INTRODUCTION
Predation is recognized as an important influence on evolution and biodiversity (Vermeij, Reference Vermeij1987; Kelley et al., Reference Kelley, Kowalewski and Hansen2003; Huntley & Kowalewski, Reference Huntley and Kowalewski2007; Sallan et al., Reference Sallan, Kammer, Ausich and Cook2011), regulating community composition on multiple geographical scales (e.g. Paine, Reference Paine1966; Pianka, Reference Pianka1966; Bertness et al., Reference Bertness, Garrity and Levings1981; Menge & Lubchenco, Reference Menge and Lubchenco1981; Yamada & Boulding, Reference Yamada and Boulding1996; Burrows et al., Reference Burrows, Kawai and Hughes1999; Guidetti, Reference Guidetti2007; Brazao et al., Reference Brazao, Silva and Boaventura2009; Schemske et al., Reference Schemske, Mittelbach, Cornell, Sobel and Roy2009; Silva et al., Reference Silva, Hawkins, Clarke, Boaventura and Thompson2010a, Reference Silva, Silva, Hawkins, Boaventura and Thompsonb). Predatory shell crushing crabs in particular are thought to play an important role in structuring gastropod populations (Yamada & Boulding, Reference Yamada and Boulding1996; Ray-Culp et al., Reference Ray-Culp, Davis and Stoner1999; Cannicci et al., Reference Cannicci, Gomei and Vannini2002). In order to address ecological questions regarding predator–prey interactions, we must first identify spatial and temporal patterns resulting from differences in predation intensity. Techniques that can easily provide large amounts of quantitative data are therefore needed to examine communities across habitats, and over broad geographical scales. Given that some species of crabs are subject to intense commercial fishing (e.g. Metacarcinus magister and Cancer productus), understanding the magnitude and exact nature of the effects of crab predation on intertidal communities is crucial to the successful management of this important economic resource. Due to the logistic challenges of observing predatory activity directly in modern marine environments, quantitative data regarding crab attack frequencies on gastropod populations are currently sparse.
Here we test the utility of a novel method pioneered by Thompson et al. (Reference Thompson, Jenkins and Bussell2000), utilizing wax gastropod replicas to quantify crab–gastropod interactions in situ without the need for direct observations. The method uses wax replicas secured to rock surfaces in the intertidal, and facilitates the collection of large amounts of attack frequency data of intertidal gastropods without the need for specialized or expensive equipment. Video recordings have been used to verify that crabs (Carcinus maenas, Necora puber and Cancer pagurus) attack wax limpet replicas in situ, and that traces of these attacks are preserved as easily identifiable ‘scars’ or grooves in the wax (Thompson et al., Reference Thompson, Jenkins and Bussell2000). Videos also showed that when crabs encountered wax replicas in the field, three types of behaviours were observed, resulting in scratch marks on the wax surface: scraping replica with chelae; clasping replica with one or both chelae; and prying (clasping replica and attempting to remove from the substrate). In their study, crabs showed no preference for live limpets over wax replicas, attacking both in comparable quantities, and wax models provided reliable numerical attack frequency data. Wax models should, therefore, provide an inexpensive and less time-intensive method (relative to direct observations) to collect a large amount of quantitative data, specifically crab attack frequencies, at a given locality.
Despite the promising findings of Thompson et al. (Reference Thompson, Jenkins and Bussell2000), studies utilizing this novel method are lacking, and there have been no attempts to expand the use of wax replicas beyond limpets. While limpets comprise an important part of many crab species' diets (Chapin, Reference Chapin1968; Lowell, Reference Lowell1986; Cannicci et al., Reference Cannicci, Gomei and Vannini2002; Silva et al., Reference Silva, Hawkins, Boaventura and Thompson2008, Reference Silva, Hawkins, Clarke, Boaventura and Thompson2010a), expanding the application of this technique to other gastropod groups would facilitate novel studies of crab foraging and prey preference. When attacking limpets, crabs utilize four main attack strategies: apex crushing; edge crushing; sliding; and prying (Lowell, Reference Lowell1986; Tyler et al., Reference Tyler, Leighton and Kowalewski2014). All of these strategies can be utilized when attacking wax replicas of limpets. Strategies for attacking spirally coiled gastropods, however, differ and include shell crushing and aperture peeling. Crushing is employed if a snail is small enough to fit within the gape of the crab's claw (Lawton & Hughes, Reference Lawton and Hughes1985); alternatively, if a snail is too large to fit in the claw, peeling will be employed. Peeling involves the insertion of the propus or dactyl into the aperture to hold the prey, while using the other claw to chip away at the margin of the shell (Lawton & Hughes, Reference Lawton and Hughes1985; Stafford et al., in press, b). Although crushing can still be utilized when attacking wax replicas, peeling cannot, as replicas are bolted to the substrate and cannot be removed by the crab. Replicas should, however, still record crabs' attempts to grapple prey, including preparation and orientation of prey for peeling, and it is reasonable to assume that crabs will attack replicas of other types of gastropods. If replicas are to be used to examine crab foraging and prey preference, however, this assumption must first be tested before studies simultaneously deploying spirally coiled and cap shaped gastropods can be conducted, to ensure that this is indeed the case.
As wax models provide in situ data derived from organisms in their natural habitats, these data can be used to test ecological hypotheses comparing attack frequencies, and can thus be used to obtain high resolution data on short term temporal variation in predation. Although laboratory observations or experiments can provide data under controlled conditions, organisms' behaviour can be influenced by captivity (e.g. limited food choice, unusual substrate, restricted foraging area, unfamiliar prey and inability to escape), and in many cases extrapolating laboratory based conclusions to natural populations may not be appropriate. Furthermore, as crabs do not appear to discriminate between wax models and live prey (Thompson et al., Reference Thompson, Jenkins and Bussell2000) the experimental design should not alter behaviour, in contrast to other in situ methods, such as exclusion caging and prey tethering (Barbeau & Scheibling, Reference Barbeau and Scheibling1994; Zimmer-Faust et al., Reference Zimmer-Faust, Fielder, Heck, Coen and Morgan1994; Kneib & Scheele, Reference Kneib and Scheele2000). Unlike direct in situ observations, wax models also facilitate the acquisition of large amounts of data, and do not require specialized or expensive equipment. As direct field observations are either restricted to brief intervals when organisms are exposed (at low tide), or require the use of SCUBA or video equipment (e.g. Lowell, Reference Lowell1986; Wootton, Reference Wootton1992; Iwasaki, Reference Iwasaki1993; Thompson et al., Reference Thompson, Jenkins and Bussell2000), these techniques result in monitoring of only a few individuals during a brief interval. Furthermore, both low tide and SCUBA observations require fair weather, calm ocean conditions, and are typically only conducted during daylight hours. As many predatory crabs are more active at night (Stevens et al., Reference Stevens, Armstrong and Hoeman1984; Robles et al., Reference Robles, Sweetnam and Dittman1989; Holsman et al., Reference Holsman, McDonald and Armstrong2006), observations restricted to daylight foraging may bias data regarding crab predation. Alternatively, recording equipment can be deployed for continuous, unobtrusive monitoring; however, such equipment is expensive and difficult to deploy, and can produce hundreds of hours of viewing material with only a few observations. Wax models can thus be used to obtain high resolution data on short term temporal variation in predation.
Due to the difficulties described above, predation proxies have been frequently employed when testing hypotheses requiring large amounts of quantitative data derived directly from natural populations over broad geographical regions or repeated sampling over longer time scales (e.g. Vermeij et al., Reference Vermeij, Schindel and Zipser1981; Vermeij, Reference Vermeij1987; Schindler et al., Reference Schindler, Johnson, MacKay, Bouwes and Kitchell1994; Cadee et al., Reference Cadee, Walker and Flessa1997; Alexander & Dietl, Reference Alexander, Dietl, Kelley, Kowalewski and Hansen2003; Stafford & Leighton, Reference Stafford and Leighton2011; Moody & Aronson, Reference Moody and Aronson2012). There are many types of predation proxies (variables correlating with predation mortality or attack frequency) such as predator population censuses (including the identities, abundances and size distributions of predator species), prey morphology (defensive prey characteristics, such as thick shells or spines) or predation traces on prey skeletons (such as repair scars or drill holes).
In intertidal gastropod communities, repair scars are a common proxy for crab predation intensity (e.g. Schindler et al., Reference Schindler, Johnson, MacKay, Bouwes and Kitchell1994; Cadee et al., Reference Cadee, Walker and Flessa1997; Preston & Roberts, Reference Preston and Roberts2007; Stafford et al., in press, a). Repair scars are distortions of the growth lines where prey that has survived an attack has replaced damaged shell material, recording non-fatal shell-crushing attacks (frequently by crabs) on molluscs. It is important to note that although repair scars do record attacks, they only provide quantitative information regarding attacks that failed (i.e. excluding mortality). Differences in repair frequency can thus result from either an increase in the number of attacks, or in a decrease in attack success rate (Vermeij, Reference Vermeij1987). Nevertheless, repair scars record actual attacks and can be used to produce quantitative, high-resolution data for comparing crab predation among localities or environments (Molinaro et al., in press; Stafford et al., in press, a). Repair scars also provide data on attacks over the lifetime of an organism, averaging out the ‘noise’ of short-term ecological fluctuations (e.g. seasonal variation and anomalous conditions) that may inhibit the recognition of trends in predation. Furthermore, predator success rate between prey morphologies will influence repair frequency differences between populations (Cadee et al., Reference Cadee, Walker and Flessa1997), and comparisons of repair frequencies between morphologically distinct populations are thus a factor of both attack frequency and predator success rates. Therefore, as repair scars (1) record only failed attacks, (2) cover the organisms' entire lifespan, and (3) possibly should only be compared between morphologically similar species, wax replicas can be used when hypothesis testing requires higher temporal resolution data of actual number of attacks. Replicas would also be well suited to studies requiring short-term ecological data.
Wave-exposed habitats are known to be less hospitable to crushing predators, as strong waves can dislodge foraging predators and disrupt feeding (Robles et al., Reference Robles, Alvarado and Desharnais2001) resulting in fewer attacks. Intertidal gastropods thus experience lower predation intensity at wave exposed sites relative to more sheltered localities, where durophagous predators, such as crabs, occur in greater densities (Raffaelli & Hughes, Reference Raffaelli and Hughes1978; Raffaelli, Reference Raffaelli1982; Boulding & Van Alstyne, Reference Boulding and Van Alstyne1993; Iribarne et al., Reference Iribarne, Fernandez and Armstrong1994; Yamada & Boulding, Reference Yamada and Boulding1996; Leonard et al., Reference Leonard, Levine, Schmidt and Bertness1998; Boulding et al., Reference Boulding, Holst and Pilon1999; Robles et al., Reference Robles, Alvarado and Desharnais2001; Molinaro et al., in press). As a result, gastropod populations at protected sites may also have larger and thicker shells and smaller apertures to resist predation (e.g. Kitching et al., Reference Kitching, Muntz and Ebling1966; Heller, Reference Heller1976; Boulding et al., Reference Boulding, Holst and Pilon1999). Protected environments also provide more favourable foraging conditions and longer foraging periods (Leonard et al., Reference Leonard, Levine, Schmidt and Bertness1998; Boulding et al., Reference Boulding, Holst and Pilon1999). Thus predatory attack frequency, and repair scar frequency is typically greater at wave protected localities (Molinaro et al., in press; Stafford et al., in press, a). By comparing data derived from repair scars to that of wax replicas between low and high energy environments, we can determine the effectiveness of wax replicas at capturing known predation gradients.
This study aims to test the effectiveness of utilizing wax gastropod replicas to capture crab attack frequency in gastropod prey populations, other than limpets, in rocky intertidal habitats of Vancouver Island, British Columbia (Canada). Wax replicas of three species of gastropods, Chlorostoma funebralis (Gmelin), Nucella ostrina (Gould) and Nucella lamellosa (Adams), were deployed at two localities, one wave-protected and one exposed. The gastropod populations were also surveyed and repair scar frequencies were obtained at both localities. If wax replicas accurately capture crab attack frequency, predation trace frequencies on wax replicas should be consistent with repair scar data, in that both wax traces and repair scar frequency should be greater at wave protected localities.
MATERIALS AND METHODS
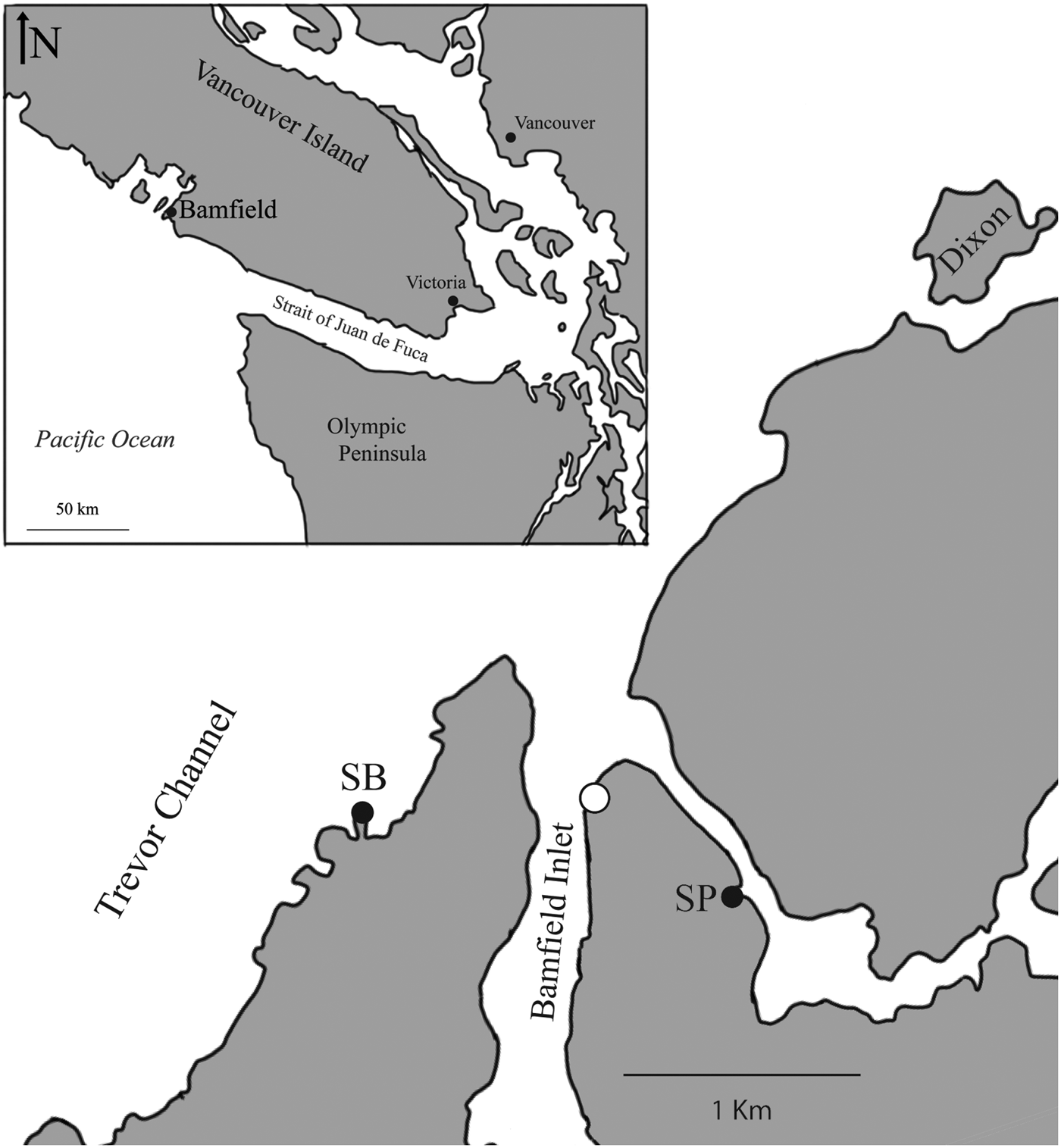
To evaluate the effectiveness of wax replicas in capturing crab attack frequency on intertidal gastropods, wax replicas were deployed and monitored at two localities near the Bamfield Marine Sciences Centre on Vancouver Island (British Columbia, Canada) on the southern side of Barkley Sound: Scott's Bay, and Strawberry Point (Figure 1). Water velocities at Scott's Bay are dominated by waves; the exposed site faces the open water of Barkley Sound and thus experiences significant wave forces (mean maximum daily wave forces of 1.85 m s−1; Robles et al., Reference Robles, Sweetnam and Dittman1989). Strawberry Point is a wave protected habitat, and maximum water velocities are driven by the incoming tides (mean maximum daily wave forces of 0.07 m s−1; Bates et al. unpublished dataset via A.R. Palmer, personal communication). This protected site is located within an inlet, and is sheltered from large waves.

Fig. 1. Study area and location of screen deployment sites. Study area is located in the city of Bamfield marked on the inset in the top left. SB, Scotts Bay (high energy); SP, Strawberry Point (low energy). White circle denotes location of the Bamfield Marine Sciences Centre.
Moulds of three abundant species of Pacific north-west intertidal gastropods were made to produce wax replicas: Chlorostoma funebralis (Turbinidae; formerly Tegula funebralis); Nucella ostrina (Muricidae); and Nucella lamellosa (Muricidae). Chlorostoma funebralis is a medium-sized, globose gastropod with a round aperture, ~10–30 mm shell height in our study area. Chlorostoma funebralis has a thick shell with no ornament (Figure 2A), and tends to aggregate at the base of boulders or among cobbles. Chlorostoma funebralis is present in both exposed and protected rocky intertidal habitats. Nucella ostrina is medium sized, has a well-developed spire, and strongly developed spiral ridges, ~12–30 mm shell height in our study area. Nucella lamellosa is a medium-sized, moderately high-spired gastropod, ~15–40 mm shell height in our study area. Nucella lamellosa has a thick shell with a narrow, elongate aperture with a thickened lip (Figure 2B). The shell surface ranges from smooth to strongly frilled. Nucella ostrina is found among boulders and on rock faces predominantly at wave exposed sites, while N. lamellosa is found among boulders and cobbles in protected habitats with abundant barnacles. These species are common prey for intertidal crabs in the area, most commonly Cancer productus (Abbott & Haderlie, Reference Abbott, Haderlie, Morris, Abbott and Haderlie1980; Appleton & Palmer, Reference Appleton and Palmer1988; personal observation).

Fig. 2. Gastropod species: (A) Nucella ostrina; (B) Nucella lamellosa; (C) Chlorostoma funebralis. The large curved indentations distorting growth lines on the N. lamellosa and C. funebralis specimens are repair scars.
Moulds were made for each species using liquid latex; replicas were made by pouring cream coloured crafting wax into moulds and placing a screw in the centre of the replica such that the tip of the screw was projecting out of the aperture, so that replicas could be bolted to plastic mesh screens for deployment in the field. Before field deployment, we established the lack of crab response to replica colour in laboratory experiments using the cancrid crab Cancer productus (Randall, 1839). Crabs were directly observed attacking both painted and bare wax replicas in equal proportions in the laboratory; wax was, therefore, left bare for field deployment, in contrast to Thompson et al. (Reference Thompson, Jenkins and Bussell2000) where wax models were painted to resemble live limpets. In addition to establishing that crabs do indeed attack wax replicas, laboratory observations also confirmed that crab attacks leave strikingly characteristic grooves on the wax replicas, and that these grooves can be easily identified.
Replicas were deployed in two field seasons during the summers of 2009 and 2010. At each locality, two 0.5 by 1 m screens were deployed, to which replicas were bolted in four rows (Figure 3). Screens were placed on the low shore in areas densely populated by Nucella, Chlorostoma and other gastropod species, and were fully submerged during high tides. As chemosensation is important in crab foraging (Rittschof, Reference Rittschof1992), placing screens within dense patches of gastropods ensured that waterborne prey cues signalling the presence of prey were in close proximity to wax replicas. Screen placement in the low shore ensured that crabs could access replicas throughout the tidal cycle when foraging, as adult crabs forage during times of submergence (Robles et al., Reference Robles, Sweetnam and Dittman1989; Yamada & Boulding, Reference Yamada and Boulding1996).

Fig. 3. Setup of wax replica deployment: an example of the screen setup in the field, with wax models attached. Image taken during low tide at Scott's Bay.
Screens were monitored every three days; all replicas were examined, and any replicas with scratch marks, divots, or any kind of damage were removed and replaced with identical, unmarked replicas of the same species (Figure 3). A pilot study utilizing daily monitoring and replacement indicated that monitoring every third day accurately captured cumulative daily attack frequencies (i.e. using the same number of replicas, total attacks for a single three-day trail were comparable to the cumulative total of three daily trials). Although an individual replica may be attacked multiple times in any three-day period, our estimates of attack frequencies are conservative, as only the number of marked individuals was counted.
In 2009, both Nucella ostrina and Nucella lamellosa replicas were deployed, 19 and 38 mm in length, respectively (measured from apex to base of aperture). Screens were monitored every three days for 15 days. 15 N. ostrina and 15 N. lamellosa were attached to each screen, with sizes alternated within rows, for a total of 30 replicas per screen, and 60 per locality. In 2010 N. lamellosa and Chlorostoma funebralis were deployed (N. lamellosa 38 mm and C. funebralis 19 mm). Replicas of N. ostrina were not used, as attack frequencies from the 2009 deployment did not differ across N. ostrina and N. lamellosa replicas in 2009 pooled across both localities (Yates's χ2 = 3.5, P = 0.06). One screen with 17 N. lamellosa and 18 C. funebralis and the other with 18 N. lamellosa and 17 C. funebralis were deployed at each locality for a total of 35 replicas per screen, and 70 per locality. Species were alternated within rows. Screens were monitored every three days for 27 days.
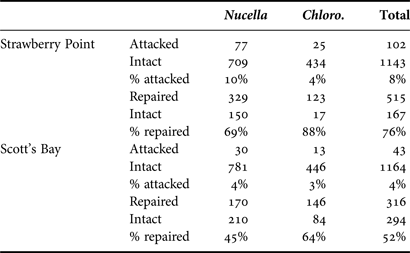
To determine the relative predation pressure from crabs at each locality, surveys of repair scars on both gastropod species were conducted at each locality examining gastropods living at 1–2 m above mean lower low water (Table 1). Repair data were collected at each locality over the course of four summer field seasons (2009–2012), resulting in a minimum of 600 individuals surveyed at each locality. As repair frequency should be greater at the wave protected locality (Stafford et al., in press, a), if wax replicas accurately reflect attack frequencies, attack frequencies will be congruent with the repair scar data and the proportion of marked replicas at the wave protected locality should also be greater.
Table 1. Attack and repair frequency by locality.

Both attack and repair frequencies are greater overall at wave protected Strawberry Point compared with the higher energy Scott's Bay, and for both Nucella spp. and Chlorostoma funebralis (Chloro.) individually. Both attacked and repaired categories are calculated using number of individuals, and not number of traces. Intact replicas are models that showed no traces of having being attacked (i.e. replica is complete and has a smooth, unaltered surface).
Repair scars are jagged disruptions in the shell surface, disrupting growth lines or shell ornament (Stafford et al., in press, b). Although repair scars can result from non-predatory breakage, scars resulting from crab predation are distinctive, and can be identified by the following criteria (Leighton, Reference Leighton, Carlson and Sandy2001; Kowalewski, Reference Kowalewski, Kowalewski and Kelley2002; Dietl & Kosloski, Reference Dietl and Kosloski2013; Stafford et al., in press): scar shape is non-random (e.g. trapezoidal) reflecting shape of attack structure (e.g. chelae); scar is not concentric with growth lines; and/or matching scars observed on both sides of the shell (suggests that predator enclosed and attempted to crush prey). We noted whether each specimen bore at least one repair scar or was free of scars, and repair frequency was calculated as the total number of specimens bearing at least one repair, divided by the total number of specimens, a widely used and conservative calculation (Kowalewski, Reference Kowalewski, Kowalewski and Kelley2002; Leighton, Reference Leighton2002; Alexander & Dietl, Reference Alexander, Dietl, Kelley, Kowalewski and Hansen2003; Dietl & Kosloski, Reference Dietl and Kosloski2013).
A χ2 test was used to determine whether attack and repair frequency were significantly different between wave protected and wave exposed localities. Marks interpreted as traces made by crab walking legs may overestimate attack frequency, and it is possible that some of these marks may not be the product of intentional attacks. The χ2 test was, therefore, repeated using only traces identified as being made by crab chelae, which are distinctive evidence of attempts to crush the prey. Statistical analyses were performed using R (v.2.15.1).
RESULTS
Screens with wax replicas attached were monitored a total of 15 times during the course of the study, resulting in 2452 observations (Table 1; Figure 4) with 102 individuals attacked at Strawberry Point out of 1245 observations, and 43 attacked individuals at Scott's Bay out of 1207 observations.

Fig. 4. Number of attacked individuals for every three-day monitoring period for both localities: (A) Strawberry Point 2009; (B) Strawberry Point 2010; (C) Scott's Bay 2009; (D) Scott's Bay 2010. Each field season is shown separately for each locality; note that in 2010 both Nucella lamellosa (black solid line), and Chlorostoma funebralis (blue dashed line) were deployed.
Crab attacks resulted in characteristic grooves in the wax surface, and in the majority of cases, attacks made with the chelae could be distinguished from those made by the walking legs (Figure 5). Chelae attacks typically consisted of very deep, wide grooves in the wax, with matching marks on both sides of the gastropod, presumably resulting from crabs attempting to grasp or crush the prey replica (Figure 5). Grooves produced by walking legs were typically much shallower and thinner, and most likely resulted from crabs attempting to dislodge prey replicas from the screens. 105 marked replicas were assigned to the ‘chelae’ category (Table 2), 12 were ascribed to walking legs, and 17 had both. Only 11 marks could not be confidently classified, but were considered attacks in analyses. Occasionally, when monitoring was conducted, a replica would be entirely absent from the screen; lost replicas were excluded from analyses, as the occurrence of predatory encounters could not be verified.

Fig. 5. Crab attack trace marks: (A) undamaged large Nucella lamellosa model; (G, H) undamaged small Nucella lamellosa models; (B, D, E, F) matching marks on either side of the replicas were typical of marks identified as being made by crab chelae; (C) the three small circular indentations visible on the main whorl are typical of marks identified as being made by the walking legs. Scale bars are 10 mm.
Table 2. Attacks by assigned appendage type.

Marks made by crab chelae make up the greatest proportion of attacks. Note that the number of marked individuals that could not be classified is very low (‘No ID’).
When all attack traces are pooled, attack trace frequency differs significantly between the two localities (χ2 = 21, P ≪ 0.0001), and is greater at Strawberry Point (Table 1). Similarly, when the data are conservatively restricted to chelae attack traces, attack frequency differs significantly between the two localities (χ2 = 18, P ≪ 0.0001), and is greater at Strawberry Point.
Repair scar surveys of gastropod populations resulted in the examination of 1292 individuals, 831 of which had a minimum of one repair scar (Table 1). The number of repairs differs significantly between localities (χ2 = 58, P ≪ 0.0001) and, consistent with attack frequency, is greater at Strawberry Point.
DISCUSSION
Attack frequencies were greater at the sheltered locality (Table 1), consistent with repair frequency data and previous research (Raffaelli & Hughes, Reference Raffaelli and Hughes1978; Raffaelli, Reference Raffaelli1982; Boulding & Van Alstyne, Reference Boulding and Van Alstyne1993; Iribarne et al., Reference Iribarne, Fernandez and Armstrong1994; Yamada & Boulding, Reference Yamada and Boulding1996; Leonard et al., Reference Leonard, Levine, Schmidt and Bertness1998; Boulding et al., Reference Boulding, Holst and Pilon1999; Robles et al., Reference Robles, Alvarado and Desharnais2001), indicating that wax replicas were an effective means of capturing and quantifying attack frequencies. Crab attacks produced characteristic and readily identifiable marks on the wax, which, in many cases, could be used to distinguish which appendages the predator used (chelae vs walking legs). Studies of crab foraging suggest that crabs feeding on molluscs may not rely exclusively on vision, or may merely respond to the strongest stimuli, either tactile or visual (Hughes & Seed, Reference Hughes and Seed1995). In encounters with gastropod prey, tactile stimuli could thus be the dominant sensing method, and cancrid crabs may, therefore, attack replicas because they ‘feel’ like their live counterparts, as replicas are identical in shape and size. Alternatively, cancrid crabs may be unable to visually distinguish live gastropods from wax replicas. At both localities, attacks made using the chelae greatly outnumbered marks made by the walking legs, suggesting that crushing and/or peeling is the preferred method of attack with regards to these species of gastropod prey, consistent with previous studies (Lawton & Hughes, Reference Lawton and Hughes1985; Yamada & Boulding, Reference Yamada and Boulding1998). Although sites were not monitored daily in this study, protocols employing daily monitoring could be logistically feasible depending on their duration and geographical scope. Although this method has only been attempted using limpets (Thompson et al., Reference Thompson, Jenkins and Bussell2000) and muricid and trochid gastropods (this study), the results of both studies suggest that wax replicas should also prove fruitful in investigations of crab predation with respect to other species of gastropods. Thompson et al. (Reference Thompson, Jenkins and Bussell2000) observed that 40% of limpet replicas deployed were marked over the course of 28 days (Port St Mary), and observed a daily attack frequency of 9% (Prawle Point).
While the number of attacks recorded by wax replicas was dramatically lower than the number of repaired individuals in surveyed populations, this is to be expected, as repair scars provide cumulative data over the lifetime of a gastropod. Chlorostoma funebralis, for example has a potential maximum age of over 30 years (Frank, Reference Frank1975), and would, therefore, be likely to encounter predators frequently during its lifetime. These data also emphasize the variability in attack frequencies over short time periods; although mean attack frequency at Scott's Bay in 2009 (Figure 4C) was 3.3, 18 attacks were recorded in the first 3 day trial, while only two attacks were recorded over the following 15 days, while consistently low numbers of attacks were observed over the study interval at the same locality in 2010. Although repair scars are records of failed attacks, repair frequency is primarily a function of attack frequency in this particular predator–prey system (Stafford et al., in press, a). The consistency between attack frequency and repair frequency, in terms of the relative proportion between the two localities (i.e. both repair and attack frequency were greater at Strawberry Point), therefore confirms that replicas are accurately recording short-term predation intensity.
Although there is some evidence that crabs may become habituated to the use of inedible models, habituation, when observed, has resulted in a reduction in handling or grappling time (Hughes & Seed, Reference Hughes and Seed1995) and not in a decrease in number of attacks. Furthermore, as habituation has been examined using repeated feeding of individual crabs in laboratory settings, it is unclear whether crabs would become habituated in natural settings, where their foraging range is unrestricted. The likelihood of habituation may be dependent on the frequency with which individual crabs return to the same prey patch, which in turn may be related to the size of the crabs' foraging range or prey density at a given locality. As crab attack frequencies did not decline over the course of the study, either habituation did not occur, or the crab population was sufficiently dense to produce a steady supply of ‘new’ crabs (i.e. crabs that had not yet foraged on the patch of wax replicas).
CONCLUSIONS
Crabs attacked wax replicas of gastropods, leaving characteristic marks in the wax. The appendage used in the attack could be readily identified from the traces left on the wax (i.e. chelae vs walking legs). The effectiveness of wax replicas in recording predator–prey interactions was verified using surveys of repair scar frequencies. The frequency with which replicas were attacked was consistent with data derived from repair frequencies of the gastropod populations; predation intensity was greater at the wave protected, quiet water locality. This method allows for the collection of a large amount of quantitative data documenting crab–gastropod interactions without the need for expensive equipment or hours of direct observation. This study confirms the value of wax replicas in investigations of gastropod predation, and illustrates the potential of this method for evaluating predation intensity among environments, across geographical gradients and through time.
ACKNOWLEDGEMENTS
We thank several field assistants including K. Barclay, P. Twerdy, B. Collins, A. Webb, M. Ahmed, E. Richards, N. Chojnacki and C. Schneider, and S. Roberts for assistance with photography. We thank the staff of Bamfield Marine Sciences Centre for facilities, resources, and guidance. E. Boulding and R. Palmer provided valuable input during this project. Thanks also to P. Kelley and C. Visaggi for constructive reviews which improved the quality and clarity of this manuscript.
FINANCIAL SUPPORT
This research was funded by an NSERC Discovery Grant and a National Geographic Discovery Grant to Leighton. Funding was provided by the NSF (EAR-1243484) to Tyler. Additional funding was provided by a Paleontological Society Ellis L. Yochelson Student Research Award, a Conchologists of America Academic Grant, and a Geological Society of America Student Research Grant to Stafford.