Introduction
Catfishes comprise diverse marine, estuarine and freshwater species, with varied habitat use and migratory patterns worldwide (Figueiredo & Menezes, Reference Figueiredo and Menezes1978; Nelson, Reference Nelson2006). Some of them migrate for thousands of kilometres, for example the large Amazonian catfish, Brachyplatystoma rousseauxii (Duponchelle et al., Reference Duponchelle, Pouilly, Pécheyran, Hauser, Renno, Panfili, Darnaude, García-Vasquez, Carvajal-Vallejos, García-Davila, Doria, Berail, Donard, Sondag, Santos, Nunez, Point, Labonne and Baras2016) and others migrate for hundreds of kilometres, for example the marine catfish, Genidens barbus, in southern Brazil (Milani & Fontoura, Reference Milani and Fontoura2007; Fontoura et al., Reference Fontoura, Schulz, Alves, Silveira, Pereira and Antonetti2019). The Ariidae family inhabits numerous coastal and fluvial systems in tropical and subtropical regions (Barletta & Blaber, Reference Barletta and Blaber2007). As they tolerate strong salinity variations (Dantas et al., Reference Dantas, Barletta, Costa, Barbosa-Cintra, Possatto, Ramos, Lima and Saint-Paul2010) and present complex patterns of habitat use (Duponchelle et al., Reference Duponchelle, Pouilly, Pécheyran, Hauser, Renno, Panfili, Darnaude, García-Vasquez, Carvajal-Vallejos, García-Davila, Doria, Berail, Donard, Sondag, Santos, Nunez, Point, Labonne and Baras2016; Avigliano et al., Reference Avigliano, Leisen, Romero, Carvalho, Velasco, Vianna, Barra and Volpedo2017), each population needs specific studies to have their nursery and breeding areas understood, to provide crucial information for their conservation (Chittaro et al., Reference Chittaro, Finley and Levin2009; Hegg et al., Reference Hegg, Giarrizzo and Kennedy2015).
The Guri sea catfish Genidens genidens (Cuvier, 1829) occurs along the east coast of South America from north-eastern Brazil to northern Argentina (Marceniuk & Menezes, Reference Marceniuk and Menezes2007), especially in estuaries and coastal lagoons. It may also be sporadically found in low numbers along marine regions adjacent to coastal lagoon systems (Araújo, Reference Araújo1988, Reference Araújo1996; Denadai et al., Reference Denadai, Bessa, Santos, Fernandez, Santos, Feijó, Arcuri and Turra2012). As usually reported for other marine catfishes, the male incubates eggs and larvae in its mouth (Chaves, Reference Chaves1994; Garcia et al., Reference Garcia, Vieira and Burns2006) during a period of 2–3 months (Barbieri et al., Reference Barbieri, Santos and Andreata1992). This parental care usually takes place in estuaries, which represents their main breeding areas throughout their geographic distribution (Gomes et al., Reference Gomes, Araújo, Azevedo and Pessanha1999; Rocha & Freire, Reference Rocha and Freire2009). Previous studies based on size-structure data have included G. genidens in the ‘estuarine resident’ guild (Garcia et al., Reference Garcia, Vieira and Winemiller2001; Gomes & Araújo, Reference Gomes and Araújo2004; Schmidt et al., Reference Schmidt, Martins, Reigada and Dias2008; Vieira et al., Reference Vieira, Garcia, Moraes, Seeliger, Odebrecht and Castello2010; Denadai et al., Reference Denadai, Bessa, Santos, Fernandez, Santos, Feijó, Arcuri and Turra2012). A recent study, however, reported that artisanal fisheries usually capture marine catfishes (up to 10% of total landings) in the northern-freshwater portion of the Patos Lagoon, Brazil, and that G. genidens is one of the usually landed species (Ceni et al., Reference Ceni, Fontoura and Cabral2016). These observations motivated us to hypothesize that G. genidens may consistently use the full freshwater reaches of the lagoon and may even reproduce in this area far away from the estuarine/freshwater boundary.
Patos Lagoon is the largest coastal lagoon in South America (10,360 km2) and G. genidens is a frequent and abundant species in its estuarine area (Chao et al., Reference Chao, Pereira, Vieira and Yáñez-Arancibia1985; Vieira et al., Reference Vieira, Garcia, Moraes, Seeliger, Odebrecht and Castello2010), being rare along the adjacent marine coast (Rodrigues et al., Reference Rodrigues, Cabral and Vieira2015). Araújo (Reference Araújo1988) showed that G. genidens occurs from the higher salinity conditions in the estuarine mouth (lower estuary) to the lower salinity conditions upstream (upper estuary), with higher abundances observed towards lower salinities. Reproduction occurs within the lagoon, in the boundary between estuarine and fresh water, and juveniles have been reported to live in the estuarine region (Araújo, Reference Araújo1988). Although G. genidens has been observed in the upstream freshwater reaches of the lagoon hundreds of kilometres away from its connection with the sea (Fontoura et al., Reference Fontoura, Schulz, Alves, Silveira, Pereira and Antonetti2019), no reproduction has ever been registered in full freshwater conditions.
Diverse ecological tools may help to examine the way G. genidens uses freshwater habitats. For example, the chemical analysis of otoliths has been successfully applied to evaluate displacement patterns in migratory fishes (Thorrold et al., Reference Thorrold, Latkoczy and Swart2001; Wells et al., Reference Wells, Rooker and Itano2012; Aschenbrenner et al., Reference Aschenbrenner, Ferreira and Rooker2016) and to distinguish fish stocks (Campana et al., Reference Campana, Chouinard, Hanson, Frechet and Brattey2000; Tabouret et al., Reference Tabouret, Bareille, Claverie, Pécheyra, Prouzet and Donard2010; Fortunato et al., Reference Fortunato, Galán, Alonso, Volpedo and Durà2017). This technique allows reconstruction of the chemical conditions experienced by migratory teleost fishes during their development (Campana, Reference Campana1999; Elsdon et al., Reference Elsdon, Wells, Campana, Gillanders, Jones, Limburg, Secor, Thorrold and Walther2008; Avigliano & Volpedo, Reference Avigliano and Volpedo2016) and has been increasingly used over the past 20 years (Secor et al., Reference Secor, Henderson-Arzapalo and Piccoli1995; Patterson et al., Reference Patterson, Kingsford and McCulloch2005; Albuquerque et al., Reference Albuquerque, Miekeley and Muelbert2010; Arai et al., Reference Arai, Kotake, Harrod, Morrissey and McCarthy2019). Strontium (Sr) and barium (Ba) concentrations in otoliths usually correlate positively and negatively with salinity, respectively (Elsdon & Gillanders, Reference Elsdon and Gillanders2002; Elsdon et al., Reference Elsdon, Wells, Campana, Gillanders, Jones, Limburg, Secor, Thorrold and Walther2008; Jessop et al., Reference Jessop, Shiao and Iizuka2013). Therefore, fish inhabiting marine habitats tend to present higher otolith concentrations of strontium and lower barium compared with freshwater fish. Another useful analytical tool to understand species' habitat use is the microscopic analysis of their gonads, which can reveal if reproduction occurs in a given habitat (e.g. Condini et al., Reference Condini, Fávaro, Varela and Garcia2014a). For example, the presence of atretic oocytes, with distended ovarian membrane and disruption of reproductive tissues suggest recent spawning, thus indicating reproduction areas (McMillan, Reference McMillan2007; Jakobsen et al., Reference Jakobsen, Fogarty, Megrey and Moksness2009). Thus, associating otolith microchemistry and microscopic analysis of gonads may significantly improve our understanding of the way G. genidens uses estuarine and freshwater habitats.
In this study, we associated otolith microchemistry and gonadal analyses to evaluate nursery origins (estuary vs fresh water) and freshwater use by G. genidens inside Patos Lagoon, the largest coastal lagoon in South America. We hypothesize that some G. genidens individuals consistently use fresh water and may even reproduce in the northern freshwater portion of the lagoon, fairly distant (~180 km) from the estuarine boundary.
Materials and methods
Study area and sampling
The Patos Lagoon (PL) is located on the coastal plain of southern Brazil (Figure 1). It constitutes the largest lagoon in South America (Kjerfve, Reference Kjerfve and Wolfe1986) with a total area of 10,360 km2 and presents an estuarine portion of ~1000 km2 (Seeliger, Reference Seeliger, Seeliger and Kjerfve2001). Freshwater environments represent about 80% of the PL area year-round (Seeliger, Reference Seeliger, Seeliger and Kjerfve2001). The PL receives fresh water from rivers located in the northern reaches of the coastal plain of Rio Grande do Sul state and from the Mirim Lagoon, which together comprise a drainage basin of ~14,000 km2 (Möller & Fernandes, Reference Möller, Fernandes, Seeliger and Odebrecht2010; Fontoura et al., Reference Fontoura, Vieira, Becker, Rodrigues, Malabarba, Schulz, Möller, Garcia and Vilella2016). The PL connects to the ocean through a 1.5–0.5 km wide channel fixed by two 5 km long rocky jetties (Seeliger et al., Reference Seeliger, Odebrecht, Castello, Seeliger, Odebrecht and Castello1997).

Fig. 1. Patos Lagoon (10.360 km2) in the state of Rio Grande do Sul, southern Brazil, showing the two sampling areas (asterisks): (1) the estuarine region (hatched area) and (2) the freshwater region at the northern reaches of the lagoon (2).
A total of 137 individuals were collected in the PL from 2012 to 2013. From these, 48 fish (19 from fresh water and 29 from estuarine water) were analysed for otolith chemistry and 89 (from fresh water) were analysed for gonadal development. Estuarine fish were sampled with bottom trawls close to Rio Grande city (Figure 1, site 1). Freshwater fish were sampled through multi-mesh gillnets at the north portion of the Patos Lagoon, about 180 km from the estuary (Figure 1, site 2). Biometric data, such as total length (TL, mm) and total weight (TW, g) were measured for all individuals in the laboratory.
To characterize mean salinities along the sampling area we used data from two different datasets. The first dataset included estuarine salinities obtained monthly from 2012 to 2014 by the Brazilian Long Term Ecological Research (B-LTER) project developed in Rio Grande city (Figure 1, site 1). The second dataset included the freshwater region of Patos Lagoon (Figure 1, site 2), where salinities were obtained also monthly from 2012 to 2014 by researchers from the PUC-RS University.
Preparation of samples for otolith microchemistry analysis
A sub-sample of 48 individuals (29 from estuarine and 19 from fresh water) sampled from January 2012 to February 2013 had their otolith chemically analysed. The pair of otoliths lapillus was chosen for chemical analysis because they are the most developed in Ariid catfishes (Acero & Betancur, Reference Acero and Betancur2007). After extraction, otoliths were cleaned, dried, embedded in crystal polyester resin and sectioned in 0.3 mm thick sections using a metallographic saw (Buehler, Lake Bluff, IL). The sections were fixed in a histological slide with quick drying glue and were polished, washed with Milli-Q water, rinsed with deionized water and stored in a laminar flow cabinet prior to the chemical analysis.
Each otolith was chemically analysed along a transect between its core and edge using a Nd-YAG laser ablation system (CETAC, LSX 100) operating at 266 nm wavelength and output power of 0.3 to 0.4 mJ per pulse. This system was coupled to an LA-ICPMS (Perkin Elmer NEX-Ion) operating with a power of 1200 watts and using Argon as carrying gas. The abundances of calcium (43Ca), strontium (86Sr) and barium (138Ba) were analysed. Blank ablations (background intensities) were measured during 50 s after every 10 otolith ablations. Strontium and calcium counts per second (cps) were subtracted from the background level, and element:Ca ratios were then calculated for all otoliths. Before the analysis, the instrument was optimized by daily performance using the maximum intensities to analysis and with minimum interference through the use of oxides and double charged ions. The accuracy of the analysis was evaluated by estimating the relative standard deviation of 10 standard consecutive measurements NIST 1834, resulting in relative standard deviations (RSD) of 3.99, 6.91 and 12.43% for 43Ca, 86Sr and 138Ba, respectively.
Determination of gonadal development
A sub-sample of 89 individuals obtained from January 2012 to May 2013 at the freshwater site had their gonads analysed to evaluate whether reproduction may occur in the freshwater portion of Patos lagoon, about 180 km far from the estuary. Given that estuarine spawning and reproduction were already well documented (Barbieri et al., Reference Barbieri, Santos and Andreata1992), we did not analyse gonads from estuarine-sampled fish. In the laboratory, fish were dissected with a ventral incision to extract their gonads, which were fixed in 10% formalin. Gonads were processed manually, dehydrated in a series of increasing alcohol concentrations, and embedded in paraffin, then sectioned in slices of 7 µm thickness with the aid of a rotational microtome and, subsequently, fixed in histological slides. Each section was stained with haematoxylin-eosin (HE). Stages of gonadal development were determined both by macroscopic inspection and microscopic identification using transmitted light under a dissecting microscope at 15–30× magnification, following the maturation scale used by Brown-Peterson et al. (Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-Barbieri2011). Gonadosomatic index (GSI) of G. genidens was determined following the equation: GSI = (GoW/TW-GoW)×100, where TW is the fish total weight (g) and GoW is the weight of the gonad (g).
Data analysis
Fish age was inferred from the analysis of growth increments in otoliths. All procedures for otolith processing followed Condini et al. (Reference Condini, Albuquerque and Garcia2014b). The periodicity of increment formation in otoliths was not validated for G. genidens in a subtropical region. Validation studies for other species (Micropogonia furnieri, Mugil liza, Epinephelus marginatus), including the congeneric species G. barbus have shown that the formation of one increment per year seems to be the rule along the Patos Lagoon (Reis, Reference Reis1986; Schwingel & Castello, Reference Schwingel and Castello1990; Garbin et al., Reference Garbin, Castello and Kinas2014; Condini et al., Reference Condini, Albuquerque and Garcia2014b). Therefore, we assume growth increments form annually for G. genidens. Differences on mean water salinities between estuary and fresh water were evaluated through one-way Kruskal–Wallis test, given that our data followed a non-parametric distribution.
Mean Sr:Ca and Ba:Ca ratios in otolith core and edge were compared between estuary and fresh water using a one-way non-parametric Kruskal–Wallis test, as data were also non-parametric. The otolith edge was defined as the ~30 µm nearest to the edge of the otolith, whereas the core region was defined as the first 60 µm away from the centre of the otolith nucleus. Given that fish size may affect otolith elemental composition in some species (Campana, Reference Campana2001; Albuquerque et al., Reference Albuquerque, Miekeley and Muelbert2010; Condini et al., Reference Condini, Tanner, Reis-Santos, Albuquerque, Saint'Pierre, Vieira, Cabral and Garcia2016), we repeated the non-parametric Kruskal–Wallis test on a subset of 15 fish (7 from the estuary and 8 from freshwater lagoon) with similar size (290–340 mm). Finally, we assessed life-long conditions experienced by each fish from Sr:Ca and Ba:Ca ratios measured along core-to-edge otolith profiles. These core-to-edge chemical profiles were used as proxies for inferring habitat use along the freshwater and estuarine regions in the study area. The chemical threshold between freshwater and estuarine habitats was defined as the mean Sr:Ca ratio measured in the edge of freshwater otoliths, plus one standard deviation (as suggested by Mohan et al., Reference Mohan, Halden and Rulifson2015 and Fuji et al., Reference Fuji, Kasai, Ueno and Yamashita2016). Therefore, Sr:Ca ratios in otoliths over this threshold were interpreted as estuarine use during a given amount of time.
Results
Out of the 48 individuals of G. genidens collected for otolith microchemistry, total length (TL) of estuarine fish ranged from 58–345 mm (0+ to 15 years), whereas the freshwater fish ranged from 293–465 mm TL (5 to 24 years). As expected, according to the historical salinity datasets, mean salinity in the estuary (7.87 ± 9.56) was significantly higher than in fresh water (0.11 ± 0.04) (KW-H(1, 149) = 17.9857; P < 0.001) (Figure 2). During the fish samplings (2012–2013), salinity ranged from 2–7 in the estuary and remained always below 0.2 in the freshwater portion of the Patos Lagoon, at about 180 km from the estuarine region.

Fig. 2. Average salinity values (± SD) in the estuarine and freshwater sites of Patos Lagoon where fish were sampled.
Elemental ratios in the otolith cores (which represent early life) were similar between estuarine and freshwater fish (KW-H(1, 469) = 1.5392, P > 0.05 for Sr:Ca, and KW-H(1, 469) = 4.2880, P > 0.05 for Ba:Ca; Figure 3A, B). In contrast, the edge compositions (which represent recent deposition) were significantly different between sampling areas, following the salinity gradient along the Patos Lagoon. Mean Sr:Ca ratios in otolith edges (Figure 3C) were significantly higher in fish sampled in estuarine waters (0.68 ± 0.13) than those caught in fresh water (0.33 ± 0.09) (KW-H(1, 224) = 136.8922; P < 0.001). An opposite pattern was observed for Ba:Ca ratios in the otolith edges (Figure 3D), which were higher in those fish caught in freshwater (0.12 ± 0.03) than in those from the estuary (0.08 ± 0.04, P < 0.001) (KW-H(1, 224) = 32.2094; P < 0.001). The same pattern was observed in the subset of otoliths from fish with similar sizes (Figure 3E, F).
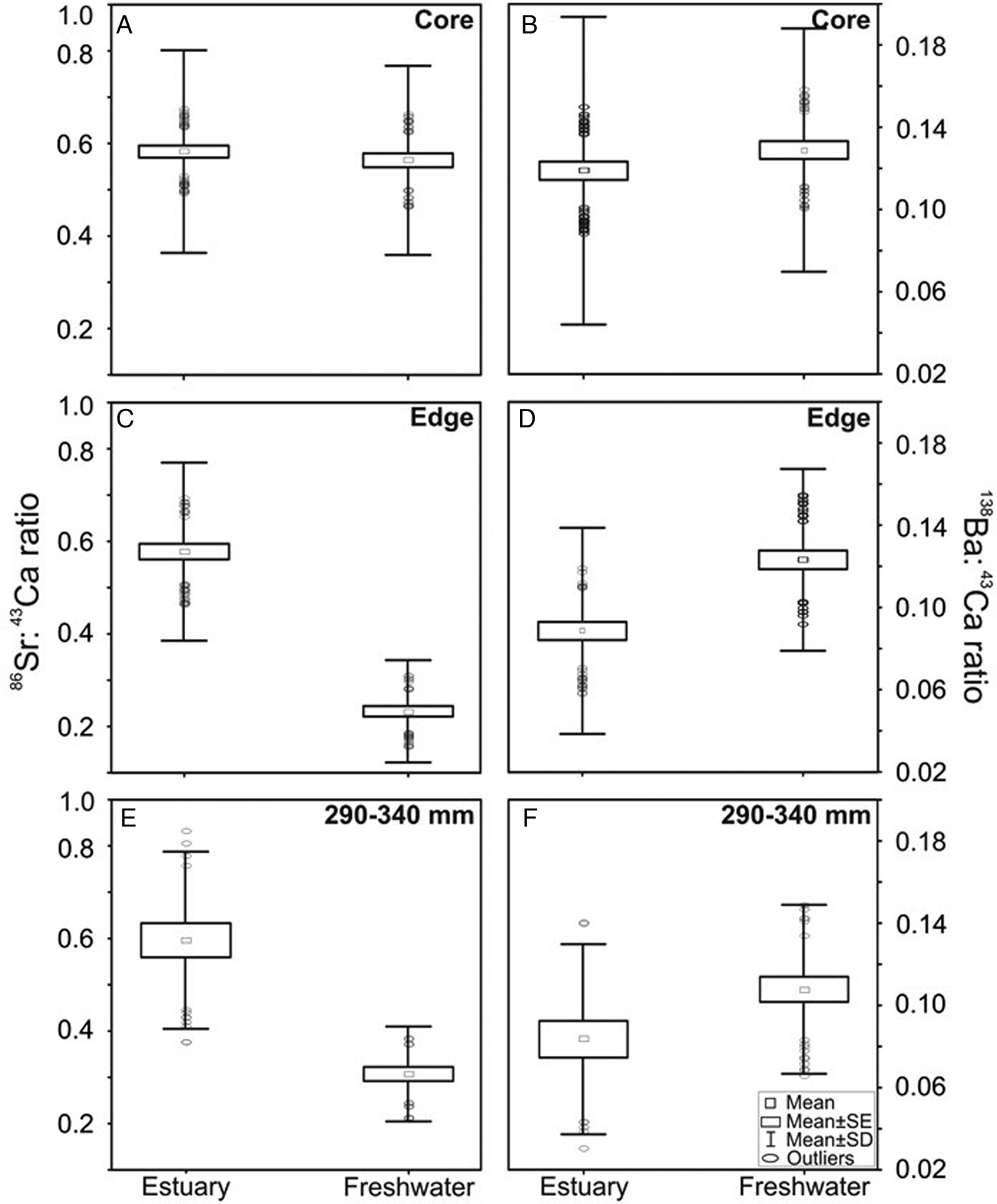
Fig. 3. Mean values (± SD) of the concentrations of Sr:Ca (left) and Ba:Ca (right) ratios at the core (panels A, B) and the edge (panels C, D) sections of the otoliths of individuals of Genidens genidens caught in the estuarine and freshwater sites of Patos Lagoon. Average values (±SD) of the concentrations of Sr:Ca (left) and Ba:Ca (right) ratios from the edge of the otoliths of individuals of G. genidens from similar size classes (between 290 and 340 mm TL) (panels E, F).
Strontium signatures in otolith edges differed significantly and did not overlap between estuarine and fresh water (Figure 3C). Barium signatures, instead, overlapped between estuarine and fresh water, despite their mean values differing statistically (Figure 3D). As this chemical overlapping may confound our ability to interpret fish movements from otolith composition, we discarded Ba:Ca ratios and opted to use Sr:Ca = 0.44 as the threshold between fresh and estuarine water.
The analysis of Sr:Ca life-time profiles in otoliths of adult fish revealed that the Guri sea catfish may present at least two clearly distinct patterns of habitat use between estuarine and fresh water along Patos Lagoon (Figure 4). The habitat use pattern here named ‘type 1’ (found in 89.5% of the sampled individuals, Figure 4A) includes fish with Sr:Ca ratios in the otolith core above the fresh water threshold (0.44) suggesting they were born and had early development in the estuarine area. Some of them appear to have migrated to fresh water after early life (see Sr:Ca ratio profiles of individuals presented in the supplementary Figure, e.g. M, Q, EE). The second pattern, named ‘type 2’ (9.5%, Figure 4B), includes fish whose otolith cores presented Sr:Ca ratios below the fresh water threshold, thus suggesting fresh water origin. All fish under this classification appear to migrate towards the estuary during early life, sometimes migrating back to fresh water later in life (e.g. see samples KK to SS in the supplementary Figure). Regardless of sampling site, otolith composition suggests all fish experienced estuarine or mixohaline waters during at least part of their juvenile life (Figure S1).

Fig. 4. Core-to-edge profiles of Sr:Ca ratios (black line) in otoliths of catfish Genidens genidens sampled in the Patos Lagoon. The dashed line indicates the threshold value between freshwater and estuarine habitats. The arrows indicate the age (years) along the otolith profiles. To assist in viewing the fluctuation of Sr:Ca ratio along the profiles of the otoliths, the absolute values has been converted into 3-order moving averages.
Out of the 89 specimens collected in the freshwater region of the lagoon, 53 were females with sizes (TL) ranging between 272 and 450 mm and 36 were males with sizes between 123 and 435 mm (Table 1). Microscopy analysis of gonads revealed that sampled females were developing (N = 14) or spawning capable (N = 15), with stages of oocytes development clearly discernible. The advanced maturation was characterized by the predominance of ovarian follicles with yolk granules associated with pre-vitellogenic follicles and distinct stages of development, such as reserve stock oocytes, oocytes with full vitellogenesis and nest of young germinative cells (Figure 5A). Based on microscopy identification of gonads and GSI analysis, we distinguished developing (mean GSI = 2.5, ranging from 0.9–3.8, Table 1) from spawning capable (GSI = 4.9, ranging from 4.0–6.5) females. Also, 24 females presented atretic oocytes, regressing phase (GSI = 0.6, ranging from 0.3–1.2) (Figure 5B), with distended membranes and disruption of reproductive tissue, thus indicating recent spawning and supporting the second pattern of habitat use (Type 2). Microscopic analysis of male gonads revealed 22 fish (Table 1) with a massive presence of seminiferous tubules filled with sperm (Figure 5C), thereby indicating sexual maturity and spawning capability. We also found 14 regressing males with empty testes presenting cell breakdown and the presence of lumen (Figure 5D), thus indicating recent spawning.
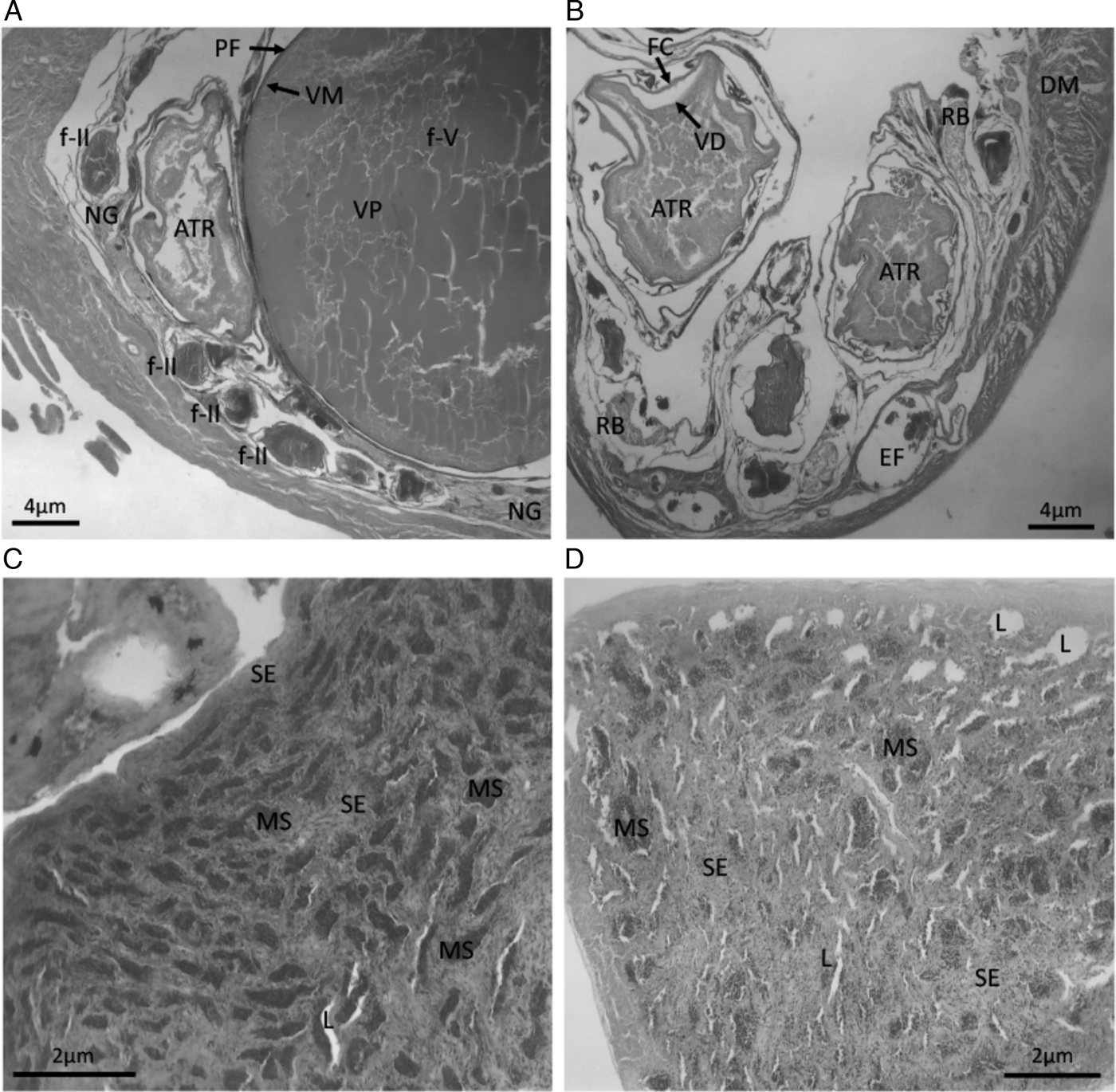
Fig. 5. Cross section of Genidens genidens ovaries and testes in different stages of development, obtained from individuals captured at the freshwater portion of Patos Lagoon. (A) Spawning capable ovary with oocytes in distinct stages of development; (B) Empty ovary presenting disarranged tissues with empty space due to oocytes released; (C) Mature spawning capable testes presenting filled seminiferous tubules; (D) Empty testes presenting few remaining sperm; ATR, atretic oocytes; f-II, Reserve stock oocytes; f-V, oocytes with full vitellogenesis; NG, Nest of young germinative cells; VM, vitelline membrane; PF, palisade of follicular cells; VP, vitelline platelets; DM, ovary distended membrane; FC, follicular cells; RB, residual body; EF, empty follicle; SE, seminiferous epithelium; L, lumen and MS, mature spermatozoa.
Table 1. Total number of individuals sampled (N), mean and range (min-max) values of total length (mm), total number of individuals with gonad analysed (n), mean and and range (min-max) of gonadosomatic index (GSI, g) in three development stages (developing, spawning capable, regression) of the Guri catfish (Genidens genidens)

Discussion
Our findings revealed that G. genidens individuals may present different patterns of habitat use along estuarine and fresh water reaches of the largest coastal lagoon in South America. Moreover, the combination of otolith microchemistry and gonadal analysis supports our initial hypothesis that some individuals of G. genidens may spawn in fresh water, which challenges its previous classification as an estuarine resident (Mishima & Tanji, Reference Mishima and Tanji1981, Reference Mishima and Tanji1983; Araújo, Reference Araújo1988; Rabitto & Abilhôa, Reference Rabitto and Abilhôa1999; Gomes & Araújo, Reference Gomes and Araújo2004; Schmidt et al., Reference Schmidt, Martins, Reigada and Dias2008; Denadai et al., Reference Denadai, Bessa, Santos, Fernandez, Santos, Feijó, Arcuri and Turra2012).
Several findings support the rationale that otolith elemental composition reflects the surrounding water composition and, indirectly, salinity patterns in the sampled region. First, mean Sr:Ca and Ba:Ca ratios in the edges of otoliths of G. genidens differed significantly between individuals caught at estuarine and freshwater sites. We observed high Sr:Ca ratios in otoliths towards estuarine/marine waters and high Ba:Ca ratios towards fresh water, as has already been demonstrated for other marine and estuarine species in this same region (Albuquerque et al., Reference Albuquerque, Miekeley, Muelbert, Walther and Jaureguizar2012; Mai et al., Reference Mai, Condini, Albuquerque, Loebmann, Saint'Pierre, Miekeley and Vieira2014; Condini et al., Reference Condini, Tanner, Reis-Santos, Albuquerque, Saint'Pierre, Vieira, Cabral and Garcia2016; Avigliano et al., Reference Avigliano, Leisen, Romero, Carvalho, Velasco, Vianna, Barra and Volpedo2017). Second, Sr:Ca ratios in the water along the Patos Lagoon increase with salinity, particularly from zero to 15 PSU (Albuquerque et al., Reference Albuquerque, Miekeley, Muelbert, Walther and Jaureguizar2012), which supports that Sr:Ca variations in otoliths may indicate fish movements along the local salinity gradient. Third, we analysed a subsample of individuals with standardized sizes and the general findings remained nearly identical to the ones obtained for the whole sample, which supports the absence of significant ontogenetic influence on Sr:Ca and Ba:Ca incorporation into G. genidens otoliths. Based on these three lines of evidence, the fluctuations of Sr:Ca in otoliths will hereafter be interpreted as fish movements across the salinity gradient along the Patos Lagoon.
The analysis of individual core-to-edge chemical profiles in otoliths suggests G. genidens may present two distinct patterns of habitat use associated with their origins (early development) in estuarine waters (type 1) and fresh water (type 2). The Sr:Ca profiles also revealed that all sampled fish show estuarine signatures during a consistent part of their juvenile life. Hence, even fish born in fresh water appear to migrate to estuarine waters, where they grow for a while. Based on our findings, we hypothesize that G. genidens remains in the estuarine region for about one to five years. After that, most individuals would stay in the estuary throughout their whole lifespan, whereas others may migrate towards fresh water, where they may stay for extended periods. Therefore, G. genidens show a quite plastic and variable use of estuarine and freshwater habitats. Our findings corroborate the results of Araújo (Reference Araújo1988), which suggests that young fish (recruits) are found exclusively in estuarine areas. Future studies should evaluate if this pattern may represent a strategy to explore the high autochthonous estuarine production found in this lagoon (Seeliger et al., Reference Seeliger, Odebrecht, Castello, Seeliger, Odebrecht and Castello1997).
Such intraspecific variation in habitat use and natal habitats (estuary vs freshwater) could be explained in the light of the partial migration theory, which claims the existence of groups of individuals behaving as residents or migrants in the same population (Chapman et al., Reference Chapman, Skov, Hulthén, Brodersen, Nilsson, Hansson and Brönmark2012a). Apparently, the decision to stay or migrate is linked to distinct environmental conditions or biological interactions (e.g. competition and/or predation pressure) experienced by each individual fish within the population (Chapman et al., Reference Chapman, Skov, Hulthén, Brodersen, Nilsson, Hansson and Brönmark2012b). We speculate that some individuals of G. genidens may seek freshwater reaches of the Patos Lagoon to ameliorate interspecific competition with the congeneric species G. barbus and G. planifrons (Araújo, Reference Araújo1988), which also use the estuarine zone of this lagoon as feeding grounds (Araújo, Reference Araújo1984; Pereyra et al., Reference Pereyra, Mont'Alverne and Garcia2016). Partial migration and the occurrence of contingents were also reported for the congeneric marine catfish G. barbus, in the adjacent La Plata River, Argentina and south Brazil (Avigliano et al., Reference Avigliano, Leisen, Romero, Carvalho, Velasco, Vianna, Barra and Volpedo2017). This congeneric marine catfish from La Plata estuary and other southern Brazilian estuaries also exhibited complex life histories with four different displacement patterns, ranging from estuarine-residency to freshwater-residency (Avigliano et al., Reference Avigliano, Leisen, Romero, Carvalho, Velasco, Vianna, Barra and Volpedo2017).
Associating otolith chemical profiles to gonad analysis provided us with useful insights on habitat use and displacement patterns along salinity gradients. Our results uncovered a marked plasticity in habitat use by G. genidens that has not been previously described. Many studies have suggested that reproduction was restricted to estuarine areas (Mishima & Tanji, Reference Mishima and Tanji1981; Chao et al., Reference Chao, Pereira, Vieira and Yáñez-Arancibia1985; Araújo, Reference Araújo1988; Gomes & Araújo, Reference Gomes and Araújo2004; Schmidt et al., Reference Schmidt, Martins, Reigada and Dias2008; Denadai et al., Reference Denadai, Bessa, Santos, Fernandez, Santos, Feijó, Arcuri and Turra2012), but our findings revealed that this species also spawns in truly freshwater sites fairly distant (~180 km) from the estuary. This consistent use of fresh water was also demonstrated by the low Sr:Ca ratios in the otolith core of some individuals (e.g. ‘type 2’ habitat use pattern). This pattern was independently corroborated by microscopic gonadal analysis, which revealed clear signs of spawning and post-spawning for both sexes in freshwater sites at the extreme north portion of Patos Lagoon.
We recognize that the observed otolith chemical profiles may also be influenced by specific climatic events that alter the regular freshwater discharge of the Patos Lagoon into the estuary. For example, strong El Niño events (e.g. 1982–83 and 1997–98) have been associated with high inflow of fresh water at Patos Lagoon estuary due to increased rainfall over its drainage basin (Garcia et al., Reference Garcia, Vieira, Winemiller and Grim2004). Also, numerical simulations suggested that El Niño events may decrease the frequency and intensity of ocean water intrusions into the estuary, leading to abnormally low salinities in the area (Barros et al., Reference Barros, Marques and Kirinus2014). However, our samples comprise fish from age zero to 20 years, thus decreasing the importance of sporadic climatic events on the composition of G. genidens otoliths. Thus, we assume these climatic events did not hinder our ability to interpret fish movements from otolith chemistry. Furthermore, we emphasize that the consistent use of limnic regions of Patos Lagoon by G. genidens was supported not only by otolith chemistry, but also by direct sampling in fresh water and gonadal histological analysis.
By accepting the hypothesis that part of the G. genidens population consistently uses fresh water for reproduction and early development, we challenge the current classification of this marine catfish species as estuarine-resident, as suggested by several authors (Mishima & Tanji, Reference Mishima and Tanji1981, Reference Mishima and Tanji1983; Araújo, Reference Araújo1988; Rabitto & Abilhôa, Reference Rabitto and Abilhôa1999; Gomes & Araújo, Reference Gomes and Araújo2004; Schmidt et al., Reference Schmidt, Martins, Reigada and Dias2008; Denadai et al., Reference Denadai, Bessa, Santos, Fernandez, Santos, Feijó, Arcuri and Turra2012). In the light of our evidence, we suggest that the ‘estuarine and freshwater guild’ (Potter et al., Reference Potter, Tweedley, Elliott and Whitfield2013) may be more appropriated to encompass the plasticity in displacement patterns and spawning habitats observed for G. genidens in the present study. The ‘estuarine and freshwater guild’ includes species in which the individuals complete their life cycles within the estuary, but also includes fish who consistently use freshwater habitats (Potter et al., Reference Potter, Tweedley, Elliott and Whitfield2013). Moreover, it is important to consider that G. genidens may present distinct habitat-use patterns along other estuarine systems since these are dynamic environments with usually specific environmental variations. Therefore, additional studies may be required to better understand the role of G. genidens and other catfishes to estuarine and freshwater ecosystems along South America.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000493
Author ORCIDs
Mario V. Condini, 0000-0003-4917-7782; Gianfranco Ceni, 0000-0001-6236-1221; Nelson F. Fontoura, 0000-0001-9978-2812
Acknowledgements
We thank the Pelagic Fisheries Resources Laboratory at FURG for logistical support and otolith processing. This study was supported by the programme ‘Long Term Ecological Research (LTER) site 8: Estuary of the Patos Lagoon and adjacent marine region’ of the Ministry of Science and Technology (MCT) and the National Scientific and Technological Development Council (CNPq); and CNPq productivity scholarship (number: 310141/2015-0) and CNPq scholarship (number: 434466/2016-6).








