INTRODUCTION
Age is a fundamental parameter in understanding the biology of an organism as it forms the basis of determining growth, timing of maturity, recruitment, mortality and longevity (Campana, Reference Campana2001). Such information is essential in characterizing the demography and dynamics of a population and facilitates fisheries management. Historically, age and growth has been estimated by following modal peaks in length–frequency data through time (King, Reference King1995). However, identification of age-specific cohorts is difficult for species with extended spawning seasons and large variation in growth (Jackson et al., Reference Jackson, Alford and Choat2000). More recently, an organism's age has been estimated from counting periodic growth increments found on hard anatomical structures. Annuli were first identified in the otoliths (‘ear bones’) of the flatfish Pleuronectes platessa, in 1899 (Jones, Reference Jones, Stevenson and Campana1992) and have also been identified in fish spines (Marzolf, Reference Marzolf1955), fin rays (Cass & Beamish, Reference Cass and Beamish1983), vertebrae (Brown & Gruber, Reference Brown and Gruber1988), and scales (Carlander, Reference Carlander, Summerfelt and Hall1987). Similarly, daily increments have been identified in juvenile fish otoliths (Panella, Reference Panella1971), cephalopod statoliths (Lipinski, Reference Lipinski1978; Spratt, Reference Spratt1978), gladii (Arkhipkin & Bizikov, Reference Arkhipkin, Bizikov, Jereb, Ragonese and Von Boletzky1991) and stylets (Doubleday et al., Reference Doubleday, Semmens, Pecl and Jackson2006). Of these structures, fish otoliths have gained wide acceptance as their increments are relatively well defined, grow continuously, and are not regenerated or lost over time.
Cephalopod statoliths are functionally and structurally analogous to fish otoliths and over the past 30 years have been extensively relied on to age a variety of squid species (Arkhipkin, Reference Arkhipkin2005). The proliferated interest in ageing cephalopods has largely been in response to the increased global fishing pressure on cephalopod resources as commercial fisheries progressively fish down trophic food webs (Pauly et al., Reference Pauly, Christensen, Dalsgaard, Froese and Torres1998). Consequently, there is a fundamental need to understand cephalopod population dynamics to ensure that they are sustainably harvested (Jackson et al., Reference Jackson, Alford and Choat2000). The life-history of squid is very different to that of teleost fish with squid exhibiting rapid indeterminate growth within a life span of generally less than a year (Forsythe & Hanlon, Reference Forsythe and Hanlon1989; Rodhouse & Hatfield, Reference Rodhouse and Hatfield1990; Jackson, Reference Jackson1994). Furthermore, the rate of this ‘live fast, die young’ strategy is highly dynamic where similar size individuals may have considerably different ages (Forsythe, Reference Forsythe1993). This high degree of plasticity in growth is likely governed by variation in ambient temperature (Forsythe, Reference Forsythe2004) and food availability (Moltschaniwskyj & Martinez, Reference Moltschaniwskyj and Martinez1998). It is not known whether this inherent plasticity is manifested within the statolith's microstructure and the potential in which it may compromise the reliability of using statoliths to infer age.
It is generally assumed that the fine increments in statoliths are formed on a daily basis (Durholtz et al., Reference Durholtz, Lipinski and Field2002). In many cases this assumption is based on limited evidence or without species-specific validation (Arkhipkin, Reference Arkhipkin2005). A few studies have produced alternative results where increments have been formed on a less than daily basis (Morris, Reference Morris, Jereb, Ragonese and Von Boletzky1991, Reference Morris1993; Lipinski & Durholtz, Reference Lipinski and Durholtz1994; Villanueva, Reference Villanueva2000a; Sato et al., Reference Sato, Kasugai and Munehara2008). Such conflicting evidence suggests that there is uncertainty about the periodicity of increment formation in squid statoliths and, as such, it has been recommended that age estimations should be treated with caution until species-specific validation of the growth increments is undertaken (Lipinski, Reference Lipinski2001). Rearing known age individuals in captivity is one of the most successful and commonly used age validation techniques (Yang et al., Reference Yang, Hixon, Turk, Krejci, Hulet and Hanlon1986; Geffen, Reference Geffen, Stevenson and Campana1992). Individuals are generally reared from hatching, thereby allowing the formation of stress marks associated with the early life history, such as hatch checks or settlement marks. Verification of these checks is essential as they often represent the starting point of age counts. Not all organisms deposit a hatch check upon hatching, therefore, it is important that the formation of this anomaly is verified on a species-specific basis (Smith & Walker, Reference Smith and Walker2006). Artificially marking calcified structures with a fluorescent calcium-binding chemical such as oxytetracycline (OTC), calcein or alizarin complexone has also been successfully used in age validation studies (Jackson, Reference Jackson1990; Campana, Reference Campana2001; Jackson & Forsythe, Reference Jackson and Forsythe2002). This method relies on the animal incorporating the compound into the growing surface of any calcified structure to produce a ‘date-specific marker’ that is visible under fluorescent light. Through comparing the number of increments between the mark and the structure's subsequent growth with the elapsed time period it is possible to evaluate the periodicity of increment formation.
Southern calamary, Sepioteuthis australis, is a squid species endemic to southern Australian and New Zealand waters. Current age estimates, using statolith increment analysis, have indicated that this species has a maximum age of 280 days (Triantafillos, Reference Triantafillos2001). Like many other inshore squid, southern calamary is of increasing commercial significance, contributing to multi-species, marine fisheries in all southern Australian states, particularly South Australia and Tasmania. Age estimates for this species are integral in determining growth rates (Pecl et al., Reference Pecl, Moltschaniwskyj, Tracey and Jordan2004), back-calculating hatch-dates (Moltschaniwskyj & Pecl, Reference Moltschaniwskyj and Pecl2007), and in developing pre-recruit indices (Steer et al., Reference Steer, Lloyd and Jackson2007). Despite their commercial importance, age has not been comprehensively validated. There has, however, been a small ad hoc study that validated the ‘one increment–one day’ hypothesis for summer-reared calamary hatchlings, but this study did not extend the investigation to determine the temporal stability of the relationship (Pecl, Reference Pecl2004). Given the plastic nature of growth observed for southern calamary (see Pecl, Reference Pecl2004), it is essential that age validation studies account for potential seasonal and ontogenetic variation.
Through the combination of two age validation methods, captive rearing of known age individuals and date-specific marking, this study aims to: (1) establish a zero point for increment counts for the commercially important southern calamary, Sepioteuthis australis by identifying the hatch check; (2) validate the periodicity of increment formation across this species' entire life history; and (3) explore the effect of varying temperature regimes on increment deposition by considering two seasonal extremes.
MATERIALS AND METHODS
Sample collection
Twelve recently laid egg masses, each consisting of approximately 250 egg strands, and 17 adult squid were collected from the Adelaide metropolitan coast during summer and winter of 2007. Egg masses were collected via SCUBA and squid were hand jigged. Once collected, eggs and squid were housed in separate, insulated boxes that were continuously filled with fresh seawater to ensure adequate water exchange and transported to aquarium facilities at SARDI Aquatic Sciences. Two data loggers (Onset StowAway® and Tidbit®) were set to record ambient water temperature on an hourly basis throughout the duration of the experiment. Average daily water temperatures ranged from 19.3–22.6°C (average 20.8°C) and 13.7–14.7°C (14.1°C) for the summer and winter regimes, respectively.
Captive rearing of known-age hatchlings
Egg masses were divided into smaller clusters of five to ten egg strands and suspended by nylon line from plastic racks into a 100 l tank connected to a flow-through seawater system. Tanks were held within a controlled environment room with water temperature and day-length set to reflect ambient conditions. Air stones were placed beneath the eggs to ensure maximum oxygenation for embryo development (Steer et al., Reference Steer, Moltschaniwskyj and Jordan2003). The development of the embryos was checked on a daily basis and any new hatchlings were transferred to a separate date-specific tank where they were maintained on a diet of live mysidacean shrimp for as long as possible. All dead hatchlings were removed each day and stored in date-specific vials containing 75% ethanol until further processing.
Date-specific chemical marking
Adults were injected at the base of their dorsal arms with 1 ml of calcein-binding solution at a concentration of 10 g l−1 of seawater. Calcein was selected as OTC and alizarin complexone were ineffective in producing a reliable mark in S. australis statoliths (Triantafillos, Reference Triantafillos2001) and it has been successfully used for a congeneric species, S. lessoniana (Jackson, Reference Jackson1989). Adults were maintained in a 40,000 l tank attached to a flow through system with supplemental aeration. Individuals were exposed to ambient temperatures and natural lighting throughout the experiment and fed predominantly live sea mullet (Mugil cephalus) and yellow-finned whiting (Sillago schomburgkii) collected from local waters. Squid diet was also supplemented with freshly frozen southern garfish (Hyporhamphus melanochir), sand trevally (Pseudocaranx wrighti) and western king prawns (Melicertus latisulcatus).
Statolith preparation
Hatchling statoliths were considerably smaller than adult statoliths and, as such, were processed differently. Once removed, hatchling statoliths were immersed in commercial-grade bleach for 60 minutes to remove adhering tissue. One statolith from each pair was then washed in 75% ethanol and whole mounted on a glass slide in Crystal Bond® thermoplastic cement. The lateral surfaces of the statoliths were lightly ground with 30 µm lapping film and polished using aluminium powder on wet suede to expose the internal microstructure. Surface scratches on the polished section were cleared with a drop of immersion oil. The width of the hatch check and the number of post-hatch increments were ascertained from digitized images captured through a high-resolution camera mounted on a Leica compound microscope at 600 × magnification using Optima (version 6.5) image analysis software. Hatch checks were not measured from hatchlings less than a day old (0-day hatchlings) as the hatch check and statolith boundary were indistinguishable from each other. A sequence of a dark and light band outside the hatch check represented one post-hatch increment.
Adult statoliths were whole-mounted in Crystal Bond® with the dorsal dome projecting over the edge of the glass slide. The statolith was ground along a transverse plane, using 30 µm lapping film, until the plane passed through the nucleus. The ground surface was polished with aluminium powder on wet suede. The polished surface was mounted so the rostrum aligned perpendicular to the slide's surface. The statolith was ground and polished to a section thin enough for examination. The extent and intensity of grinding was continually monitored using a binocular light microscope. Prepared adult sections were examined at 400 × magnification using an Olympus BX51 light microscope connected to ImagePro analysis software. Two digitized images were captured for each section, one illuminated with transmitted light and the other under fluorescent light to expose the date-specific calcein stain. The position of calcein stain was established from the fluorescent image and post-stain increments were counted from the corresponding transmitted light image to the edge of the statolith.
Post-hatch increments in both hatchling and adult statoliths were counted by two independent, experienced readers. If counts differed by more than two increments, a third count was done and the outlier discarded. Statoliths were rejected if counts continued to differ by more than two increments or if the hatch check could not be confidently identified.
Statistical analysis
The relationship between days elapsed in captivity and statolith increment counts for summer and winter-reared individuals (hatchlings and adults) were explored using linear regression analysis. Analysis of covariance was used to test whether the slope and intercepts of these regression analyses differed between seasons. Season was treated as a fixed factor. Homogeneity of variances was determined using Levene's test and through visual inspection of residual plots. No data transformations were required.
RESULTS
Captive rearing of known-age hatchlings
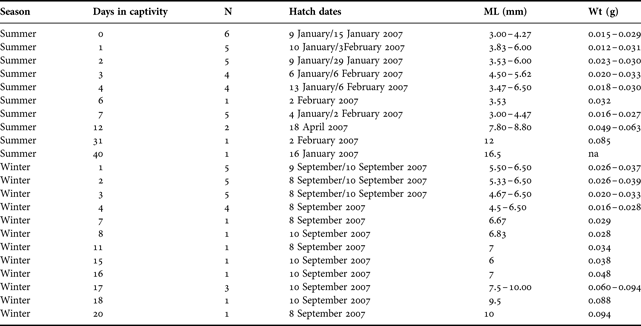
A total of 57 hatchlings (34 in summer and 29 in winter) were reared in captivity. Hatch dates ranged from 6 January to 18 April 2007 for summer-reared hatchlings and 8 to 10 September 2007 for winter-reared hatchlings (Table 1). In each case, hatchlings suffered high mortality rates with only 13 animals surviving >7 days in captivity. The oldest hatchlings survived until 20 and 40 days in the summer and winter regimes, respectively (Table 1).
Table 1. Details of the squid used for the known-age component of the study.
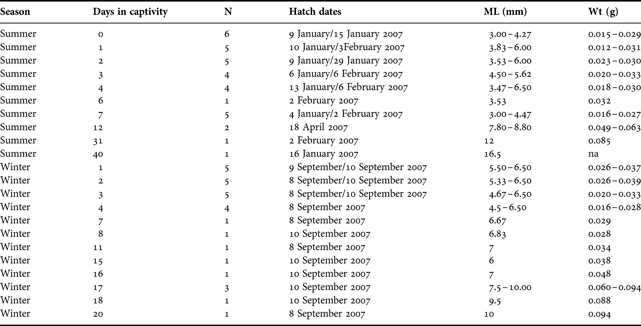
na, not applicable.
At least one statolith was dissected from each hatchling and successfully prepared. Each statolith exhibited a distinct hatch check, the mean radius of which was 6.0% greater in winter-reared hatchlings compared to those reared in summer (c.f. 0.094 ± 0.0017 mm and 0.088 ± 0.0015 mm, respectively). Strong positive linear relationships between the average increment count and the number of days elapsed for both summer and winter-reared hatchlings were evident (Figure 1A). The elapsed time in captivity explained more than 99% of the variability in the average increment count. Analysis of covariance indicated no significant difference between the slopes of the regression lines (Fslope = 0.004; df = 1, 63; P = 0.952) signifying a similar pattern between the two seasons. The intercepts, however, were significantly different (Fintercept = 10.584; df = 1, 63; P = 0.002), where the average increment count was 0.13 increments higher for summer-reared hatchlings than winter-reared hatchlings. The magnitude of this difference, however, was considered biologically negligible.

Fig. 1. (A) Relationship between average increment count and days elapsed for captive reared summer (•) and winter (○) hatchlings; (B) relationship between average post-injection increment count and days elapsed for captive reared summer (•) and winter (○) adults.
Date-specific chemical marking
A total of 17 adults (8 in summer and 9 in winter), ranging between 145 and 312 mm mantle length (ML), were collected and injected with calcein. These animals were successfully reared for more than 8 days with two individuals euthanized 54 days post-injection (Table 2). At least one statolith was successfully prepared from 15 (88%) squid. Calcein was an excellent marker for southern calamary adults as a distinct stain was detected in 14 (93%) of the statoliths when viewed under fluorescent light. Post-stain increments could also be clearly identified when the sections were viewed under transmitted light (Figure 2).

Fig. 2. Calcein stained statolith viewed at 400 × magnification under (A) fluorescent light and (B) transmitted light with a polarizing filter. Arrow indicates fluorescent calcein mark.
Table 2. Details of the squid used for the chemical marking component of the study.

Post-stain increment counts did not correspond to the number of days in captivity (Figure 1B). Subsequently, no seasonal effect on the ratio between average increment count and days elapsed in captivity could be detected. The average rate of increment formation was one for every 10 days in captivity. At one day post-staining, the average increment count was 49% (2.5 increments) greater in summer than winter indicating a greater degree of underestimation in winter adults.
DISCUSSION
This study explored the rate of increment formation in statoliths over seasonal and ontogenetic extremes for Sepioteuthis australis. The ‘one increment–one day’ hypothesis was verified for captive-reared hatchlings (up to 40 days) and remained temporally stable. This result was consistent with that found by Pecl (Reference Pecl2004) for summer-reared S. australis hatchlings (<120 days) in Tasmania. This relationship, however, was not evident in the calcein-injected adult squid, as inconsistencies, of up to 44 days, between the number of days post-stain and average number of increments were detected. Yang et al. (Reference Yang, Hixon, Turk, Krejci, Hulet and Hanlon1986) observed similar results in captive-reared Loligo opalescens where increments were deposited daily during the first 65 days of growth but were less-than-daily beyond that age. This suggests that either the periodicity of increment formation changes later in life or that negative effects associated with rearing squid in captivity or statolith preparation methods have contributed to this discrepancy.
Changes in metabolic rates, either through ontogenetic processes or variation in temperature, can significantly affect statolith growth (O’Dor & Wells, Reference O'Dor, Wells and Boyle1987; Villanueva, Reference Villanueva2000b). In some cases, this has been manifested as less-than-daily increments within the statolith's microstructure (Morris, Reference Morris, Jereb, Ragonese and Von Boletzky1991, Reference Morris1993; Villanueva, Reference Villanueva2000a), a phenomenon that has also been observed in larval fish otoliths (Szedlmayer & Able, Reference Szedlmayer and Able1992; Ahrenholz et al., Reference Ahrenholz, Fitzhugh, Rice, Nixon and Pritchard1995). Loliginid squid typically exhibit a two-stage pattern of growth and do not appear to reach an asymptote (Jackson, Reference Jackson1994). They grow rapidly (exponentially) for a substantial period of their life followed by a relatively short period of decreased growth (power) at the end of ontogenesis (Forsythe & Van Heukelem, Reference Forsythe, Van Heukelem and Boyle1987; Arkhipkin & Roa-Ureta, Reference Arkhipkin and Roa-Ureta2005). Given this variation in growth and the documented reduction in incrementation rates associated with reduced metabolic activity, it is plausible that the ‘one ring–one day’ relationship deteriorates with age. However, it is equally plausible that this variation in growth has compromised the interpretation and relative readability of the statolith's microstructure to produce erroneous age estimates. For example, faster growing squid typically exhibit broadly spaced increments that are relatively easy to interpret. As growth slows down the spacing of these increments is reduced and becomes difficult to discern, often resulting in ‘blurred’ zones (Durholtz & Lipinski, Reference Durholtz and Lipinski2000). Consequently, the level of interpretability of increments that are laid down towards the end of the squid's life may fall below the resolving power of light microscopy and they may appear to have been deposited at a less-than-daily rate. This may also explain why a greater discrepancy was found in the marginal region of the statoliths collected from winter-caught adults.
The level of increment interpretation may be further compromised in squid that have been maintained in captivity for extended periods (Jackson et al., Reference Jackson, Arkhipkin, Bizikov, Hanlon, Okutani, O'Dor and Kubodera1993). This is because captive squid have displayed stunted growth (Pecl & Moltschaniwskyj, Reference Pecl and Moltschaniwsky1999), abnormal statolith development (Lipinski & Durholtz, Reference Lipinski and Durholtz1994), reduced increment widths (Durholtz & Lipinski, Reference Durholtz and Lipinski2000) and faint statolith microstructure (Jackson et al., Reference Jackson, Arkhipkin, Bizikov, Hanlon, Okutani, O'Dor and Kubodera1993). Although scanning electron microscopy (SEM) has been successfully used to clarify increments in previous studies (Lipinski, Reference Lipinski1986; Lipinski & Durholtz, Reference Lipinski and Durholtz1994) initial attempts to clarify the interpretation of increments for S. australis as part of this study were unsuccessful (Hunt, personal observation). Chemically etching the prepared statoliths with various concentrations of hydrochloric acid and nitric acid prior to SEM analysis enhanced the structural properties of the hatch check, but failed to resolve any increments (Hunt, personal observation). Villanueva (Reference Villanueva2000a) was also unable to determine increments in embryonic statoliths of L. vulgaris using SEM, even though stress marks resulting from the transfer of animals between tanks were clearly visible. Efforts were made to reduce captive biases throughout the experiment, such as stocking large 40,000 l tanks with a maximum of nine adults, minimizing observer interference and feeding animals live prey ad libitum that are known to comprise part of their natural diet. Some squid were successfully maintained in captivity for up to 54 days post-staining indicating a level of captive conditioning. They were also observed to feed within minutes of being transferred into the tanks and readily spawned, further indicating that they were adequately habituated.
Assessing the increment periodicity in calcein-injected adults was based entirely on the interpretation of the microstructure in the marginal regions of the statolith. It is possible that some of these increments were lost due to over grinding during the preparation process, consequently providing an underestimation of the number of increments post-injection. This may explain why a calcein mark was not detected in an individual that died eight days post-staining, even though exceptional fluorescent marks were evident in all other statoliths examined. To counter this, it is suggested that individuals are injected with calcein at two separate intervals and the rate of increment formation between consecutive stains on the statoliths be examined (see Jackson, Reference Jackson1989; Villanueva, Reference Villanueva2000a; Sato et al., Reference Sato, Kasugai and Munehara2008).
Given the potential complications associated with rearing squid in captivity and potential statolith processing artefacts, it is difficult to definitively validate the ‘one ring–one day’ hypothesis throughout the entire life-span of S. australis. It can, however, be confirmed that the rate of increment formation is daily for recent hatchlings and juvenile calamary that are less than 40 days old and that this relationship is temporally stable across seasonal extremes. Further work is required to resolve incrementation rates in adult calamary. Validating age in adult squid has been a consistent challenge, as it is a costly exercise to either maintain sufficient numbers of squid in adequate aquaculture systems for extended periods (Yang et al., Reference Yang, Hixon, Turk, Krejci, Hulet and Hanlon1986; Durholtz & Lininski, 2000), or recapture enough chemically tagged squid from the wild (Lipinski et al., Reference Lipinski, Durholtz and Underhill1998). Although still costly, there may be scope to carry out validation studies in sea-cages, similar to those that are commonly used in aquaculture ventures, as this would reduce some of the biases associated with land-based aquaria.
ACKNOWLEDGEMENTS
This research was supported by funds and facilities provided by SARDI Aquatic Sciences and the School of Earth and Environmental Sciences of the University of Adelaide. The authors are indebted to Matt Lloyd, Bruce Jackson, Phillipa Wilson, Andrew Munro and the Adelaide Microscopy staff for field and laboratory assistance. Thanks also to three anonymous referees for their constructive comments. This study complies with the current collection laws and code of ethics of South Australia: PIRSA animal ethics approval No. 13/06 and the Adelaide University ethics clearance S-093-2006.