INTRODUCTION
The Mediterranean Sea is a well-known area for the analysis of benthic foraminiferal taxonomy. In 1826, d'Orbigny was the first to describe numerous current taxa from the Mediterranean region. Studies on foraminifera multiplied, among others Parker & Todd (1958) in the Eastern and Western Mediterranean Sea, Hofker (1960) in the Gulf of Naples, Blanc-Vernet et al. (1979) in the Gulf of Gabes, Jorissen (1987) in the Mediterranean Sea, Cimerman & Langer (1991) in the Adriatic and Tyrrhenian Seas, Sgarrella & Moncharmont Zei (1993) in the Gulf of Naples, Rasmussen (2005) in the southern Aegean Sea, Avşar et al. (2006) at the Sea of Marmara, Frezza & Carboni (2009) in the Mediterranean Sea and Milker & Schmiedl (2012) in the western Mediterranean Sea.
Foraminifera are very useful to geologists. They use them as powerful tools for relative age determination (biostratigraphy and chronostratigraphy), correlations between different locations and for palaeoenvironmental reconstruction. Foraminifera are especially valuable tools in the geology-geobiology arsenal of ocean-climate change research. For one, there are planktic (floating near-surface dwelling) and benthic (bottom-dwelling) forms. These foraminifera allow us to simultaneously consider the character of the upper water column and the conditions at the seafloor based on time-averaged sediment assemblages. We can study foraminifera assemblages as palaeobiological communities. Foraminifera can inform us about the ancient ocean-climate system, the depositional environment, the biotic response to global change, and the patterns of evolution. In addition, we can analyse the geochemistry of their calcium carbonate shells, and this will provide a variety of proxies (indirect evidence) of ancient ocean conditions (Leckie, Reference Leckieno date).
The foraminifera group has typically been included in the Protozoa. Based mainly on molecular phylogenetics, specialists claimed to be certain of their belonging to the Rhizaria major group, within the Protozoa (Cavalier-Smith, Reference Cavalier-Smith2004). This microfauna plays a crucial role in ecological and palaeo-ecological studies, thanks to their high numerical density in aquatic sediments and the excellent preservation potential of their shells. Benthic foraminifera show a great diversity with more than 10,000 modern taxa (Sen Gupta, Reference Sen Gupta2003). Some foraminifera are ubiquitous, while others live only in specific environments: sand, rocky substrates, algae, coral lagoons or seagrass (Debenay et al., Reference Debenay, Pawlowski and Decrouez1996). Seagrass systems are characterized by high biodiversity. Their leaves offer substrata suitable for the settlement and the growth of a number of micro- and macro-colonists that form stratified assemblages characterized by a high diversity of species (Mabrouk et al., Reference Mabrouk, Ben Brahim, Hamza, Mahfoudhi and Bradai2014).
Seagrass Cymodocea nodosa are the second largest habitat of seagrass in the Mediterranean Sea after those of Posidonia. A newly introduced seagrass species from the Red Sea in the Gulf of Gabes is Halophila stipulacea, and it is encountered over very limited specific scopes (Anonymous, 2012). Epiphytic fauna of seagrass consists of a multitude of different organisms. We can encounter some ciliates, foraminifera, nematodes, polychaetes, tunicates (mostly colonial), bryozoans and hydrozoans (Kerneis, Reference Kerneis1960; Novak, Reference Novak1984; Chimenz et al., Reference Chimenz, Taramelli, Cironi, Contessini, Gravina, Maggiore, Maj, Motta, Somaschini, Boudouresque, Meinesz, Fresi and Gravez1989; Aladro-Lubel & Martínez–Murillo, Reference Aladro-Lubel and Martinez-Murillo1999). The last two groups are also the dominant group of the epifauna of seagrass leaves, where sometimes foraminifera are also noticed (Chimenz et al., Reference Chimenz, Taramelli, Cironi, Contessini, Gravina, Maggiore, Maj, Motta, Somaschini, Boudouresque, Meinesz, Fresi and Gravez1989). Concerning epiphytic foraminifera, Blanc-Vernet et al. (Reference Blanc-Vernet, Clairefond, Orsolini, Burollet, Clairefond and Winnock1979) was interested in the eastern part of the archipelago of Kerkennah (Gulf of Gabes) studying epiphytic assemblages of Posidonia meadows, Cymodocea and Caulerpa. These epiphytic foraminifera have been widely studied in the Mediterranean Sea (Brasier, 1975; Baden, 1990; Langer, 1993; Ribes et al., 2000; Wilson, 2007; in Mateu-Vicens et al., Reference Mateu-Vicens, Antonio, Salud and Beatriz2010). Up until now, current epiphytic foraminifera are insufficiently studied on Tunisian shores (Mabrouk et al., Reference Mabrouk, Hamza, Brahim and Bradai2011) as compared with benthic ones. It is probably caused by the complexity of environmental and anthropogenic factors that govern the ecosystem. In general, these factors create a pressure, leading benthic communities to several reactions; some species are disturbed and cannot develop naturally, and others, more resistant, impose their opportunistic strategy of life and exclude more sensitive and less competitive species (Aloui-Bejaoui & Afli, Reference Aloui-Bejaoui and Afli2012).
The aim of this study is to identify benthic and epiphytic foraminifera species in the studied region, and to highlight the affinity between benthic or epiphytic foraminifera and seagrass species, along with studying the physico-chemical characteristics of the area.
MATERIALS AND METHODS
Study area
Kerkennah is a 160 km2 archipelago composed of two principal islands (Chergui and Gharbi), located in the Gulf of Gabes (Tunisia) and 20 km off the coast of Sfax.
Kerkennah is known for its large covered Posidonia meadow lining its shallows. It is situated in a region with a mild, predominantly arid Mediterranean climate and is mainly characterized by its shallow, typical morphology and important marine hydrology (Ben Mustapha, Reference Ben Mustapha2007).
Indeed meadows cover a very large area, all around the Kerkennah shallow-waters. The meadows are less dense in the eastern and southern limits than those growing in the northern region, but both have a density (in shoots m−2) typical of type I and II meadows. Moreover, Kerkennah islands are an area of converging currents: Atlantic origin, current entering through the Strait of Chebba in the north and a Levantine one entering through the Strait of Sfax – Sidi Youssef (Ben Mustapha, Reference Ben Mustapha2007). At the Kerkennah reef, the amplitude of these wave and tidal movements are moderate and Posidonia meadows prevent water ebbing away easily, so that the tidal currents converge during rising tide and diverge when the tide lowers. Kerkennah shoals undergo the action of wave currents, which generally is 2–5 times more intense than that caused by tide current (Ben Mustapha, Reference Ben Brahim, Hamza, Ben Ismail, Mabrouk, Bouain and Aleya2007). This intense hydrological action induces a dynamic transport of sediment, facing the Gulf of Gabes and then joining the dominant littoral drift. The reef of Kerkennah receives 197,700 m3 of medium sand and much mud and plant debris annually. It also plays the role of a sediment trap, given its very shallow depth and its Posidonia seagrass beds that have a combined action in damping waves (Ben Mustapha, Reference Ben Mustapha2007).
The Kerkennah islands are of great importance for the Tunisian fishing sector and are influenced by regional water circulation (Ben Brahim et al., Reference Ben Brahim, Hamza, Ben Ismail, Mabrouk, Bouain and Aleya2013). The archipelago has shallow coastal waters with an average depth ranging from 0 to 5 m (Ben Brahim et al., Reference Ben Brahim, Hamza, Ben Ismail, Mabrouk, Bouain and Aleya2013). The islands’ sea bottom morphology is highly complex. It is characterized by mudholes, marine tide channels and P. oceanica beds of different shapes (Ben Brahim et al., Reference Ben Brahim, Hamza, Ben Ismail, Mabrouk, Bouain and Aleya2013).
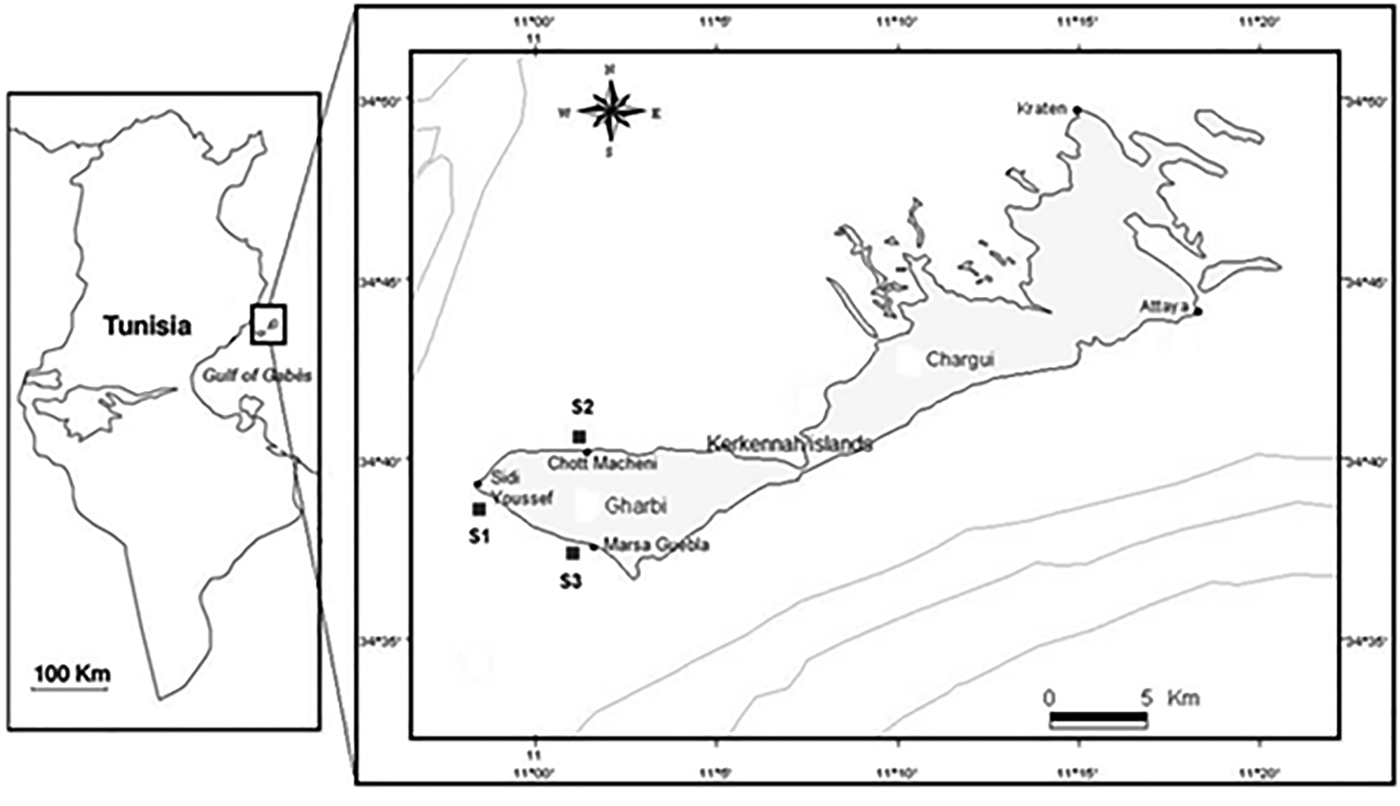
During the month of January 2012, the sampling campaign was carried out. In S2, a newly introduced species Halophila stipulacea (Forsskal) were detected, after reports that it had recently colonized the Tunisian coast (Sghaier et al., Reference Sghaier, Zakhama-Sraieb, Benamer and Charfi-Cheikhrouha2011) (Figure 1) (Table 1).
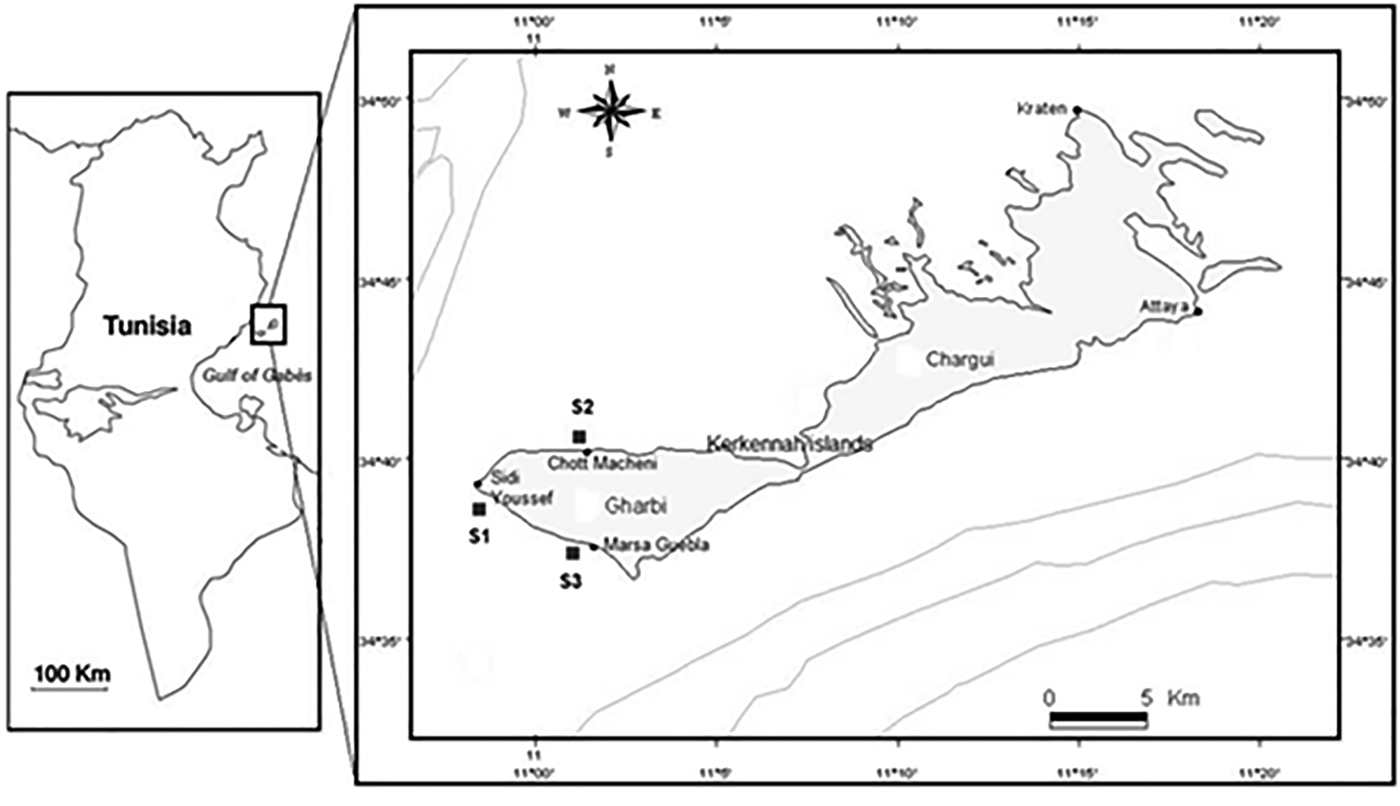
Fig. 1. Location map of the study area and sampling sites (Gharbi-Kerkennah islands), Mediterranean Sea.
Table 1. Sampling sites characteristics.

Sampling operation
The sampling operations were carried out with seawater (utilizing 1-litre plastic bottles ), seagrass leaves were collected using a metal quadrat (20 × 20 cm) and sediment with the help of a standard Van Veen grab. A constant volume of the top-soft sediment from each station (a slice 1 cm thick) was picked and stained with a double volume of Rose Bengal solution (2 g l−1 ethyl alcohol) for at least 2 weeks for biocenotic recognition (Walton, 1952; in Buosi et al., Reference Buosi, Cherchi, Ibba, Marras, Marrucci and Schintu2013) in order to study benthic foraminifera. Seagrass leaves were collected and immediately immersed in a solution of distilled water and ethanol to preserve both the organic matter and the carbonate matter. In vitro, leaves were then carefully scraped with a razor blade (Libes, Reference Libes1986) and conserved in a double volume of Rose Bengal solution in order to examine the epiphytic foraminifera with a binocular lens. All the samples were conserved at 4°C until specific analyses.
Data analysis
The pH and salinity of the seawater were measured in the laboratory using a multi-parameter 340i/SET. For nutrients, the seawater samples were stored at −20°C. Analyses involved nitrite NO2−, nitrate NO3−, ammonium NH4+, total nitrogen TN, phosphate PO43−, total phosphate TP and silicates and were conducted through autoAnalyzer Bran Luebbe type 3 based on the calorimetric method.
The soil's organic matter is composed of living organisms, residues of plants and animals and decomposition products. It is calculated as follows: a mass of the dry sample (greater than 2 g and whose particle size is less than 100 µm) sustains a loss to heat for 2 h at 550°C. The formula of the organic matter (TOM) is:
Pe: Sample size or mass of the dry sample, M0: Mass of the container containing the sample before the heat loss, M1: Mass of the container after the ignition loss.
The measurement of Total Organic Carbon (TOC) is done with the Nelson & Sommers conversion method (1996). For soils, a conversion factor of 1.724 has been used to convert organic matter to organic carbon based on the assumption that organic matter contains 58% organic C (Schumacher, Reference Schumacher2002).
The Kjeldahl method is a means of determining the nitrogen content of organic and inorganic substance or sediment in this case (Perchet, Reference Perchet2008).
The particle size test by screening, is a test to determine the weight distribution of grain or particles depending on their size. After drying, a sample of 500 g is placed in a series of superimposed sieves placed on a Vibro-sieve. Following this vibration, the rejected mass of each sieve was weighed and the fraction of sample retained on each sieve is plotted against the amount of the total sample. A series of sieves with a progression according to the AFNOR standard was used in this study.
From the cumulative granulometric distribution curve, the average particle size (Mzi) reflecting the average grain size, and the classification index (σ) characterizing the sorting degree of grains, can be calculated:
 $$\eqalign{ {\rm Mzi} &= \displaystyle{{({\rm \varphi} 16 + {\rm \varphi} 50 + {\rm \varphi} 84)} \over 3}; \cr {\rm \sigma} &= \displaystyle{{({\rm \varphi} 84 - {\rm \varphi} 16)} \over 4} + \displaystyle{{{\rm \varphi} 95 - {\rm \varphi} 5} \over {6.6}} ({\rm Leeder}, 1982)}$$
$$\eqalign{ {\rm Mzi} &= \displaystyle{{({\rm \varphi} 16 + {\rm \varphi} 50 + {\rm \varphi} 84)} \over 3}; \cr {\rm \sigma} &= \displaystyle{{({\rm \varphi} 84 - {\rm \varphi} 16)} \over 4} + \displaystyle{{{\rm \varphi} 95 - {\rm \varphi} 5} \over {6.6}} ({\rm Leeder}, 1982)}$$![]() ${\rm \varphi}$: grain sizes for different percentages taken from cumulative curves calculated for various stations.
${\rm \varphi}$: grain sizes for different percentages taken from cumulative curves calculated for various stations.
In order to study the foraminifera species, each sample of sediment was washed on two superimposed sieves (500 and 250 µm). Each fraction was dried at 40°C and placed in 100 ml dishes. The sorting operation of foraminifera was made on an optic binocular microscope (OLYMPUS type B061). The seagrass leaves samples were viewed with the optical binocular microscope to record the living assemblage that consists of epiphytic foraminifera, still in their living position, and photographed with a digital camera. Species encountered during the sorting operation were identified according to previous works (Glaçon, Reference Glaçon1963; Lee et al., Reference Lee, Muller, Stone, Enery and Zucker1969; Blanc-Vernet et al., Reference Blanc-Vernet, Clairefond, Orsolini, Burollet, Clairefond and Winnock1979; Cimerman & Langer, Reference Cimerman and Langer1991; Hottinger et al., Reference Hottinger, Halicz and Reiss1993; Montaggioni & Camoin, Reference Montaggioni and Camoin1993; Sgarrella & Moncharmont-Zei, Reference Sgarrella and Moncharmont-Zei1993; Ishman et al., Reference Ishman, Graham and D'Ambrosio1997; Debenay et al., Reference Debenay, Favry, Guelorget and Perthuisot1998, Reference Debenay, Millet and Angelidis2005; Basso & Spezzaferri, Reference Basso and Spezzaferri2000; Avşar & Meriç, Reference Avşar and Meriç2001; Avşar & Ergin, Reference Avşar and Ergin2001; Debenay, Reference Debenay2001; Holzmann et al., Reference Holzmann, Hohenegger, Hallock, Piller and Pawlowski2001; Javaux & Scott, Reference Javaux and Scott2003; Murray, Reference Murray2003; Rasmussen, Reference Rasmussen2005; Melis & Violanti, Reference Melis and Violanti2006; Sohrabi-Mollayousefy, 2006; Yalçin et al., Reference Yalçin, Meriç, Avşar, Tetiker, Barut, Yilmaz and Dinçer2006; Devi & Rajashekhar, Reference Devi and Rajashekhar2009; Meriç et al., Reference Meriç, Avşar, Nazik, Barut, Bergin, Balkis, Oncel and Kapan-Yesilyurt2010; Aloulou et al., Reference Aloulou, Elleuch and Kallel2011; Milker & Schmiedl, Reference Milker and Schmiedl2012).
For each studied seagrass ecosystem and sediment fraction, we calculated the following faunal parameters: the total foraminiferal distribution (standardized for a 10 cm2 sediment surface), the abundance rate and the Shannon and Weaver index diversity of benthic and epiphytic species. The Shannon–Weaver index links the number of species to the assemblage density (Barras et al., Reference Barras, Jorissen, Labrune, Andral and Boissery2014).
 $$H^{\prime} = - \sum\limits_{i = 1}^S {P_i {\rm In}P_i} $$
$$H^{\prime} = - \sum\limits_{i = 1}^S {P_i {\rm In}P_i} $$S: total number of species, P i: Population size of species i.
As a statistical study, PRIMER software enabled us to obtain a dominance plot (Clarke & Warwick, Reference Clarke and Warwick1994). We used parametric Pearson test (software R) in order to correlate the obtained data. Differences were considered significant when P < 0.05. A principal component analysis (PCA) was performed with XLstat under Excel. It was used to determine the community's relationship to abiotic parameters and was carried out for the ordination of sample locations based on the matrix constructed using the relative abundances of species. According to the dominance of the three foraminifera forms (epiphytic, juvenile benthic and adult benthic forms), the distribution of seagrass ecosystems was shown in a ternary diagram drawn in Microsoft Excel, to visualize compositional data characterized by these three ecosystems (component 1), which are amalgamated to the three foraminifera forms (component 2).
RESULTS
The pH, temperature and salinity measurements of seawater showed that the three sites had very similar values (Table 2). We deduced that S1 was the richest in orthophosphate and nitrite and S3 presents high values in nitrates, phosphate, total nitrogen (TN), silicate and ammonium.
Table 2. Physical and chemical parameters of the studied stations.

Posidonia oceanica’s sediment (S1) was the richest in total organic matter (TOM), total organic carbon(TOC) and total Kjeldahl nitrogen (TKN), while Cymodocea nodosa’s sediment contained the lowest TOM, TOC and TKN (Table 3). It is noteworthy that Posidonia oceanica’s sediment and Halophila stipulacea’s substrate composition in TOM, TOC and TKN are very similar.
Table 3. Sediment characterization.

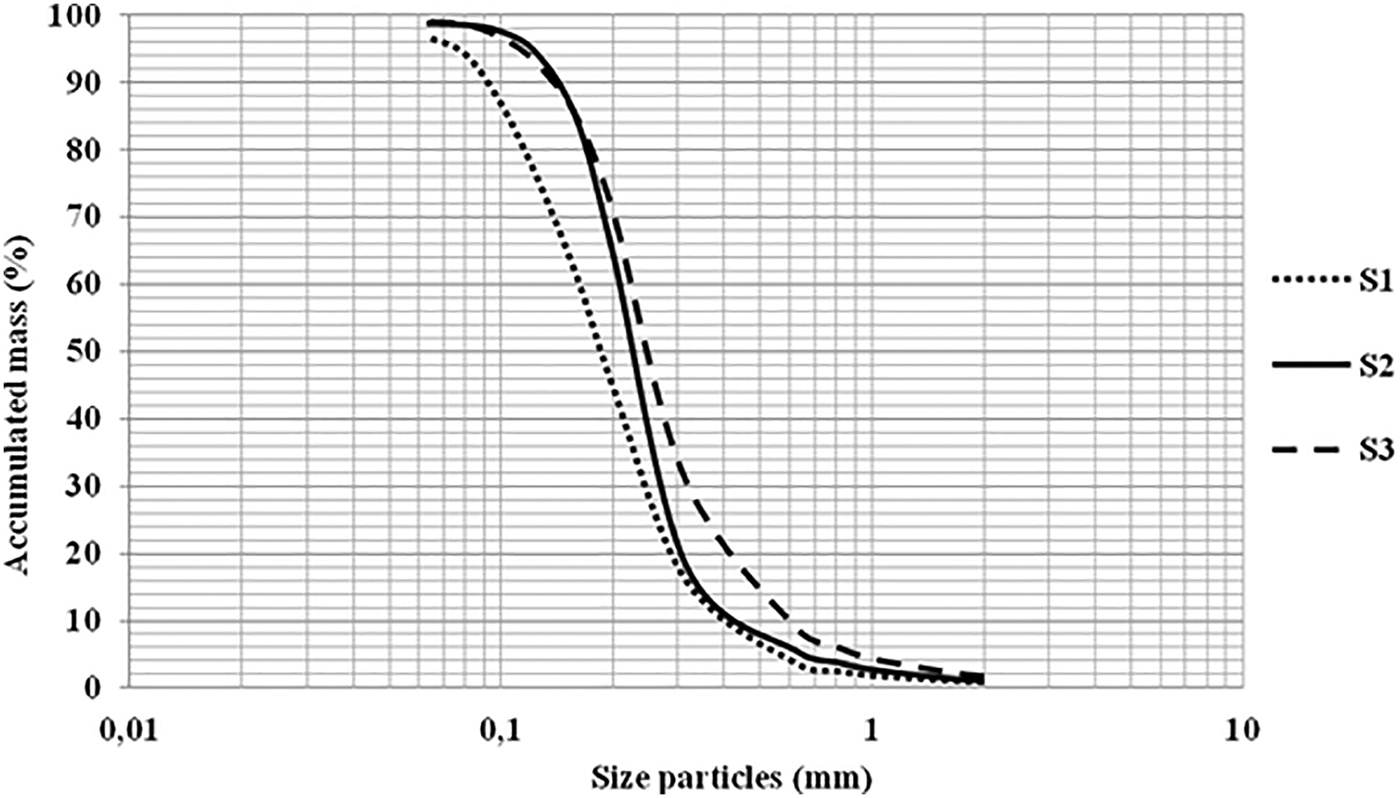
The particle size parameters of the three sediments were determined from the particle size distribution curve (Figure 2).
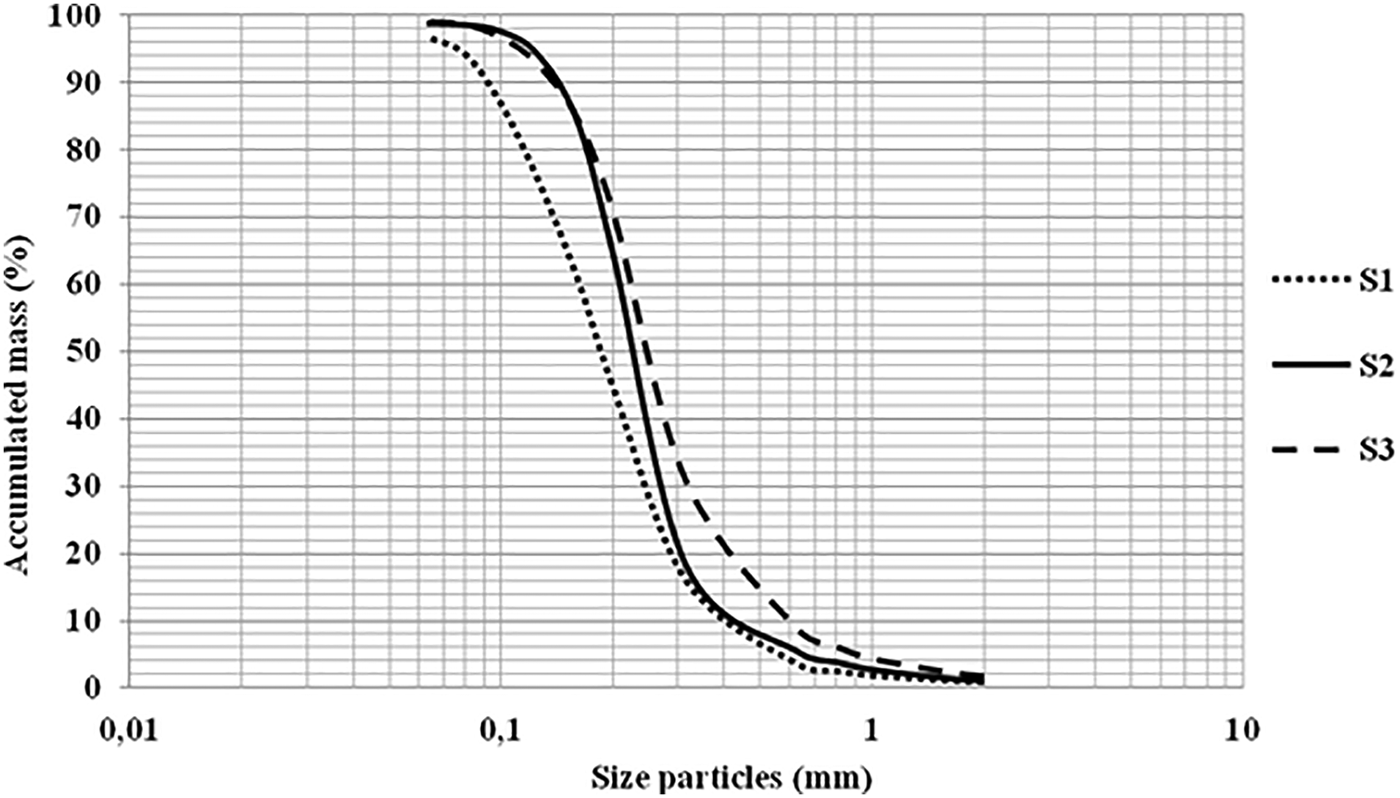
Fig. 2. Cumulative granulometric distribution curve.
After calculating the particle size parameters, we were able to conclude that S1 (Posidonia oceanica’s sediment) was a fine and moderately graduated sand (Mzi Ф = 2.46, σ = 0.81 Ф). S2 (Halophila stipulacea’s sediment) was a very fine and moderately well classified sand (Mzi Ф = 2.09, σ = 0.59 Ф). However, S3 (Cymodocea nodosa’s sediment) proved to be a medium and moderately graded sand (Mzi Ф = 1.86, σ = 0.82 Ф). The sand of Posidonia meadows was the finest followed by that of the Halophila ecosystem, while the sand of Cymodocea nodosa was found to be the coarsest of the three. Also Halophila stipulacea’s sand was the most graduated of the three studied.
Foraminifera study
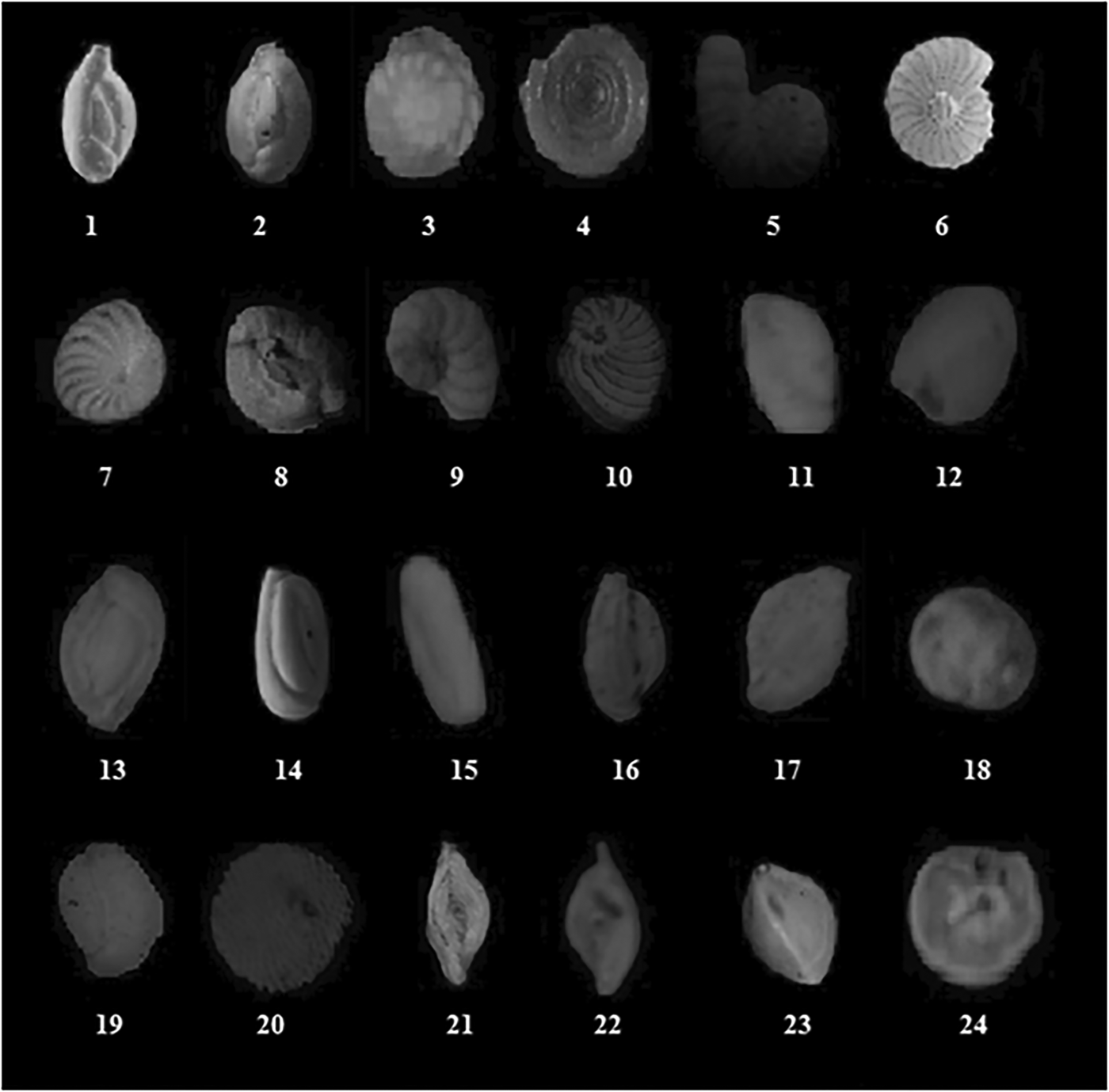
Twenty-four foraminifera species were identified among the three studied ecosystems: Ammonia beccarii (Linnaeus, 1758), Cornuspira foliacea (Philippi, 1844), Trochulina mira (Cushman, 1922), Elphidium aculeatum (d'Orbigny, 1846), Elphidium crispum (Linnaeus, 1758), Massilina secans (d'Orbigny, 1826), Peneroplis pertusus (Forskal, 1775), Peneroplis planatus (Fichtel & Moll, 1798), Pseudotriloculina laevigata (d'Orbigny, 1826), Quinqueloculina agglutinans (d'Orbigny, 1839), Siphonaperta aspera (d'Orbigny, 1826), Quinqueloculina berthelotiana (d'Orbigny, 1839), Quinqueloculina bosciana (d'Orbigny, 1839), Adelosina cliarensis (Heron-Allen & Earland, 1930), Quinqueloculina lata (Terquem, 1876), Quinqueloculina poeyana (d'Orbigny, 1839), Adelosina pulchella (d'Orbigny, 1826), Quinqueloculina variolata (d'Orbigny, 1878), Rosalina globularis (d'Orbigny), Sorites variabilis (Lacroix, 1941), Coscinospira arietina (Batsch, 1791), Spiroloculina antillarum (d'Orbigny, 1839), Spiroloculina angulosa (Terquem, 1878) and Triloculina marioni (Schlumberger, 1893) (WoRMS Editorial Board, 2015). Selected specimens were photographed and are illustrated in Figure 3.

Fig. 3. Identified foraminifera plates. 1: Adelosina cliarensis (Heron-Allen & Earland, 1930); 2: Adelosina pulchella (D'Orbigny, 1826); 3: Ammonia beccarii (Linnaeus, 1758); 4: Cornuspira foliacea (Philippi, 1844); 5: Coscinospira arietina (Batsch, 1791); 6: Elphidium aculeatum (Orbigny, 1846); 7: Elphidium crispum (Linnaeus, 1758); 8: Massilina secans (Orbigny, 1826); 9: Peneroplis pertusus (Forskal, 1775); 10: Peneroplis planatus (Fichtel & Moll, 1798); 11: Pseudotriloculina laevigata (d'Orbigny, 1826); 12: Quinqueloculina agglutinans (Orbigny, 1839); 13: Quinqueloculina berthelotiana (Orbigny, 1839); 14: Quinqueloculina bosciana (Orbigny, 1839); 15: Quinqueloculina lata (Terquem, 1876); 16: Quinqueloculina poeyana (Orbigny, 1839); 17: Quinqueloculina variolata (Orbigny, 1878); 18: Rosalina globularis (Orbigny); 19: Siphonaperta aspera (Orbigny, 1826); 20: Sorites variabilis (Lacroix, 1941); 21: Spiroloculina antillarum (d'Orbigny, 1839); 22: Spiroloculina angulosa (Terquem, 1878); 23: Triloculina marioni (Schlumberger, 1893); 24: Trochulina mira (Cushman, 1922).
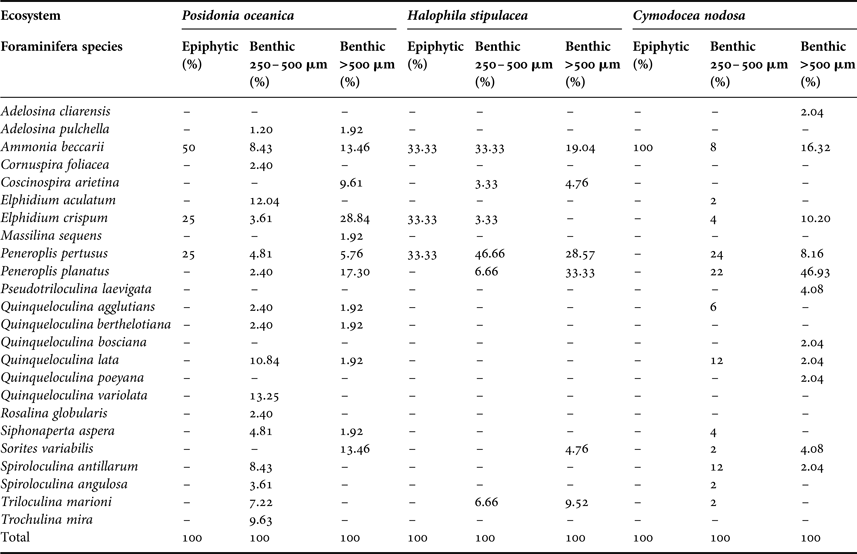
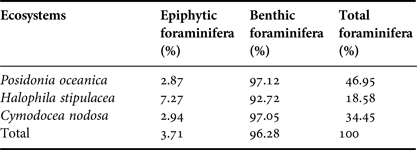
The distribution of the identified foraminifera is presented according to the micro-habitat of these microorganisms in the three seagrass ecosystems (Posidonia oceanica, Halophila stipulacea, Cymodocea nodosa): fine fraction of the sediment (250–500 µm; juvenile foraminifera forms), coarse fraction of the sediment (>500 µm; adult forms) and on the seagrass leaves or epiphytic foraminifera in the latter case (Table 4).
Table 4. Foraminifera distribution in the studied seagrass ecosystem.

Ammonia beccarii proved to be an epiphytic species found in the three studied seagrasses (with 50% in S1; 33.33% in S2 and 100% in S3) (Table 4). It was the only epiphytic foraminifera species found on the Cymodocea nodosa leaves (100%). This species was also present in the sediment of the three stations (with 8.43% and 13.46% in S1; 33.33% and 19.04% in S2; 8% and 16.32% in S3). However, Elphidium crispum was found to be an epiphytic species for both Posidonia oceanica (25%) and Halophila stipulacea (33.33%). This species were also detected in both fractions of sediment. Peneroplis pertussis proved to be an epiphytic species, mostly for Posidonia oceanica (25%) and Halophila stipulacea (33.33%). These species were distributed over all sediment stations in the two fractions.
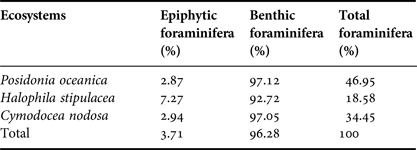
The most abundant foraminifera species are Ammonia beccarii (31.32%), Peneroplis pertusus (19.59%), Peneroplis planatus (14.29%) and Elphidium crispum (12.03%). By analysing the collected data, we could evaluate the characteristics of the epiphytic population of foraminifera by comparing it with the benthic one. Indeed, the results showed a much lower abundance of these microorganisms on seagrass leaves (3.71%) than benthic ones (96.28%) (Table 5). The species of foraminifera (benthic and epiphytic) were more abundant in the Posidonia oceanica ecosystem (46.95%) in comparison with the two other study sites.
Table 5. Foraminifera abundance rate per cm2 of sediment.
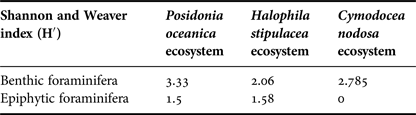
According to the Shannon and Weaver index, we can deduce that the Posidonia oceanica ecosystem had the most diverse benthic foraminifera (3.33); while the Halophila stipulacea had a more diversified epiphytic foraminiferal fauna (1.58) (Table 6).
Table 6. The Shannon and Weaver index of diversity.

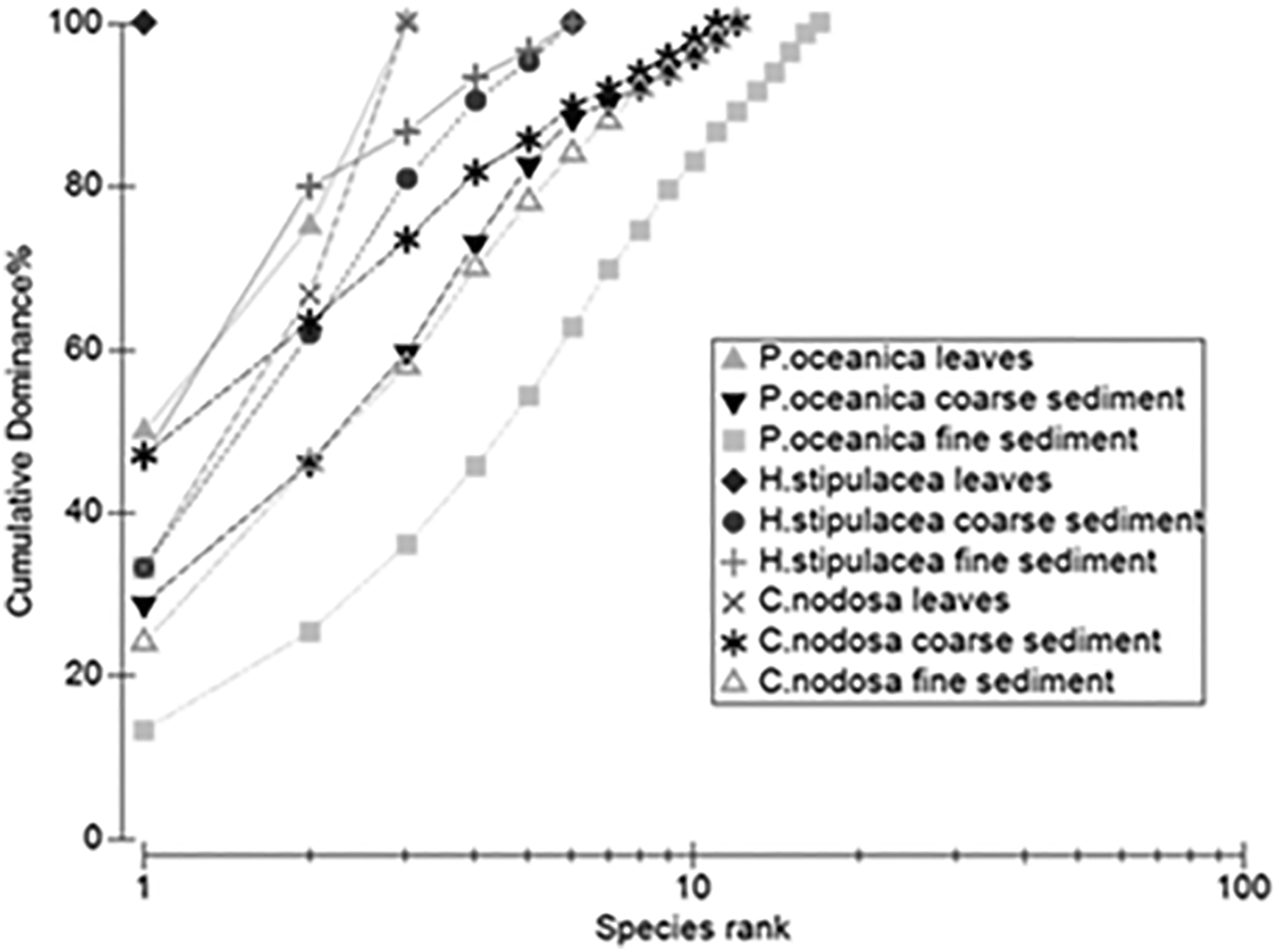
The dominance plot was obtained using the software PRIMER, which shows that the foraminifera segregation in Posidonia oceanica fine sediment was the most diversified one, while epiphytic foraminifera distribution on the Halophila stipulacea leaves was shown to be the most homogeneous one (Figure 4).

Fig. 4. Cumulative dominance of foraminifera species.
With Pearson's correlation, a significant negative affinity (r2 = −0.682, P = 0.043) between Quinqueloculina bertholiana, Adelosina pulchella and NH4, NT and SiO4 was distinguished. This indicated that these species were nitrophilous ones. Ammonia beccarii is found to have a significant positive correlation (r 2 = 0.739, P = 0.023) with TKN, which means that it was also a nitrophilous species.
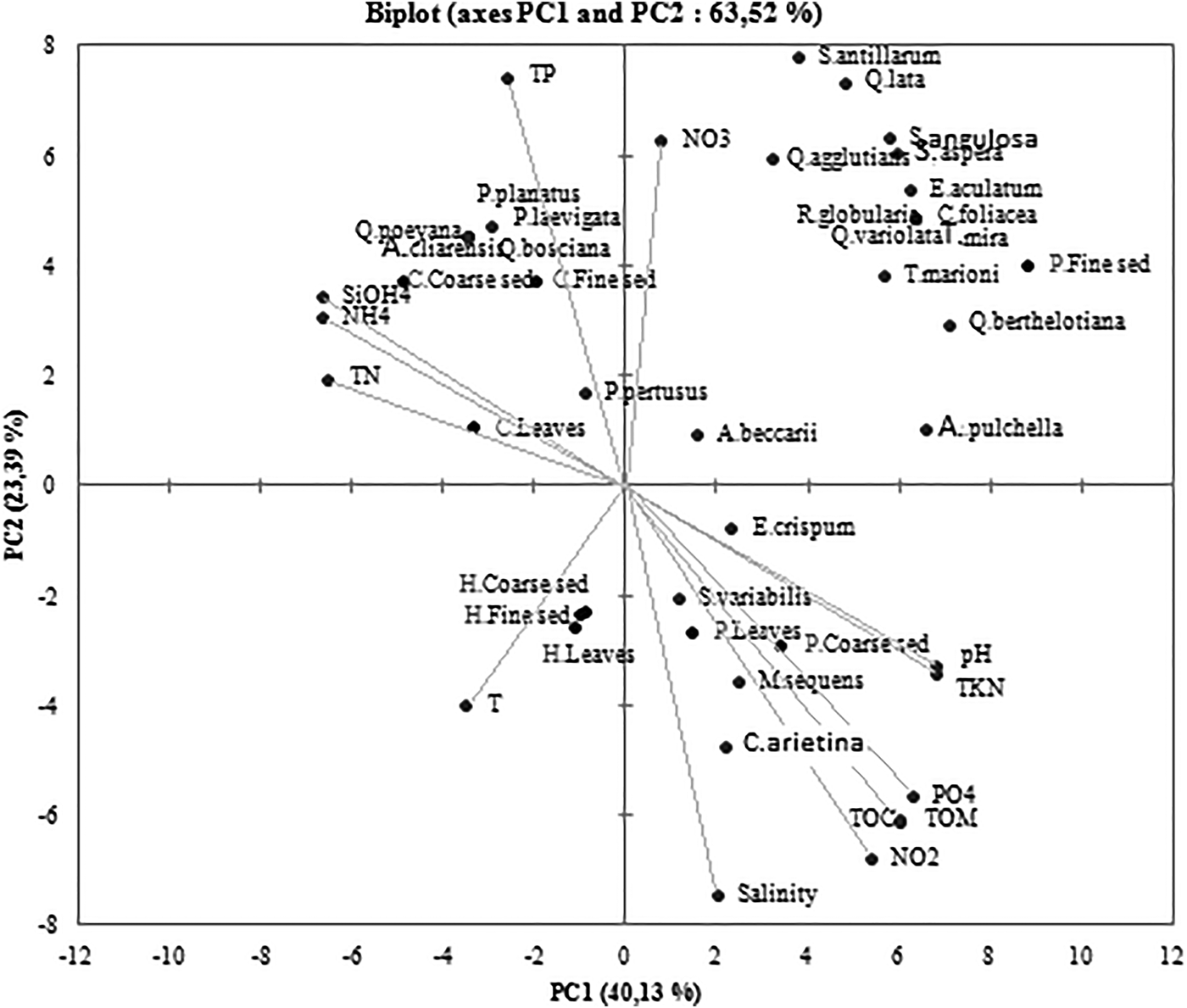
The PCA analysis allowed us to distinguish two principal components that explain 63.52% of the total data variance (Axis 1: 40.13% and Axis 2: 23.39%). Posidonia’s fine sediment was positively correlated with C. foliacea, D. mira, E. aculatum, Q. aspera, Q. bertholiana, Q. lata, A. pulchella, Q. variolata, R. globularis, S. antillarium, S. angulosa and T. marioni. These 12 foraminifera species were not positively linked with any abiotic factor. However, the Posidonia leaves and coarse sediment were not linked with any foraminifera species. These ecosystems were the most sensitive ones to abiotic conditions. In addition, they present a positive dependence to pH, TKN, PO4, TOM, TOC, NO2 and salinity. The sediment of Cymodocea nodosa’s ecosystem was positively correlated to TN, NH4, TP and SiOH4. Still, the Halophila stipulacea ecosystem wasn't linked with any specific foraminiferal species or abiotic factor (Figure 5).
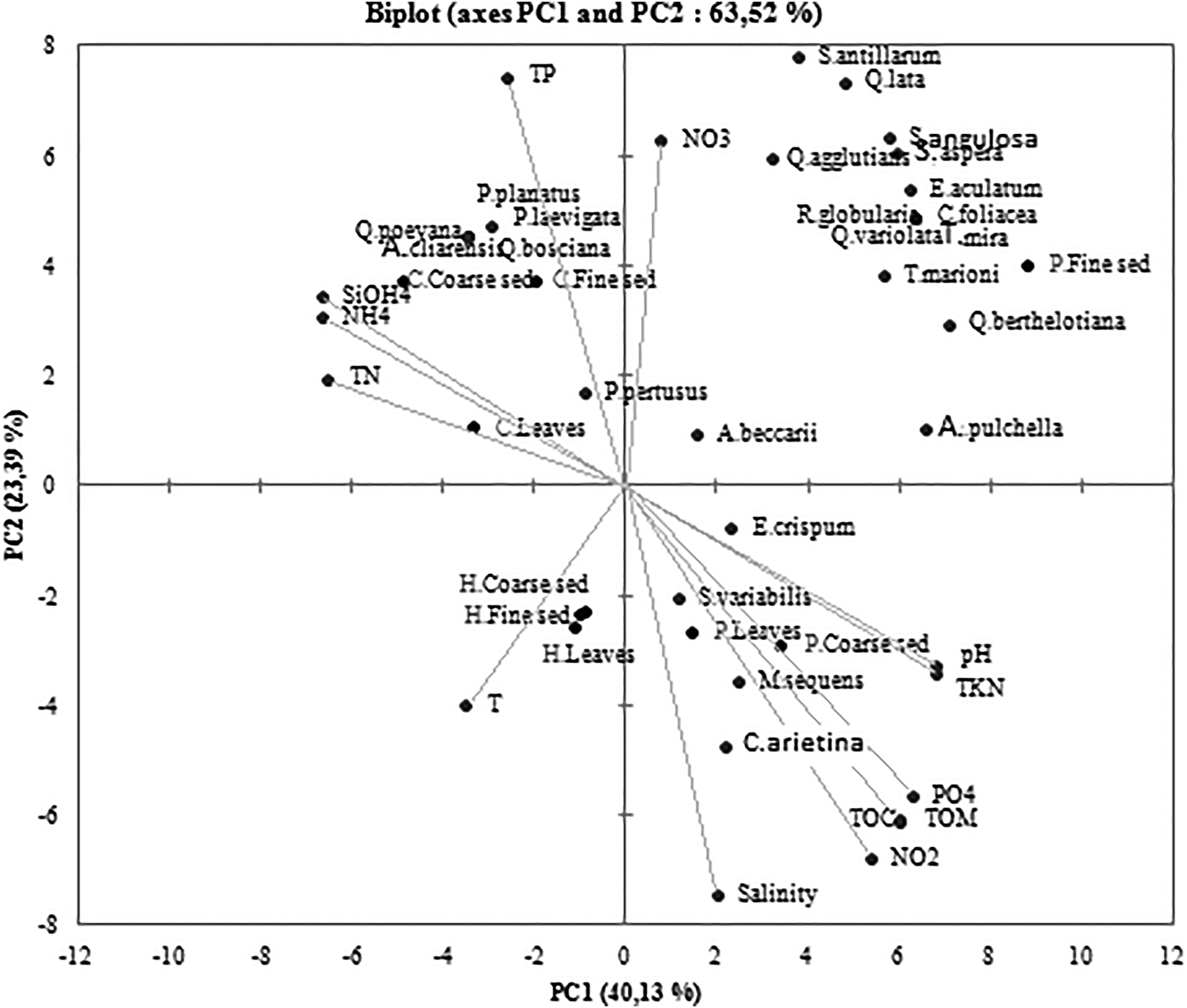
Fig. 5. Loading plots of the two components obtained with PCA for the data set.
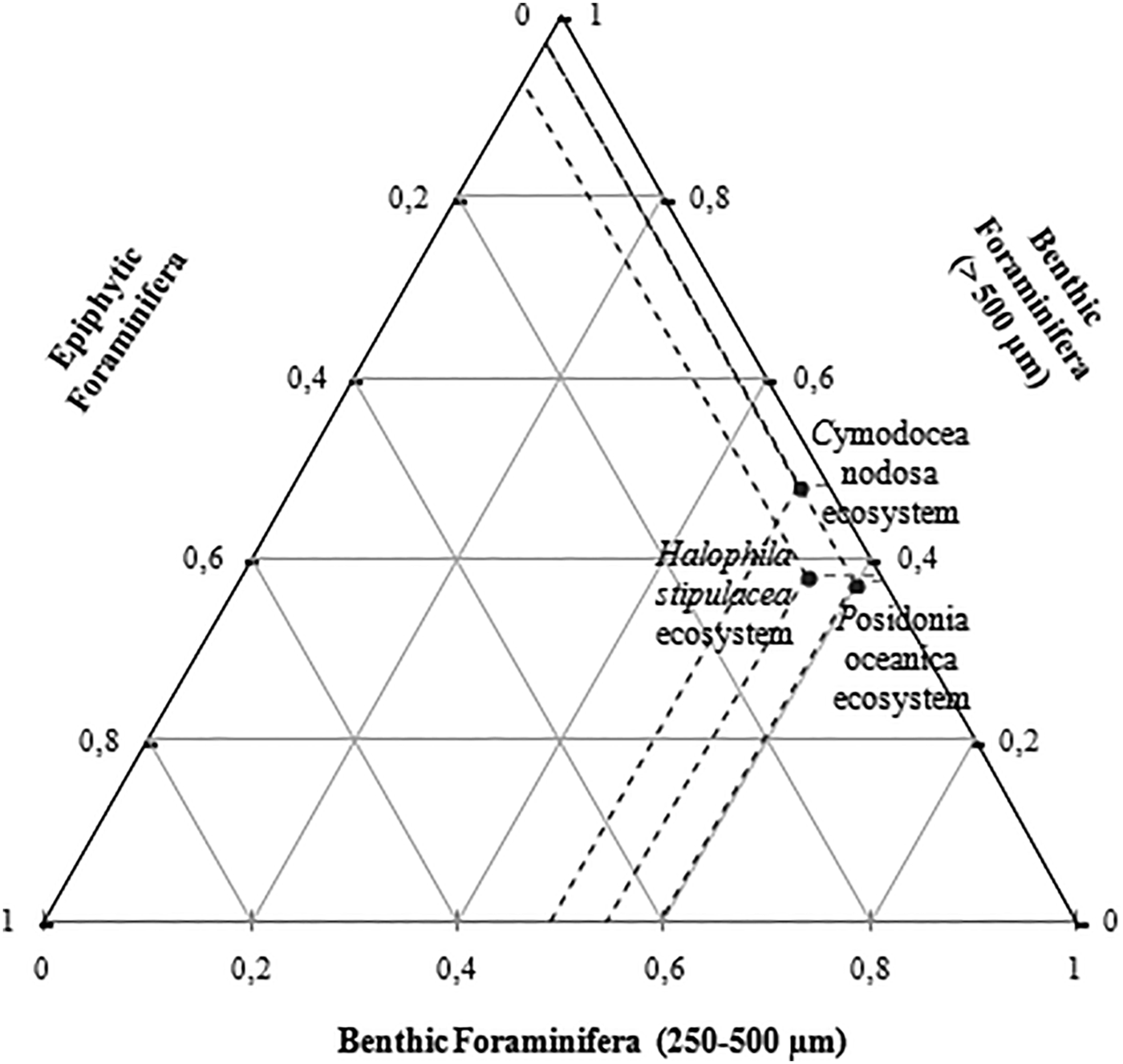
The ternary diagram presents the distribution of seagrass ecosystems (Posidonia oceanica, Halophila stipulacea and Cymodocea nodosa) according to the foraminifera types and range of size (epiphytic, juvenile benthic forms (250–500 µm) and adult benthic forms (>500 µm)). Stations S1 (Posidonia oceanica ecosystem) and S2 (Halophila stipulacea ecosystem) were characterized by a dominance of juvenile benthic foraminifera, with a much higher percentage in S1, whereas station S3 was composed in majority of adult benthic foraminifera. Posidonia oceanica ecosystem (S1) and Cymodocea nodosa ecosystem (S3) had equal percentage of epiphytic species, while S2 showed a relatively more abundant epifauna (Figure 6).
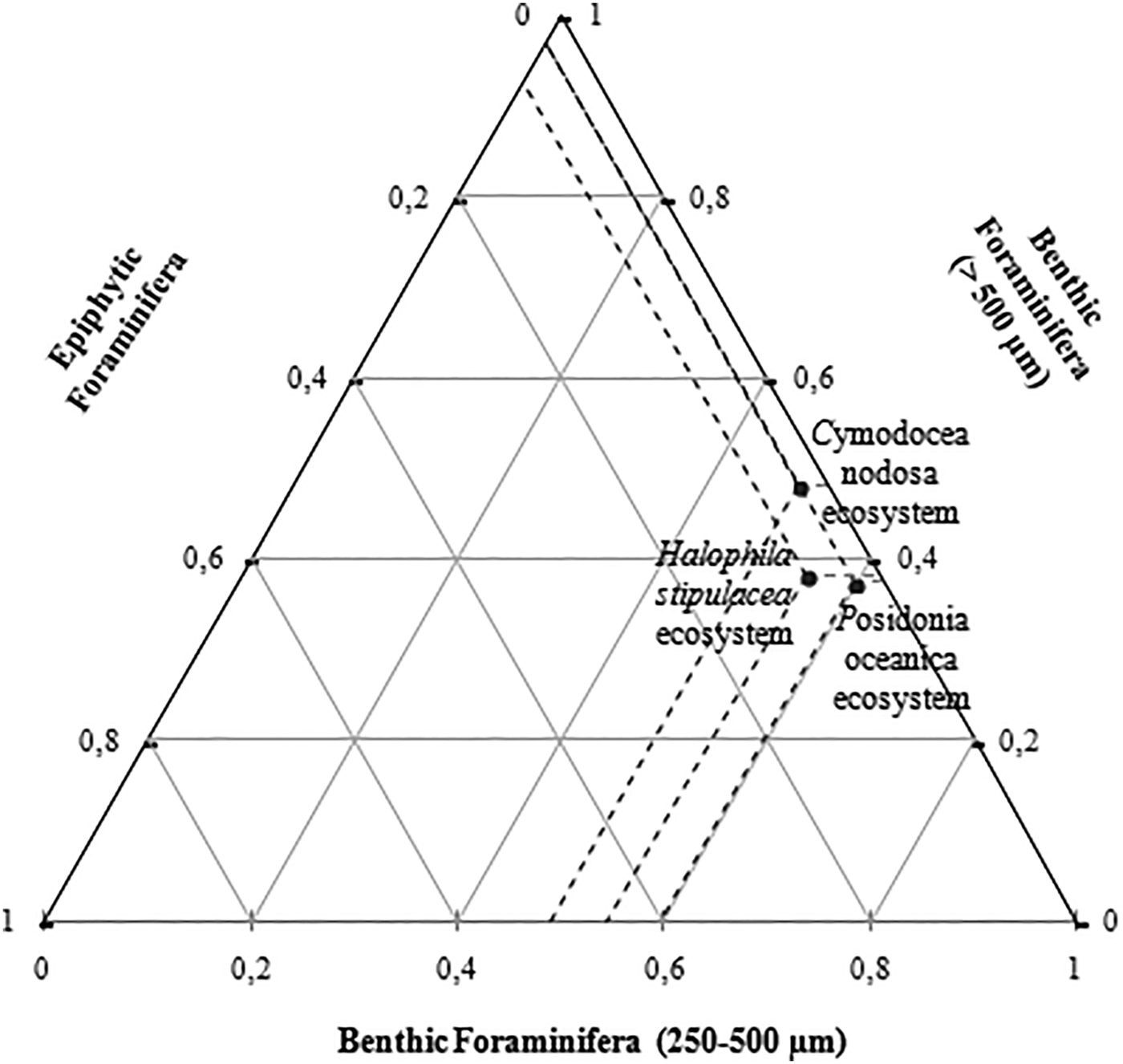
Fig. 6. Ternary diagram of the distribution of the different foraminifera communities in the studied ecosystems.
DISCUSSION
The fact that Posidonia oceanica’s ecosystem had the most important total organic matter content can be explained by the fact that TOM is essentially composed of much larger debris of macro-algae and seagrass (roots, leaves). It is logical because it is known that Posidonia leaves have the largest fixing surface among the three studied seagrasses. We found that substrates of all three studied areas covered by seagrass were sand dominated, while the mud was almost insignificant. In nearly 30 years, the sediment quality had evolved (Ammari, Reference Ammari1984); S1 and S2 turned from fine sand and very fine sand to medium and fine sand respectively, whereas S3 conserved the same granulometric status. This can be explained by the monumental effect of contamination due to the advance of industrialization and shipping activities between Sfax and Kerkennah in the late 1980s. It has already been reported that heavy metals pollution have modified Posidonia oceanica's seagrass and have changed the benthic foraminiferal ecosystem in the Mediterranean Sea (Caruso et al., Reference Caruso, Cosentino, Tranchina and Brai2011; Cosentino et al., Reference Cosentino, Pepe, Scopelliti, Calabrò and Caruso2013). Similar sand-dominated textures have been observed on seagrass-dominated substrates elsewhere (Edel Province in Shark Bay, Australia and Inhaca Island, Mozambique; in Mateu-Vicens et al., Reference Mateu-Vicens, Antonio, Salud and Beatriz2010).
Foraminifera study
The species found in these study areas were in complete accordance with those reported in the Mediterranean Sea. Major foraminifera species represented in this study were Ammonia beccarii, Peneroplis pertusus, Peneroplis planatus and Elphidium crispum, which was the case of a study conducted along the northern coast of the Gulf of Gabes in 2010 (Aloulou et al., Reference Aloulou, Elleuch and Kallel2011). Those authors found that major families present were Rotaliidae, Elphididae, Peneroplidae and Spiroloculindae. Foraminifera were particularly well represented in the Posidonia oceanica ecosystem and in this biotope reached higher percentages than those that were in the surrounding biotopes. During the campaigns of ‘Mer Pélagienne’ conducted by Blanc-Vernet et al. (Reference Blanc-Vernet, Clairefond, Orsolini, Burollet, Clairefond and Winnock1979) in the Gulf of Gabes, the authors explained this phenomenon by the fact that the microfauna find a large fixing surface on Posidonia leaves. Indeed epiphytic communities are more sensitive to environmental changes than the hosting plant itself (Prado et al., 2008; Montefalcone, 2009; Giovannetti et al., 2010; in Ben Brahim et al., Reference Ben Brahim, Hamza, Ben Ismail, Mabrouk, Bouain and Aleya2013). It is suggested that the low diversity indexes in the epiphytic microfauna were partly the result of the direct exposure of Kerkennah islands to pollution affecting the coast of Sfax and the consequence of some tidal asymmetries leading to nutrient-rich inputs from the city (Ben Brahim et al., Reference Ben Brahim, Hamza, Ben Ismail, Mabrouk, Bouain and Aleya2013). Light levels can also influence foraminifera distributions, especially for mixotrophic species such as Elphidium crispum (Sanders, Reference Sanders1991). Identified species of foraminifera were not represented in all the studied seagrass ecosystems.
Indeed, it is clear that the Cymodocea nodosa ecosystem was the least diverse site of this trio. Qualitatively, the list of benthic foraminifera species varies from one area to another. There were also differences in quantity terms. Indeed, the diversity distribution proportions of these foraminifera species are a characteristic of their different biotopes.
The low diversity in the epifaunal assemblages of Posidonia oceanica can be explained by the maturity and the hostility of the biotope, since this study site is close to a port area. In the literature, the Posidonia oceanic ecosystem is known to be dominated by a few, well-adapted foraminiferal species (Mateu-Vicens et al., Reference Mateu-Vicens, Antonio, Salud and Beatriz2010).
The Halophila stipulacea leaves seemed to be the most diversified ecosystem in the epiphytic foraminifera. We think that it is mainly due to some adaptation issues as this seagrass is a newly introduced species, so it has no specific microfauna yet.
By comparing the results of this study with those conducted in 1979 by Blanc-Vernet, we could notice a few similarities in species of foraminifera identified in the sediment, but with some differences, especially in terms of abundance, certainly due to the variation of the period, the sea depth and the increasing pollution rate. The Ammonia identified in this work has a special affinity for Cymodocea nodosa, which was hardly the case in 1979 according to Blanc-Vernet. In fact, these types of seagrass were mainly populated by Miliolidae, Sorites and Rosalina (Blanc-Vernet et al., Reference Blanc-Vernet, Clairefond, Orsolini, Burollet, Clairefond and Winnock1979).
Murray (Reference Murray2006) considered Ammonia species as strictly infaunal which is not the case in this study; as Ammonia beccarii seems to be an epifaunal species for the three biotopes (Seuront & Bouchet, Reference Seuront and Bouchet2015).
This new preference is a very interesting behaviour that should be characterized by conducting further studies taking into consideration influent parameters.
According to the PCA study, Halophila stipulacea’s ecosystem could not be related to any specific foraminiferal species, while epiphytic ones were much more abundant in this biotope than any other one. The authors suggest that this is a matter of adaptation. Benthic and epiphytic foraminifera were more abundant in the Posidonia oceanica ecosystem, with Posidonia leaves providing a large fixing surface for microfauna. This biotope had the most diverse benthic foraminifera.
Concluding, the low rate of epiphytism is probably the consequence of the constraints of the marine ecosystem such as the heating of the water column and hydrodynamics in Kerkennah's coasts.
This study focused on various aspects of the ecology, chemistry and geology. This approach is beneficial. Since the factors governing marine life in coastal areas include both environmental and anthropogenic disturbances, studying their effects on ecosystems will require more multidisciplinary approaches (Aloui-Bejaoui & Afli, Reference Aloui-Bejaoui and Afli2012). Further anthropogenic studies are necessary.
ACKNOWLEDGEMENTS
The authors wish to thank the entire team of Research Unit 3E (Energy, Water Environment) (ENIS-Sfax-Tunisia) and INSTM (Sfax-Tunisia), for their support and warm welcome within their institutions. The authors are also thankful to Mrs Rim Trabelsi (English teacher), Mr Wassim Kammoun (English professor), Mrs Najla Ayadi (researcher) and Mrs Ihsen Zghal (palaeontologist) for having proofread the manuscript.