (IZ) (44°00′42″N 15°08′21″E) and it extended from 6 to 35 m of the depth. The sites VR and ME were situated towards the open sea, the same as the sites SK and BR, but these meadows were growing in large bays. The other four sites (SA, FU, IZ and MR) were situated in a channel with strong sea bottom currents. The Posidonia meadow near the Fulija Islet was under the influence of a tuna farm (at a distance of approximately 300 m). This tuna farm, together with the tuna farm near the Kudica Islet (3 km north from the Fulija Islet), produce up to 1500 tons of tuna fish per year. At the study site SA the Posidonia meadow was under the influence of local small village sewage whereas the other investigated sites were not affected by human activities.

Fig. 1. Map of the Dugi Otok Island and the positions of the sampling sites (in abbreviations).
Sampling methods
Sampling of Posidonia oceanica shoots was made by means of SCUBA diving. The density of the sea grass bed (number of shoots per m2) was estimated at each site using 50 × 50 cm quadrates, with minimum 10 replicates per site. The quadrates were randomly chosen along the meadow, all at the same depth of 10 m. Meadows were assigned to one of the categories proposed by Pergent et al. (Reference Pergent, Pergent-Martini and Boudouresque1995). For estimation of the biometric features of the leaves, ten shoots were collected randomly from each investigated site during the summer period and preserved in seawater–formalin (5%) solution. The leaf width from the orthotropic shoots was measured using calipers. The samples were also examined for number of leaves (adult and intermediate), leaf surface per shoot (cm2 per shoot) and leaf area index (m2 per m2) (Pergent et al., Reference Pergent, Pasqualini, Pergent-Martini, Skoufas, Sourbes and Tsirika2003). The number of epiphytic algae taxa (Rhodophyceae, Fucophyceae and Chlorophyceae) and their coverage (expressed as percentage of leaf surface) (Piazzi et al., Reference Piazzi, Balata and Cinelli2004) were also determined. The epiphytic algae from the rhizome and the leaf base were also investigated.
From the same collected shoots epiphytic algae were scraped with a scalpel from the entire adult and intermediate leaves (5 leaves per shoot, minimum 10 replicates), isolated from epiphytic fauna under a stereomicroscope, dried for 48 h at 75°C and the total dry weight per shoot was determined (Cancemi et al., Reference Cancemi, De Falco and Pergent2003).
Onset Computers data loggers were used for measurements of light intensity at all of the study sites at 10 m depth. Sea temperature was measured with Onset Computers temperature data loggers at 10 m depth at two sites; the Fulija Islet (FU) in the channel and at Meanj (ME) towards the open sea in the period of one year (from 1.1.2004 to 1.1.2005).
Data of the investigated parameters were only available at one sampling time, therefore a one-way ANOVA was used in order to assess differences between investigated sites followed by Tukey's honestly significant difference test. The Statistica 7.0 for Windows software was used. The similarity in macroalgal composition among sampling sites was analysed by calculating the Bray–Curtis similarity and showed by cluster analysis and multidimensional scaling (MDS) using PRIMER 5.0.
RESULTS
Sea temperature showed seasonal variability and ranged from 10.3°C to 23.1°C with no differences among sampling sites (Figure 2). At the sampling site Fulija Islet the temperature was on average 1°C higher than at the sampling site Meanj. Maximum sea temperature was recorded in August, while minimum temperature values were measured in February and March, when the water column was entirely mixed.

Fig. 2. Sea temperature at the sites Fulija (FU) and Mežanj (ME) at 10 m depth.
Significant differences in the light intensity between the sites were observed in winter and summer (one-way ANOVA, P < 0.01) (Figure 3). The highest light intensity value was measured in winter (3.48 log Lum/sqm) and summer (3.19 log Lum/sqm) at sampling site ME. The lowest light intensity values were recorded in winter (2.43 log Lum/sqm) and in summer (2.06 log Lum/sqm) at the site FU.

Fig. 3. The highest values of the light intensity in summer and winter at study sites (at 10 m depth) in log Lum/m2.
Significant differences between all investigated parameters of Posidonia oceanica meadows were detected among meadows at channel sites and meadows at open sea sites (Table 1).
Table 1. Summary of the results of the one-way ANOVAs testing the investigated parameters of Posidonia oceanica meadows between meadows at channel sites and meadows at open sea sites.

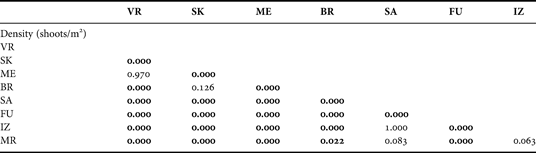
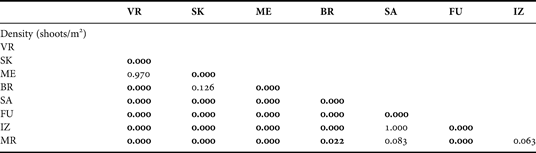
The highest density of P. oceanica meadows, measured at 10 m depth, was found at sites VR (707.7±72.4 shoots/m2) and ME (689.6±45.9 shoots/m2) (Figure 4A). The lowest values were recorded at the site FU (37.8±2.2 shoots/m2) in the vicinity of the fish farm. Significant differences were found in sea grass shoot density between sites at the outer part of the Dugi Otok Island (VR, SK, ME and BR) and sites in the channel (one-way ANOVA, P < 0.001) (Tables 1 & 2). Significant decline in sea grass density provides evidence of damage to the sea grass meadow. The Posidonia bed density values at the site FU are significantly beneath the values that are normally recorded at the 10 m depth (Tukey test P < 0.05). According to the classification scale established by Pergent et al. (Reference Pergent, Pergent-Martini and Boudouresque1995), these values are far beneath the ‘abnormal density’ of very disturbed beds. The lower values of bed density at the site FU could be associated with local hydrodynamic conditions (current velocities ranged from 6 to 25 cm s−1) that affect the deposition of organic matter from tuna cages. Sites SA, IZ and MR are classified as disturbed beds or beds tending towards regression. All these sites are from the Dugi Otok channel with strong sea bottom currents. Sites SK and BR belong to the outer part of the Dugi Otok Island, but are situated in large bays and these meadows are classified as beds in equilibrium, tending towards normal bed density. At sites VR and ME meadows are classified as dense to very dense beds or higher subnormal density.

Fig. 4. Mean values (with standard error and standard deviation) of shoot density (A), leaf length (B), leaf number (C) and leaf width (D) at study sites.
Table 2. Results of the Tukey HSD test on meadows density (shoots/m2) at the investigated sites. Significant differences (P < 0.05) are indicated in bold.
The highest values of leaf length were measured at sites VR (92.9±6.9 cm), SK (83.9±8.1 cm) and ME (83.6±6.1 cm), while the lowest value was measured at the site FU (43.6±5.3 cm) (Figure 4B). Significant differences were found in the leaf length between sites at the outer part of the Dugi Otok Island and sites in the channel (one-way ANOVA, P < 0.001).
The highest mean number of leaves (adult and intermediate) per shoot was recorded at sites ME (8.7±0.9) and BR (8.1±1.2) (Figure 4C). The lowest values were recorded at sites IZ (5.7±0.7) and FU (5.7±1.4).
The highest values of the leaf width were measured at sites ME (9.2±0.2 mm) and SK (8.9±0.3 mm) and lower values were measured at sites FU (7.3±0.4 mm) and BR (7.5±0.3 mm) (Figure 4D). Significant difference was found in the mean number of leaves and the leaf width between investigated sites (one-way ANOVA, P < 0.001).
Leaf area index decreased as the meadow density decreased, and the maximum value was observed at sites ME and VR, where the densest meadows occurred (Figure 5A). High values of leaf surface per shoot (cm2 per shoot) were recorded at the sites ME and VR (Figure 5B). Biomass of the epiphytic algae exhibited the highest values in the vicinity of the tuna farm (site FU; 198.8±74.4 mgdw/shoot), with significant differences between that and the other investigated sites (one-way ANOVA, P < 0.001). Considerably high biomass was also recorded at the site SA (74.1±16.3 mgdw/shoot) (Figure 5C). At the other investigated sites, the biomasses of the epiphytic algae were not exceeding more than 50 mgdw/shoot. Significant differences in biomasses of the epiphytic algae between these sites were not found (P > 0.05). The lowest value was measured at the site ME (12.9±4.8 mgdw/shoot). Maximum values of the epiphyte coverage of leaf surface were measured at the site FU (more than 35%) (Figure 5D).

Fig. 5. Mean values (with standard error and standard deviation) of leaf area index (A), leaf surface (B), biomass of epiphytic algae (C) and epiphyte coverage of leaf surface (D) from the study sites.
Fifty-five macroalgal species were found on leaves from the investigated sites (9 Chlorophyceae, 15 Pheophyceae and 31 Rhodophyceae) (Table 3). Macroalgal assemblages on leaves were dominated by the encrusting Corallinaceae Hydrolithon farinosum (Lamouroux) Penrose and Chamberlain, Jania rubens Lamouroux, Pneophyllum fragile Kützing and Haliptilon attenuata Garbary & Johansen and by the Pheophyceae Halopteris filicina (Grateloup) Kützing and Myronema orbiculare J. Agardh. The highest number of macroalgal species was found at the site FU (29 species) and the lowest number at the site SA (15 species).
Table 3. List of macroalgal epiphytes on leaves of Posidonia oceanica from sampling sites.

The most abundant species, on the rhizomes and the leaf base, were the Rhodophyceae Peyssonnelia rubra (Greville) J. Agardh, Polysiphonia denudata (Dillwyn) Kützing and Womersleyella setacea (Hollenberg) R.E. Norris.
The similarity of the macroalgae species growing as epiphytes, based on the presence or absence of taxa, was relatively high for sites from the Dugi Otok channel and the open sea. The cluster analysis and multidimensional scaling showed that two distinct groups exist (Figure 6). One group contains samples collected from the channel (A) and the other consists of open sea samples (B). In group B, the highest similarity was found between sites VR and ME, two sites with highest values of morphological characteristics of investigated P. oceanica meadows. The Bray–Curtis similarity was the highest between sites VR and ME (75.68%), sites IZ and FU (70.83%) and sites BR and SK (68.29%). The lowest values of similarity were found between sites FU and ME (20.41 %) and sites IZ and ME (20.51%).

Fig. 6. Results of cluster analysis and multi-dimensional scaling of similarity of the macroalgae species growing as epiphytes on Posidonia oceanica leaves at 10 m depth. Channel sites (A) and open sea sites (B).
DISCUSSION
The results presented in this paper demonstrate the differences in morphological and structural characteristics of Posidonia oceanica meadows among various sites with and without anthropogenic influence. Eutrophication, as an anthropogenic cause of P. oceanica meadows decline, is a serious problem in coastal areas of the Mediterranean Sea. Symptoms of fish farm activities, local sewage discharge and eutrophication of the surrounding areas as a result of these facilities have been documented and confirmed by various authors in the past (Delgado et al., Reference Delgado, Grau, Pou, Riera, Massuti, Zabala and Ballesteros1997; Marbà & Duarte, Reference Marbà and Duarte1997; Ruiz et al., Reference Ruiz, Pérez and Romero2001; Cancemi et al., Reference Cancemi, De Falco and Pergent2003; Marbà et al., Reference Marbà, Santiago, Dìaz-Almela, Álvarez and Duarte2006; Pitta et al., Reference Pitta, Apostolaki, Giannoulaki and Karakassis2005, Reference Pitta, Apostolaki, Tsagaraki, Tsapakis and Karakassis2006). It is well known that even minimal inputs of inorganic phosphorus into farm bottom water can drastically affect the planktonic and algae system (Maldonado et al., Reference Maldonado, Carmona, Echeverría and Riesgo2005). Released nutrients enhance phytoplankton epiphyte and macroalgal growth, which in turn reduces the light available to sea grass. Patches of green macroalgae Ulva rigida C. Agardh, recorded at the site FU at depths from 10 to 25 m, together with the genus Enteromorpha throughout the world in water contaminated with domestic sewage, like that at the site SA, indicate such effects. It is well-known that continued inputs of dissolved nutrients, from fish farming activities, usually result in drastic environmental changes that affect both planktonic and benthic communities at the local scale (Ruiz et al., Reference Ruiz, Pérez and Romero2001; Cancemi et al., Reference Cancemi, De Falco and Pergent2003).
Another impact on Posidonia meadows at sites FU and SA is accumulation of organic wastes in the seabed which is unfortunately not studied yet, although it could be confirmed by the presence of muddy composition in the sediments. A significant fraction of the organic matter input is incorporated into the sediment and mineralized there, resulting in high organic content in the sediment. The impact of organic loading in the sediment may be long lasting, even if the water quality improves over time. This is proved by the observation that the decline of Posidonia meadows continued for years after closure of the fish farm (Delgado et al., Reference Delgado, Ruiz, Pérez, Romero and Ballesteros1999). Total degradation of Posidonia oceanica meadows was observed in circumference of 120 m around the tuna cages at the site FU, where total sea grass die-off was observed, while the shoot density was significantly reduced within a radius of 500 m from the tuna cages. The effective impact derived from the fish farm is highly variable and depends on local hydrodynamics, amount of fish in the cages, fish feeding strategy and the sinking rate of faecal pellets (Karakassis et al., Reference Karakassis, Hatziyanni, Tsapakis and Plaiti1999, Reference Karakassis, Tsapakis, Hatziyanni and Pitta2001).
Meadow density of P. oceanica depends on depth and density decreases from shallower to deeper waters with decrease of light intensity (Pergent et al., Reference Pergent, Pergent-Martini and Boudouresque1995). Transparency and light intensity could be fairly stable in open water areas, but this is not the case in many coastal environments where water turbidity may fluctuate profoundly as a result of physical, chemical and biotic factors. The lower values of light intensity (both in winter and summer), measured at 10 m depth were observed at sites FU and SA, both under the influence of high organic inputs. Observed evident changes in the community around the tuna farm cages were increased abundance of epiphytes on sea grass leaves and reduced water-column transparency (decrease in light accessibility) that has impact on photosynthetic performance of P. oceanica. The number of epiphytic taxa and the biomass vary significantly and indicate both physical (temperature) and chemical (nutrient concentrations) environmental conditions (Pergent et al., Reference Pergent, Pergent-Martini and Boudouresque1995).
The 55 taxa of epiphytic macroalgae identified at the study sites are comparable with the 78 taxa reported by Blundo et al. (Reference Blundo, Di Martino and Giaccone1999) and with 74 taxa reported by Piazzi et al. (Reference Piazzi, Balata and Cinelli2002), and not on the other hand with 19 taxa recorded by Cinelli et al. (Reference Cinelli, Cormaci, Furnari, Mazzella and Bourdouresque1984). All these records were from the western Mediterranean. In the epiphytic community, the Rhodophyceae dominated in all samples. The number of identified taxa decreased in channel sites (FU, IZ, SA and MR), reflecting a similar trend for the coverage of P. oceanica leaves by epiphytes. The shoot density, leaf length, leaf width and leaf surface were increased at open sea sites (VR, ME, SK and BR), where the epiphytic load was less abundant. Leaf area index decreases at sites with decreased meadow density. The reason for differences in the examined biometric features of P. oceanica between the sites at the outer part of Dugi Otok Island could lie in the fact that sites VR and ME are situated towards the open sea and the meadows at sites SK and BR are growing in large bays. Several epiphytic macroalgae (mostly Rhodophyceae) from the investigated sites normally inhabit waters deeper than 10 m (like Womersleyella setacea (Hollenberg) R.E. Norris), but could be found in shallow areas because of decreased light intensity in lower part of Posidonia leaves. The epiphytic load in the meadow near the cages showed its maximum during the period of higher insolation and temperatures in spring and summer. Significantly higher epiphyte biomass was found at the site in the vicinity of the tuna farm (FU) and near local sewage (SA) in comparison to the other investigated sites. These results can be used as the direct evidence of the nutrient enrichment, mostly in NH4+, also noted by Cancemi et al. (Reference Cancemi, De Falco and Pergent2003). The extensive development of macroalgal epiphytes on the Posidonia leaves at the investigated sites limits the amount of light available to the plant. There are also opposite observations where the epiphyte loading was much smaller in the fish farm area than in the reference site, mostly because of high herbivore pressure found close to the fish farm (Delgado et al., Reference Delgado, Grau, Pou, Riera, Massuti, Zabala and Ballesteros1997; Pergent et al., Reference Pergent, Mendez, Pergent-Martini and Pasqualini1999; Ruiz et al., Reference Ruiz, Pérez and Romero2001). This kind of high herbivore activity was not found during our research. Light reduction is one of the most important factors responsible for sea grass decline in the eutrophied waters. The degradation of the Posidonia meadows near the Fulija Islet (FU) could be also attributed to a light reduction caused by shading near cages, and dispersion of organic detritus. However, the degradation of the Posidonia meadows also occurred because their leaves were covered by benthic mucilaginous aggregates.
Because of the algal bloom during the late spring the meadow was covered with a thick carpet that obstructs the Posidonia leaves to photosynthesize (Figure 7). The potential damage on Posidonia meadows by accumulation of these aggregates includes shading, changes in nutrient concentrations of sediment and, at the end, anoxia. The lack of oxygen can favour the activity of anaerobic bacteria and production of toxic substances, such as hydrogen sulphide (Delgado et al., Reference Delgado, Ruiz, Pérez, Romero and Ballesteros1999). The mucilage accumulation is probably related to water motion that has been reported to attenuate as a power function of depth in P. oceanica beds (Gambi et al., Reference Gambi, Buia, Casola, Scardi and Boudouresque1989). This process of regression was accompanied by a heavy bloom of pluricellular filamentous algae, principally Acinetospora crinata (Carmichael ex Harvey) Kornmann. In recent years the occurrence of benthic mucilaginous aggregates has become an increasing problem along the Adriatic coastline and in many other areas of the Mediterranean Sea (Giuliani et al., Reference Giuliani, Virno Lamberti, Sonni and Pellegrini2005). The appearance of these benthic aggregates shows a seasonal pattern becoming noticeable in the field as small, yellowish tufts in early spring and continued until the end of summer forming, under favourable environmental conditions, extensive patches at the bottom. The macroscopic development is caused by few associated filamentous species: Nematochrysopsis marina (Feldmann) Billard and Chrysonephos lewisii (Taylor), two fast growing multicellular benthic chrysophytes, together with a free-living form of the brown alga Acinetospora crinita. This kind of mucilaginous cover drastically reduced photosynthesis and it has been reported that mucilaginous carpets could suffocate sea grass beds (Den Hartog, Reference Den Hartog1994; Delgado et al., Reference Delgado, Grau, Pou, Riera, Massuti, Zabala and Ballesteros1997; Cancemi et al., Reference Cancemi, De Falco and Pergent2003; Holmer et al., Reference Holmer, Perez and Duarte2003). The Posidonia meadows at the site FU were heavily affected and the accumulation of aggregates did not vary with depth. The covering was massive and took the form of a blanket that appeared to be anchored to the upper portion of the heavily epiphytized leaves. There was no A. crinata or other filamentous algae recorded at the other investigated sites in our research.

Fig. 7. Heavy bloom of filamentous algae Acinetospora crinata (Carmichael ex Harvey) Kornmann at the site FU.
In general, all four meadows investigated in the Dugi Otok channel showed a generalized state of regression. This regression state reaches its maximum at site FU (Fulija Islet) where the meadow merely survives in an extremely degraded situation caused by aquaculture activity. Based on shoot density measurement, meadows at the outer part of the Dugi Otok Island were in a comparatively better state of health.
The results of this research show significant degradation or even disappearance of the sea grass meadows when fish farming cages are set up above or in the vicinity of a Posidonia meadow. In some areas beneath the cages at the site FU only the rhizome mattes remained. Influence of light limitation due to increased turbidity, interplay between nutrients, epiphytes and the plant tolerance to changes in sediment quality deserves further investigation to understand P. oceanica meadow decline associated with anthropogenic activities in the Mediterranean Sea.
ACKNOWLEDGEMENTS
This study was undertaken as part of the ‘JADRAN project’ coordinated by the Ruđer Bošković Institute and project ‘Mapping of Posidonia meadows in Croatian part of Adriatic Sea’ financed by the Ministry of Science and Technology of the Republic of Croatia. The author wishes to thank Dr Ante uljević from the Institute of Oceanography and Fisheries, Split, Croatia and Dr Nevenka Zavodnik from the Center for Marine Research, Institut Ruđer Bošković, Rovinj, Croatia for help with the classification and determination of epiphytic algae.